Medication Access and Continuity: The Experiences of Dual-Eligible Psychiatric Patients During the First 4 Months of the Medicare Prescription Drug Benefit
Abstract
Objective: This study attempted to systematically assess the experiences of Medicare and Medicaid “dual-eligible” psychiatric patients, including evaluating patients’ access to medications and the administrative functioning of the program, during the first 4 months of the Medicare Part D prescription drug benefit. Method: Psychiatrists (N=5,833) were randomly selected from the American Medical Association’s Physicians Masterfile. After exclusion of those not practicing and with undeliverable addresses, 64% responded; 35% met study eligibility criteria of treating at least one dual-eligible patient during their last typical workweek and reported clinically detailed information on one systematically selected patient. Results: A total of 53.4% had at least one medication access problem to report between Jan. 1 and April 30, 2006. Although 9.7% experienced improved medication access, 22.3% discontinued or temporarily stopped taking medication because of prescription drug coverage or management issues, and 18.3% were previously stable but were required to switch medications. Among those with medication access problems, 27.3% experienced a significant adverse clinical event; 19.8% had an emergency room visit. Most drug plan features studied, including preferred drug/formulary lists, prior authorization, medication dosing/number limits, “fail-first” protocols, and requirements to switch to generics, were associated with significantly higher rates of medication access problems. Conclusions: The findings indicate consequential medication access problems for psychiatric patients during the implementation of Medicare Part D. Although Centers for Medicare and Medicaid Services policies were enacted to ensure access to protected classes of psychopharmacologic medications, the high rates of medication access problems observed indicate further refinement of these policies is needed.
When the Medicare prescription drug benefit (Medicare Part D) took effect Jan. 1, 2006, 42 million Medicare enrollees for the first time obtained access to a federally sponsored prescription drug benefit. At the same time, approximately 6 million dual-eligible beneficiaries, with both Medicaid and Medicare insurance and chronic, complex medical and psychiatric conditions, went from receiving drug coverage from state Medicaid programs to receiving coverage through the new Medicare Part D program. The dual-eligible individuals, including approximately 2 million individuals with a psychiatric disorder significantly impairing their functioning, were automatically randomly enrolled in low-premium drug plans but permitted to choose a different plan to better meet their needs.
In recent years, state Medicaid programs have increasingly used prescription drug prior authorization and other management strategies, such as dispensing limits, requiring generics, and using copayments, to contain Medicaid prescription drug costs (1) . In a number of states, psychiatric medications were exempted from these requirements (2) . In these states, the therapeutic nonequivalence of psychopharmacologic medications for the treatment of heterogeneous mental disorders was acknowledged along with the concern that medication disruptions have been shown to be associated with symptom relapse or exacerbation, hospitalization, and other unintended adverse consequences among psychiatric patients (3 – 7) .
For example, Koyanagi et al. (2) reported that although 49 state Medicaid programs required prior authorization for medications, 30 states had exceptions to certain classes of psychiatric medications. Antipsychotic medications were most commonly exempted from these requirements.
The Medicare Part D program is provided under contracts with 1,429 prescription drug plans (8) that are generally private at-risk entities that may develop their own policies and formularies within parameters established by the Centers for Medicare and Medicaid Services. In implementing Part D, the Centers for Medicare and Medicaid Services acknowledged the special formulary challenges presented by the shift of psychiatric dual-eligible patients’ drug benefit to Medicare. The Part D prescription drug plan formularies were to be discretionary within guidelines developed by the U.S. Pharmacopeia, with at least two drugs having to be available in each of the established U.S. Pharmacopeia drug categories. The Centers for Medicare and Medicaid Services noted in its December 2004 formulary guidance that its expectations are that best-practice formularies contain “a majority of drugs” within the following classes: “antidepressants, antipsychotics, anticonvulsants, antiretrovirals, immunosuppressants, and antineoplastics.” A later clarification of this guidance explained that access to a “majority” of drugs within the six classes meant that the plans were, in fact, expected to provide access to “all or substantially all” of the drugs in these categories (9) . “All or substantically all,” however, did not require inclusion of all dosages or forms of the drugs. In addition, although the Centers for Medicare and Medicaid Services was prohibited by law from covering benzodiazepines, 48 state Medicaid programs agreed to provide coverage for benzodiazepines for dual-eligible individuals.
