Cortical Serotonin 5-HT 2A Receptor Binding and Social Communication in Adults With Asperger’s Syndrome: An in Vivo SPECT Study
Abstract
Objective: The cause of autistic spectrum disorder (i.e., autism and Asperger’s syndrome) is unknown. The serotonergic (5-HT) system may be especially implicated. However, cortical 5-HT 2A receptor density in adults with the disorder has not been examined, to the authors’ knowledge. Method: The authors investigated cortical 5-HT 2A receptor binding in eight adults with Asperger’s syndrome and in 10 healthy comparison subjects with single photon emission computed tomography and the selective 5-HT 2A receptor ligand 123I iodinated 4-amino-N-[1-[3-(4-fluorophenoxy)propyl]-4-methyl-4-piperidinyl]-5-iodo-2-methoxybenzamide ( 123 I-5-I-R91150). Results: People with Asperger’s syndrome had a significant reduction in cortical 5-HT 2A receptor binding in the total, anterior, and posterior cingulate; bilaterally in the frontal and superior temporal lobes; and in the left parietal lobe. Also, reduced receptor binding was significantly related to abnormal social communication. Conclusions: The authors’ findings suggest that adults with Asperger’s syndrome have abnormalities in cortical 5-HT 2A receptor density and that this deficit may underlie some clinical symptoms.
Autistic spectrum disorder (comprising the subtypes of autism, Asperger’s syndrome, and pervasive developmental disorder not otherwise specified) affects approximately 60 per 10,000 people and is characterized by pervasive abnormalities in socioemotional communication and stereotypical and obsessional behaviors.
The cause of autistic spectrum disorder is poorly understood and is most likely multifactorial; however, the serotonergic (5-HT) system may be especially implicated. In nonhuman primates and nonautistic humans, the 5-HT system modulates social behavior, amygdala response to facial emotion (1) , and repetitive behaviors (see reference 2 for a review). Furthermore, the 5-HT 2A receptor modulates personality traits, such as harm avoidance (3) . People with autistic spectrum disorder are reported to have a significant decrease in platelet 5-HT 2A receptor binding (4 , 5) , and the 5-HT 2A receptor gene is a functional candidate gene in autism (see reference 6 for a review).
Thus, people with autistic spectrum disorder may have abnormalities in 5-HT 2A receptors. However, no one has directly investigated in vivo brain 5-HT 2A receptor binding in non-mentally retarded adults, to our knowledge. Therefore, we measured cortical 5-HT 2A receptor binding in adults with Asperger’s syndrome and in comparison subjects with single photon emission computed tomography (SPECT) and the selective 5-HT 2A receptor ligand 123I iodinated 4-amino-N-[1-[3-(4-fluorophenoxy)propyl]-4-methyl-4-piperidinyl]-5-iodo-2-methoxybenzamide ( 123 I-5-I-R91150). Based on prior work (4 , 5) , we tested the hypothesis that in people with Asperger’s syndrome, cortical 5-HT 2A receptors are significantly decreased.
Method
We studied eight adult men with Asperger’s syndrome (full-scale IQ measured with the WAIS-III: >80, range=80–120; age: mean=26 years, SD=6) who were educated until they were at least 18 years and 10 healthy male comparison subjects who did not differ significantly in age or education. All volunteers were diagnosed with ICD-10 criteria and the Autistic Diagnostic Interview—Revised (7) . For people with Asperger’s syndrome, the mean scores on the Autistic Diagnostic Interview—Revised for domain A (qualitative abnormalities in reciprocal social interaction), domain B (qualitative abnormalities in communication), and domain C (repetitive behavior) were, respectively, mean=18 (SD=3), mean=13 (SD=6), and mean=6 (SD=3).
People were excluded if they had a history of physical or psychiatric disorder affecting brain function (e.g., epilepsy), a genetic disorder associated with autistic spectrum disorder (e.g., fragile X syndrome), or clinically abnormal magnetic resonance imaging. The study had ethical approval. No subject was taking any medication, and none reported past use of any psychotropic medication (e.g., selective serotonin reuptake inhibitors).
SPECT scans were performed at the Institute of Nuclear Medicine, Middlesex Hospital, University College London Medical School, with a three-headed PRISM 3000XP scanner (Phillips Medical Systems, Cleveland) with low-energy ultra-high-resolution fan-beam collimators. A bolus dose of 180 MBq of 123 I-5-I-R91150 was administered intravenously. Sequential whole-brain images were acquired through a period of stable binding at pseudoequilibrium from 120 minutes to 280 minutes postinjection.
Whole-brain images were acquired with a resolution of 9–11 mm full width at half maximum. A transmission scan template, with a corresponding 123 I-5-I-R91150 specific emission scan template, was generated. As previously described, data were analyzed with the researcher automatically blind to subject status (e.g., see reference 8). In brief, cerebellar and cortical regions of interest based on the Talairach atlas were drawn on the emission scan template brain, and subsequent automatic data analysis examined between-group differences independent of the operator. The individual reconstructed and realigned image volumes were coregistered to the template images by using a nine-parameter (three-translation, three-rotation, and three-scaling) transformation based on the transmission image. Thus, mean activity per voxel for each region of interest was generated automatically for each subject. Average cerebellar uptake was used as a reference region because it is virtually devoid of 5-HT 2A receptors. Thus, an estimated binding potential can be generated for each cortical region by calculating the cortical/cerebellar ratio for each cortical region during the period of pseudoequilibrium.
Data were first examined for normality of distribution to conform to the assumptions of the statistics employed. Between-group differences in mean 5-HT 2A estimated binding potential were examined by using a nonparametric test (Mann-Whitney U, two-tailed), and statistical significance was defined as p<0.05. Also, within those with Asperger’s syndrome, we carried out a preliminary analysis (with a nonparametric test: Kendall’s tau) of the relationship between estimated binding potential and clinical symptoms as measured by the score on each of the three domains of the Autistic Diagnostic Interview—Revised. To reduce the number of statistical comparisons, we correlated only the estimated binding potential of the brain regions that were significantly different from those of the comparison subjects.
Results
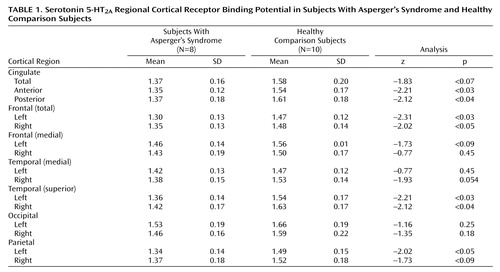
The individuals with Asperger’s syndrome had a significantly lower mean 5-HT 2A estimated binding potential than the comparison subjects in the anterior and posterior cingulate cortex, in the frontal and superior temporal cortex bilaterally, and in the left parietal cortex ( Table 1 ). Also, estimated binding potential in the anterior and posterior cingulate and the right frontal cortex was significantly correlated with increased qualitative abnormalities in reciprocal social interaction (domain A of the Autistic Diagnostic Interview—Revised); in each of these three regions, r=–0.837 and p<0.02.
Discussion
Our findings suggest that people with Asperger’s syndrome have a significant reduction in cortical 5-HT 2A receptors, supporting prior reports that people with autistic spectrum disorder have reduced serotonergic “responsivity” (4) and platelet 5-HT 2A receptor binding (4 , 5) .
However, we studied adults and used parental reports of abnormal behavior and an analysis based on regions of interest. Hence, we do not know if our findings will generalize across the autistic spectrum (e.g., to children) or if abnormalities in 5-HT 2A estimated binding potential of specific subregions of the cortex relate to particular subtypes of social behavior (e.g., gaze avoidance or processing of facial emotion). Also, we carried out a number of statistical comparisons. Nevertheless, we compared group differences in mean estimated binding potential with two-tailed nonparametric statistical tests; seven of 15 regions were significantly different, and four more were nearly significant. Therefore, our findings are unlikely to be fully explained by type 1 error.
Reduced 5-HT 2A estimated binding potential of the cingulate, frontal, and temporal cortex was correlated with qualitative abnormalities in reciprocal social interaction. These regions are crucial to human social communication. Also, in the general population, cortical 5-HT 2A receptor density is related to personality traits for harm avoidance (3) . Furthermore, as noted above, the 5-HT 2A receptor gene is close to a region on chromosome 13q reported to be linked to autism (6) . Hence, differences in cortical 5-HT 2A receptor availability may partially explain some symptoms in people with Asperger’s syndrome. Furthermore, differences in the 5-HT system are of relevance to the treatment of autistic spectrum disorder (e.g., randomized trials of 5-HT reuptake inhibitors improve global severity ratings [9] ).