Medicare prescription drug plans were permitted to use a range of management strategies that have some support in improving drug safety and containing prescription drug costs for these six protected classes of drugs (10 – 12) . In its transition policy, the Centers for Medicare and Medicaid Services recognized the difficulties that could arise for enrollees with mental illness and required plans to establish an appropriate transition process for new enrollees moving to Part D from other sources of prescription drug coverage (9) . Specifically, this policy stated that patients “who were on a stable medication regimen” should not be subject to prior authorization or step therapy protocols when they existed (in the “all or substantially all” classes).
Although Centers for Medicare and Medicaid Services policies were in place to address these issues, there was considerable uncertainty regarding this transition and how and to what extent at-risk prescription drug plans would manage access to these classes of medications within the framework of the “all or substantically all” policy. There were also concerns regarding the ability of state Medicaid programs to manage the care of these patients without ready access to pharmacy claims data. Consequently, advocacy organizations, including APA, the National Alliance on Mental Illness, the National Association of State Mental Health Program Directors, the National Council for Community Behavioral Health Care, the National Mental Health Association, and Treatment Effectiveness Now, launched a website and an 800 help line that remains operational to serve as a central resource to help clinicians, patients, and family members prepare for the transition and address any problems that may arise (www.mentalhealthpartd.org). Given the potential for adverse clinical consequences, the American Psychiatric Institute for Research and Education (APIRE) implemented a national study to monitor the functioning of Medicare Part D among a large national sample of dual-eligible patients treated by psychiatrists. This study, which monitored the experiences of this population from Jan. 1, 2006, through April 30, 2006, was designed to do the following:
1. Systematically quantify the extent and nature of any medication access or continuity problems and any improvements in medication access or continuity
2. Quantify the extent of any adverse clinical events reported to have occurred as a result of unintended medication disruptions or access problems, including hospitalizations, emergency room visits, homelessness, and injury to self or others
3. Identify specific patient groups at increased risk for medication access problems
4. Evaluate the administrative functioning and requirements of the prescription drug plans and identify the features most strongly associated with medication access problems
This descriptive practice-based research study, which entailed collecting clinically detailed data directly from physicians in real time, was undertaken in recognition that the availability, timeliness, and overall utility of claims and administrative data to evaluate these issues was questionable. Claims and administrative databases would not be able to fully assess medication access problems or differentiate clinically desired from clinically undesired medication discontinuations or switches associated with the Medicare Part D transition. Consequently, the full range of problems and clinical consequences would be difficult or impossible to track, and administrative burdens and processes could also not be captured through claims and administrative data sources.
Although this study examines only patients of psychiatrists, this group is of particular interest because psychiatrists treat the majority of the nation’s individuals receiving treatment for schizophrenia and others with the most severe forms of mental illnesses (13 , 14) . Most of these patients are on clinically complex medication regimens, receiving multiple medications (15 , 16) .
Method
A total of 5,833 psychiatrists were randomly selected from the American Medical Association’s Physicians Masterfile of all U.S. psychiatrists (N=55,000). Psychiatry residents and those not listing direct patient care as their type of practice were excluded. After exclusion of psychiatrists not currently practicing (N=291) and with undeliverable addresses (N=439), responses were obtained from 64% of the target sample (N=3,247). Of these respondents, 35% (N=1,183) met the study eligibility criteria of treating at least one dual-eligible patient during their last typical work week.
Primary data collection was conducted from January through April 2006 using mailed-in, practice-based survey research methods. Psychiatrists reported clinically detailed data on one systematically selected patient with dual eligibility. Each psychiatrist was randomly assigned one of 21 start days and times to report on their next dual-eligible patient treated during their last typical work week. The average patient in the sample had 6 weeks to accrue a medication access problem during the study data collection period. Key questions included the extent of disruptions in medication access or continuity since Jan. 1, 2006, because of prescription drug plan coverage/administrative issues, adverse consequences since any medication access problems, and administrative time associated with prescription drug benefits for clinicians and their staff. The survey included a $75 check to increase the likelihood of response.
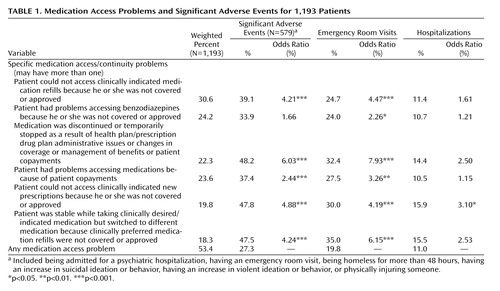
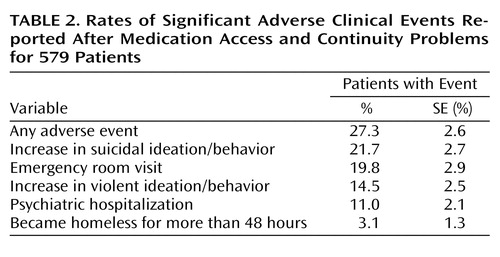
Rates of specific medication access and continuity problems reported are listed in Table 1 . Rates of significant adverse events among patients experiencing any medication access or continuity problems were examined as indicated in Table 2 to identify types of medication access problems associated with higher odds of significant adverse events, including emergency room visits and hospitalization. Odds ratios examine the relationship between specific prescription drug plan features and the likelihood of having a medication access or continuity problem reported. Finally, logistic regression identified prescription drug plan features most highly associated with medication access problems (dependent variable), with adjustment for month of visit and patient sociodemographic, diagnostic, and clinical factors.
Results
Patient Characteristics
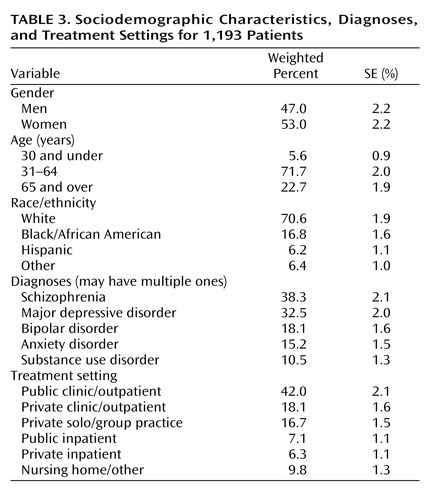
Approximately half of the patients were men, with the majority white and between 31 and 64 years of age ( Table 3 ). Nearly 40% had a diagnosis of schizophrenia, and 50% had a serious mood disorder of either major depression or bipolar disorder. Although 42.0% of the sample were treated in a public clinic or outpatient facility, 34.8% were treated in private outpatient clinics or solo/group practice settings, and 13.4% were seen in inpatient hospital settings during the sampled visit, a setting in which Medicare Part D does not apply.
Medication Access and Continuity Problems
Overall, 53.4% of the dual-eligible psychiatric patients studied had at least one problem with medication access or continuity, as indicated in Table 1 , reported between Jan. 1 and April 30, 2006. For 9.7% (SE=1.2%), the psychiatrist reported improved medication access/continuity as a result of prescription drug coverage since Jan. 1, 2006. Rates of medication access/continuity problems did not vary over time during the 4-month data collection period.
Specific medication access problems encountered are presented in column 1 of Table 1 . Most commonly reported problems included not being able to access clinically indicated medication refills (30.6%); problems accessing benzodiazepines (24.2%); and discontinuing or temporarily stopping medications as a result of health plan/prescription drug plan administrative, management, or coverage issues or because patients could not afford copayments (approximately 23% each). In addition, 18.3% of the patients were stable taking clinically indicated/desired medications but were required to switch to a different medication because clinically preferred medication refills were not covered or approved.
Among patients who could not be prescribed clinically indicated and clinically preferred medications (N=233), the most common medication classes that could not be prescribed included atypical antipsychotics (reflecting 21.9% of all clinically indicated medications that could not be prescribed), selective serotonin reuptake inhibitor antidepressants (20.7%), other antidepressants (16.5%), benzodiazepines (13.7%), and mood stabilizers/anticonvulsants (9.5%).
Adverse Clinical Events Reported After Clinically Undesired Medication Access Problems
As shown in Table 2 , 27.3% of patients with medication access or continuity problems had at least one significant adverse clinical event reported, with 1.9 (SE=0.1) significant adverse events reported per patient. Among patients who had medication access or continuity problems, 19.8% had a subsequent emergency room visit reported, and 11.0% had a hospitalization. An increase in suicidal ideation or behavior was reported for 21.7%, an increase in violent ideation or behavior occurred in 14.5%, and 3.1% became homeless for more than 48 hours.
Patients whose medication was discontinued or temporarily stopped were six times more likely (odds ratio=6.0) to have a significant adverse event (as defined in Table 1 ) and almost eight times more likely (odds ratio=7.9) to have an emergency room visit compared to patients with other types of medication access problems. In addition, four of the additional medication access problems in Table 1 were associated with significant rates of adverse events that were two to five times higher (odds ratios=2.4–4.9) than those of patients with other access problems.