5-HT acts as a trophic or differentiation factor in the brain (10) . We found that people with Asperger’s syndrome had lower estimated binding potential in regions that are also anatomically abnormal in the disorder (11) . Furthermore, in people with Asperger’s syndrome, abnormal neuronal integrity of a frontal region, including the anterior cingulate, is also related to problems in social communication (12) . Thus, our findings may be confounded by differences in brain anatomy, and/or differences in the serotonergic system may affect brain development in autistic spectrum disorder.
Others have reported a link between repetitive behaviors and the serotonergic system. We found no relationship between these behaviors and cortical 5-HT 2A density in Asperger’s syndrome. This may reflect a lack of power or our inability to detect subtle regional differences in receptor density by using approaches based on regions of interest. Also, people with Asperger’s syndrome had significantly lower estimated binding potential in all of the brain regions we examined, but this only reached statistical significance in some regions. We do not suggest that people with Asperger’s syndrome have regionally specific differences in 5-HT 2A receptions. It is more likely that they have generalized abnormality, but it may affect some brain regions more than others.
Previous work in animals with 123 I-5-I-R91150 has indicated a higher nonspecific binding in primates than in rats (13) . However 123 I-5-I-R91150 has been validated for use in humans and has been used successfully in clinical populations (e.g., see reference 14 ).
In summary, adults with Asperger’s syndrome have abnormalities in cortical 5-HT 2A receptor density; this may be related to some clinical symptoms. Further work should directly relate differences in brain 5-HT receptors to behavior in larger groups.
1. Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR: Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297:400–403Google Scholar
2. Stein D: Obsessive-compulsive disorder. Lancet 2002; 360:397–405Google Scholar
3. Moresco FM, Dieci M, Vita A, Messa C, Gobbo C, Galli L, Rizzo G, Panzacchi A, De Peri L, Invernizzi G, Fazio F: In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: a positron emission tomography study. Neuroimage 2002; 17:1470–1478Google Scholar
4. McBride PA, Anderson GM, Hertzig ME, Sweeney JA, Kream J, Cohen DJ, Mann JJ: Serotonergic responsivity in young male adults with autistic disorder: results of a pilot study. Arch Gen Psychiatry 1989; 46:213–221Google Scholar
5. Cook EHJ, Arora RC, Anderson GM, Berry-Kravis EM, Yan SY, Yeoh HC, Sklena PJ, Charak DA, Leventhal BL: Platelet serotonin studies in hyperserotonemic relatives of children with autistic disorder. Life Sci 1993; 52:2005–2015Google Scholar
6. Veenstra-VanderWeele J, Kim SJ, Lord C, Courchesne R, Akshoomoff N, Leventhal BL, Courchesne E, Cook EH Jr: Transmission disequilibrium studies of the serotonin 5-HT2A receptor gene (HTR2A) in autism. Am J Med Genet 2002; 114:277–283Google Scholar
7. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Google Scholar
8. Busatto GF, Pilowsky LS, Costa DC, Mertens J, Terriere D, Ell PJ, Mulligan R, Travis MJ, Leysen JE, Lui D, Gacinovic S, Waddington W, Lingford-Hughes A, Kerwin RW: Initial evaluation of 123I-5-I-R91150, a selective 5-HT2A ligand for single-photon emission tomography, in healthy human subjects. Eur J Nucl Med 1997; 24:119–124Google Scholar
9. McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH: A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996; 53:1001–1008Google Scholar
10. Whitaker-Azmita PM: Serotonin and brain development: role in human developmental diseases. Brain Res Bull 2001; 56:479–485Google Scholar
11. McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DGM: Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain 2002; 127:1594–1606Google Scholar
12. Murphy DGM, Critchley HD, Schmitz N, McAlonan GM, van Amelsvoort T, Robertson D, Daly E, Rowe A, Russell A, Simmons A, Murphy KC, Howlin P: Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry 2002; 59:885–891Google Scholar
13. Abi-Dargham A, Zea-Ponce Y, Terriere D, Al-Tikriti M, Baldwin R, Hoffer P, Charney DS, Leysen JE, Laruelle M, Mertens J, Innis RB: Preclinical evaluation of [I-123]R93274 as a SPECT radiotracer for imaging serotonin 5-HT2A receptors. Eur J Pharmacol 1997; 321:285–293Google Scholar
14. Jones HM, Travis MJ, Mulligan R, Bressan RA, Visvikis D, Gacinovic S, Ell PJ, Kerwin RW, Pilowsky LS: In vivo 5-HT2A receptor blockade by quetiapine: an R91150 single photon emission tomography (SPET) study. Psychopharmacology (Berl) 2001; 157:60–66Google Scholar