The likelihood of being hospitalized did not vary with type of access problem. One exception was associated with not being able to access medications for which the likelihood of hospitalization was three times higher among those with this specific problem. However, we lack temporal data on the timing of hospitalizations in relation to the prescription access problem and recognize that Medicare Part D does not apply to hospital formularies. Hence, interpretation of the relationship between medication access and hospitalizations is somewhat confounded in this study.
Patient, Setting, and Prescription Drug Plan Features Associated With Medication Access Problems
Medication access or continuity problems were not associated with patient sociodemographic or treatment setting variables in Table 3 . However, patients with major depression (60.9%) and anxiety disorders (64.7%) were significantly more likely to report medication access or continuity problems (p<0.05).
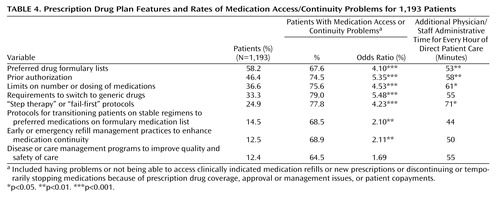
Patients with the prescription drug plan features in Table 4 reported high rates of medication access and continuity problems (74%–79%). Most of these were significantly associated with medication access or continuity problems. For plans requiring prior authorization, limits on the number or dosing of medications, requirements to switch to generics, and “step therapy” or “fail-first protocols,” medication access/continuity problems were four to five times more likely (odds ratios=4.23–5.35) compared to patients in plans without these features.
Logistic regression analyses were adjusted for month of visit and patient sociodemographic, diagnostic, and clinical factors. When these factors were controlled, patients in plans in which prior authorization was required had 2.5 times the likelihood of having a medication access problem (odds ratio=2.5, 95% confidence interval [CI]=1.4–4.2) compared to those in plans without this requirement. Likewise, those with requirements to switch to generics had 3.3 times the likelihood of reporting a medication access problem (odds ratio=3.3, 95% CI=2.0–5.6). Those with limits on the number or dosing of medications had 1.7 times the likelihood of reporting a medication access problem (odds ratio=1.7, 95% CI=1.0–3.0).
Prescription Drug Plan Administration Issues
Psychiatrists reported that they or their staff spent on average 45.6 minutes on administrative issues related to prescription drug plan coverage (including filling out paperwork and Internet, telephone, or other time with patients, prescription drug plans, pharmacies, or the Centers for Medicare and Medicaid Services) for every hour of direct patient care provided since Jan. 1, 2006. Patients with medication access problems required a little less than two times as much administrative time per hour of direct patient care (56 minutes versus 30 minutes) (p=0.0005). As indicated in Table 4 , many of the prescription drug plan features studied were associated with significantly greater administrative time.
For 29.7% of patients, the psychiatrist reported the patient having problems with prescription drug plan enrollment or changing to a desired plan. In addition, 27.4% of the patients had exceptions requests or appeals initiated on their behalf, whereas 19.3% of the psychiatrists reported changing or discontinuing clinically indicated medications rather than pursuing appeals or exceptions processes.
Study Strengths and Limitations
Although other groups have reported on the characteristics of the Medicare Part D prescription drug plans (17 , 18) and described general survey and focus group data characterizing pharmacists, physicians, state Medicaid directors, and mental health program directors’ experiences and reported problems with Medicare Part D implementation (19 – 21) , linked Medicaid and Medicare Part D claims data are not yet available to examine these issues more in depth on a clinical or patient level. Consequently, this study is unique in providing clinically detailed data on the experiences of a large national sample of dual-eligible psychiatric patients. The primary limitation is exclusive reliance on physician-reported, cross-sectional data with the potential for response, selection, and recall biases. Psychiatrists might not be fully aware of medication access/continuity problems experienced by their patients, and significant adverse clinical events were reported only for patients experiencing a medication access problem. Clinicians may be more inclined to report problems if they view the Part D program or prescription drug plans unfavorably. They are also more likely to be aware of prescription drug plan features if patients experience a medication access problem. Psychiatrists more likely to have experienced problems may have been more likely to respond or deviate from the systematic patient sampling protocol and preferentially select patients who experienced medication access problems. The physicians were, however, compensated to help minimize nonresponse bias.
To assess potential patient sampling biases, overall rates of medication access/continuity problems physicians estimated for their dual-eligible caseloads were compared to the patient-level rates of problems reported among the systematically selected dual-eligible sample presented in this study. Although these rates were generally consistent, the patient-level data indicated higher reported rates of medication access problems associated with switching previously stable patients to different medications and higher reported rates of discontinuing or temporarily stopping medications as a result of prescription drug plan administrative issues or drug coverage/management. Finally, the study did not assess levels of medication access problems before Part D nor was a comparison group available to assess the independent effect of Part D with control for temporal trends.
Discussion
To our knowledge, this study provides the first clinically detailed national data on the impact of Medicare Part D prescription drug plan management practices on psychiatric patients’ ability to obtain medications and maintain compliance as well as clinical outcomes experienced by patients with medication access problems under Part D. Although one in 10 patients was reported to have enhanced medication access as a result of the Part D benefit, approximately half of all patients were reported to have experienced at least one medication access or continuity problem. Nearly one-quarter were reported to have discontinued or temporarily stopped taking their medications, and nearly 20% were clinically stable but required to switch to a different medication during the first 4 months of Part D implementation. One-quarter of the patients with medication access problems experienced a significant adverse clinical event.
Since psychopharmacologic medications are considered the first-line treatments for individuals with the most severe forms of mental illness (22 – 24) , these findings raise concerns given high rates of symptom relapse or exacerbation, hospitalization, and other adverse consequences associated with disruptions in medication continuity (3–7). In fact, missing as few as one to 10 days of antipsychotic therapy during the course of 1 year nearly doubles the risk of hospitalization (25) .
Several factors account for the data observed. Although the Centers for Medicare and Medicaid Services policy of including all or substantially all of the three psychopharmacologic classes ensured inclusion of key medications and its policy explicitly stated that plans could not switch medications for clinically stable patients (unless the prescription exceeded Food and Drug Administration safety indications), this study indicates that the Part D prescription drug plans used formulary management protocols to restrict psychopharmacologic medication access and to switch clinically stable patients. The utilization management policies and practices that posed the most significant problems for psychiatric patients were the following:
1. Switching clinically stable patients taking clinically desired/indicated medications because medication refills were not covered or approved
2. Discontinuing clinically indicated/preferred medications
3. Denying coverage for clinically indicated medications
4. Limiting access to an indicated number or dosage of medications
In addition, lack of coverage of benzodiazepines under Part D presents access problems for these agents with clinical management implications as described below.
When used in the context of severely ill psychiatric patients, prescription drug plan utilization management protocols (as listed in Table 4 ) should balance current evidence and professional standards of care with a thorough consideration of an individual patient’s unique clinical needs and medical history to improve quality of care. Nearly all of the prescription drug plan care and utilization management protocols and features studied were positively associated with medication access or continuity problems. In addition, among patients experiencing medication access problems, all of the specific medication access problems and prescription drug clinical management approaches used by the prescription drug plans, as listed in Table 1 , were associated with a significantly greater likelihood of emergency room visits and other adverse clinical events.
Although it may be considered economically desirable to switch clinically stable patients to a lower cost medication in the same class, this practice is not supported by current Centers for Medicare and Medicaid Services policies, as described above, because clinical protocols for switching patients to different medications have not been well established (26 , 27) . In this study, among the sample of patients with medication access problems, those who were stable but were required by prescription drug plans to switch their medications had very high rates of emergency room visits and other significant adverse events highlighting how switching medications may significantly jeopardize patients’ well-being and potentially result in higher overall costs. New evidence has emerged showing that switching antipsychotic medications for patients with chronic schizophrenia is associated with greater side effects and higher discontinuation rates than maintaining patients on stable medication regimens (28) . Given the significant risks to patients, until there are well-studied, clinically acceptable, safe protocols for switching clinically stable psychiatric patients’ medications, prescription drug plans should discontinue these practices.
Prescription drug plan management practices also present significant efficiency problems in the real-time context of clinical practice. The time burden associated with many prescription drug plan features and medication access problems resulted in clinicians and their staff spending more time on prescription drug plan administrative issues than on total direct patient care. These data do not take into account the considerable time delays patients may experience in accessing their medications as a result of delays in rendering prior authorizations or medication coverage decisions. The overall quality of patient care may also diminish as a result of less time attending to patients’ other medical and psychosocial issues. Prescription drug plan practices impeding the timeliness, accessibility, or continuity of patient care need to be carefully evaluated.
A large proportion of patients, nearly one-quarter, experienced problems accessing benzodiazepines despite most all state Medicaid programs providing benzodiazepine coverage for this population. Because benzodiazepines are considered an evidence-based, recommended treatment for agitation, mood stabilization, anxiety, and sleep problems in patients with schizophrenia and other severe mental illnesses (22 – 24) , this presents a serious clinical problem. Better coordination of benzodiazepine coverage with state Medicaid programs or covering benzodiazepines directly through the Part D program is critically needed.
Conclusion
The findings from this study provide insight into the early experiences of a medically vulnerable psychiatric population under the Medicare prescription drug program administered largely by for-profit drug plans with discretion to manage benefits. APIRE has continued to track the experiences of this population under Part D, with follow-up studies conducted through the end of 2006 continuing to show significant rates of medication access problems. Although the Centers for Medicare and Medicaid Services adopted policies specifically to mitigate potential medication access problems for this population, its “all or substantially all” policy mandating inclusion of most key psychiatric drugs and prohibiting plans from switching clinically stable patients also permitted plan discretion as to prescription drug management protocols. Our findings indicate these prescription drug plan protocols (e.g., prior authorization, “step therapy,” limits on number/dosing of medications, etc.) proved to be significantly associated with medication access/continuity problems, clinically undesired medication switches, and serious adverse clinical events among many patients experiencing medication access problems. Without the Centers for Medicare and Medicaid Services “all or substantially all” policy, many key psychiatric drugs would likely not have been on prescription drug plan formulary lists at all, and it is highly likely that formulary management restrictions and medication access problems would have been greater.
The formulary challenges presented by dual-eligible psychiatric patients are significant (8 , 29) , and some prescription drug plan management protocols may prove insurmountable for medically vulnerable, cognitively impaired patients. There is a need to develop policies to facilitate timely access to clinically appropriate medications with cost management and “care management” strategies appropriate for the unique needs of this vulnerable population. Although it is vital that the Centers for Medicare and Medicaid Services maintain and better enforce the formulary protections it has established, it is also essential to develop clinically appropriate management strategies that take into account total health care costs and social costs (including homelessness and criminal justice system costs), while providing appropriate incentives for responding to the clinical needs of this severely ill patient population (30) .
1. Crowley J, Ashner D, Elam L: State Medicaid Outpatient Prescription Drug Policies: Findings From a National Survey. Kaiser Commission on Medicaid and the Uninsured, 2005 Update. http://www.kff.org/medicaid/7381.cfmGoogle Scholar
2. Koyanagi C, Forquer S, Alfano E: Medicaid policies to contain psychiatric drug costs. Health Aff (Millwood) 2005; 24:536–544Google Scholar
3. Drake RJ, Pickles A, Bentall RP, Kinderman P, Haddock G, Tarrier N, Lewis SW: The evolution of insight, paranoia, and depression during early schizophrenia. Psychol Med 2004; 34:285–292Google Scholar
4. Opler LA, Caton CL, Shrout P, Dominguez B, Kass FI: Symptom profiles and homelessness in schizophrenia. J Nerv Ment Dis 1994; 182:174–178Google Scholar
5. Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–247Google Scholar
6. Svarstad BL, Shireman TI, Sweeney JK: Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv 2001; 52:805–811Google Scholar
7. Swanson JW, Swartz MS, Borm R, Hiday VA, Wagner HR, Burns BJ: Involuntary out-patient commitment and reduction of violent behavior in persons with severe mental illness. Br J Psychiatry 2000; 176:324–331Google Scholar
8. Hoadley J, Hargrave E, Cubanski J, Neuman T: An In-Depth Examination of Formularies and Other Features of Medicare Drug Plans, The Henry J. Kaiser Family Foundation: The Medicare Drug Benefit, April 2006. http://kff.org/medicare/upload/7489.pdfGoogle Scholar
9. Centers for Medicare and Medicaid Services: Why Is CMS Requiring “All or Substantially All” of the Drugs in the Antidepressant, Antipsychotic, Anticonvulsant, Anticancer, Immunosuppressant and HIV/AIDS categories? http://www.cms.hhs.gov/PrescriptionDrugCovContra/Downloads/FormularyGuidance AllorSubAll.pdfGoogle Scholar
10. Smalley WE, Griffin MR, Fought RL, Sullivan L, Ray WA: Effect of a prior-authorization requirement on the use of nonsteroidal antiinflammatory drugs by Medicaid patients. N Engl J Med 1995; 332:1612–1617Google Scholar
11. Delate T, Mager DE, Sheth J, Motheral BR: Clinical and financial outcomes associated with a proton pump inhibitor prior-authorization program in a Medicaid population. Am J Manag Care 2005; 11:29–36Google Scholar
12. Hansen LB, Fernald D, Araya-Guerra R, Westfall JM, West D, Pace W: Pharmacy clarification of prescriptions ordered in primary care: a report from the Applied Strategies for Improving Patient Safety (ASIPS) collaborative. J Am Board Fam Med 2006; 19:24–30Google Scholar
13. Lehman AF, Steinwachs DM, Survey Co-Investigators of the PORT Project: Patterns of usual care for schizophrenia: initials results from the schizophrenia patient outcomes research team (PORT) client survey. Schizophr Bull 1998; 24:11–20Google Scholar
14. Pingitore DP, Scheffler RM, Sentell T, West JC: Comparison of psychiatrists and psychologists in clinical practice. Psychiatr Serv 2002; 53:977–983Google Scholar
15. West JC, Wilk J, Olfson M, Rae DS, Marcus S, Narrow WE, Pincus HA, Regier DA: Patterns and quality of treatment for patients with schizophrenia in routine psychiatric practice. Psychiatr Serv 2005; 56:283–291Google Scholar
16. West JC, Duffy FF, Wilk J, Rae DS, Narrow WE, Pincus HA, Regier DA: Patterns and quality of treatment for patients with major depressive disorder in routine psychiatric practice. Focus 2005; 3:43–50Google Scholar
17. Frakt AB, Pizer SD: A first look at the new Medicare prescription drug plans. Health Aff (Millwood) 2006; 25:W252–W261Google Scholar
18. Cubanski J, Neuman P: Status report on Medicare Part D enrollment in 2006: analysis of plan-specific market share and coverage. Health Aff (Millwood) 2007; 26:W1–W12Google Scholar
19. Kaiser Family Foundation: National Survey of Pharmacists and National Survey of Physicians: Findings on Medicare Part D, Medicare Policy Project and the Public Opinion and Media Research Program, September 2006. http://www.kff.org/kaiser polls/upload/7556.pdfGoogle Scholar
20. Smith V, Gifford K, Kramer S, Elam L: Observations on the Initial Implementation of the Medicare Prescription Drug Program: Perspectives of State Medicaid Directors Through a Focus Group Discussion, Kaiser Commission on Medicaid and the Uninsured, May 2006. http://www.kff.org/medicaid/upload/7520.pdfGoogle Scholar
21. Smith V, Gifford K, Kramer S, Elam L: The Transition of Dual Eligibles to Medicare Part D Prescription Drug Coverage: State Actions During Implementation: Results From a 50-State Snapshot, Kaiser Commission on Medicaid and the Uninsured, February 2006. http://www.kff.org/medicaid/upload/7467.pdfGoogle Scholar
22. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Revision. Am J Psychiatry 2000; 157(suppl)Google Scholar
23. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder, Revision. Am J Psychiatry 2002; 159(suppl)Google Scholar
24. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Schizophrenia, 2nd ed. Am J Psychiatry 2004; 161(suppl)Google Scholar
25. Weiden PJ, Kozma C, Grogg A, Locklear J: Partial compliance and risk or rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004; 55:886–891Google Scholar
26. Tse G, Thompson D, Procyshyn RM: Generic clozapine: a cost-saving alternative to brand name clozapine? Pharmacoeconomics 2003; 21:1–11Google Scholar
27. Kluznik JC, Walbek NH, Farnsworth MG, Melstrom K: Clinical effects of a randomized switch of patients from Clozaril to generic clozapine. J Clin Psychiatry 2001; 62(suppl 5):14–17Google Scholar
28. Essock SM, Covell NH, Davis SM, Stroup TS, Rosenheck RA, Lieberman JA: Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006; 163:2090–2095Google Scholar
29. Donohue J: Mental health in the Medicare Part D drug benefit: a new regulatory model? Health Aff (Millwood) 2006; 25:707–719Google Scholar
30. Polsky D, Doshi JA, Bauer MS, Glick HA: Clinical trial-based cost-effectiveness analyses of antipsychotic use. Am J Psychiatry 2006; 163:2047–2056Google Scholar