Effectiveness of Clozapine Versus Olanzapine, Quetiapine, and Risperidone in Patients With Chronic Schizophrenia Who Did Not Respond to Prior Atypical Antipsychotic Treatment
Abstract
Objective: When a schizophrenia patient has an inadequate response to treatment with an antipsychotic drug, it is unclear what other antipsychotic to switch to and when to use clozapine. In this study, the authors compared switching to clozapine with switching to another atypical antipsychotic in patients who had discontinued treatment with a newer atypical antipsychotic in the context of the Clinical Antipsychotic Trials for Interventions Effectiveness (CATIE) investigation. Method: Ninety-nine patients who discontinued treatment with olanzapine, quetiapine, risperidone, or ziprasidone in phase 1 or 1B of the trials, primarily because of inadequate efficacy, were randomly assigned to open-label treatment with clozapine (N=49) or blinded treatment with another newer atypical antipsychotic not previously received in the trial (olanzapine [N=19], quetiapine [N=15], or risperidone [N=16]). Results: Time until treatment discontinuation for any reason was significantly longer for clozapine (median=10.5 months) than for quetiapine (median=3.3), or risperidone (median=2.8), but not for olanzapine (median=2.7). Time to discontinuation because of inadequate therapeutic effect was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone. At 3-month assessments, Positive and Negative Syndrome Scale total scores had decreased more in patients treated with clozapine than in patients treated with quetiapine or risperidone but not olanzapine. One patient treated with clozapine developed agranulocytosis, and another developed eosinophilia; both required treatment discontinuation. Conclusions: For these patients with schizophrenia who prospectively failed to improve with an atypical antipsychotic, clozapine was more effective than switching to another newer atypical antipsychotic. Safety monitoring is necessary to detect and manage clozapine’s serious side effects.
Clozapine is generally considered to be the most effective antipsychotic drug. Studies of patients who had inadequate therapeutic response to conventional neuroleptic drugs have incontrovertibly demonstrated that clozapine is more effective than treatment with another conventional neuroleptic (1 – 6) . Additional studies have suggested that clozapine may be superior to other atypical antipsychotics in controlling symptoms that are not responsive to conventional drugs in patients with chronic schizophrenia (7 , 8) . Few studies, however, have examined the effectiveness of clozapine in patients who have not responded to an atypical antipsychotic drug. Moreover, because of clozapine’s burden of serious side effects, it is not known whether multiple trials involving some or all of the newer atypical antipsychotics should be undertaken before treating a patient with clozapine (9) .
Phase 2 of the Clinical Antipsychotic Trials for Interventions Effectiveness (CATIE) investigation was designed to address this question. In particular, patients who discontinued treatment with a newer atypical antipsychotic in phase 1 or 1B of the CATIE investigation because of suboptimal control of psychopathology were invited to undergo another random assignment to clozapine or to another atypical antipsychotic (olanzapine, quetiapine, or risperidone) other than what they had received in phase 1. However, such patients also had the option to select another phase 2 trial (Stroup et al., this issue), and patients who discontinued treatment in phase 1 or 1B for other reasons could also select “the clozapine trial.”
Method
Study Setting and Design
The National Institute of Mental Health initiated the CATIE investigation to determine the comparative effectiveness of antipsychotic drugs. The rationale, design, and methods of the trials have been described in detail (10 , 11) . The trials were conducted between January 2001 and December 2004 at 57 clinical sites in the United States. The patients were initially randomly assigned to treatment with olanzapine, quetiapine, risperidone, ziprasidone, or perphenazine in the phase 1 trial. Patients with tardive dyskinesia at baseline were excluded from random assignment to perphenazine. Patients who discontinued treatment with perphenazine in phase 1 could subsequently enter a trial involving random assignment to olanzapine, quetiapine, or risperidone (phase 1B) before entering phase 2. Any patient who discontinued treatment with olanzapine, quetiapine, risperidone, or ziprasidone in phases 1 or 1B was eligible to participate in one of the phase 2 trials. If the assigned phase 2 treatment was effective, patients could continue it until the completion of either 18 months of study (including time spent in phases 1 and 2) or until they completed 6 months of treatment in phase 2 (even if the 6-month period extended beyond 18 months of total study treatment). This article reports the results of the phase 2 efficacy trial, recommended to individuals who discontinued the previous phase 1 treatment because of inefficacy.
Participants
Inclusion criteria were ages 18–65 years, a diagnosis of schizophrenia (determined by the Structured Clinical Interview for DSM-IV), and decision-making capacity to provide informed consent. Exclusion criteria were mental retardation, other cognitive disorders, or past serious adverse reactions to any of the proposed treatments. Also excluded were patients experiencing their first psychotic episodes, patients with past evidence of profound treatment resistance, women who were pregnant or breast-feeding, or patients with serious, unstable medical conditions. Patients with brief prior periods of treatment with clozapine were allowed to enter the CATIE investigation as long as the reasons for stopping clozapine treatment had not been serious adverse events.
The appropriate institutional review boards approved the study at each site, and the patients or their legal guardians provided signed informed consent to participate.
Interventions
The patients assigned to clozapine (N=49) received open-label treatment. The schedule for dose titration and the maintenance doses were determined by the treating clinicians. Monitoring for agranulocytosis (weekly WBC counts) and myocardial inflammation (sedimentation rate, eosinophil count, creatine phophokinase level, and ECGs at baseline and after 1, 2, and 4 weeks of treatment) was standardized. The patients assigned to the newer atypical antipsychotics received blinded capsules containing olanzapine, 7.5 mg (N=19), quetiapine, 200 mg (N=15), or risperidone, 1.5 mg (N=16), starting with one capsule each day. Doses were adjusted by the treating clinician within the range of one to four capsules a day. Overlap administration of the antipsychotic each patient received in the preceding phase was permitted for the first 4 weeks to allow gradual transition to the new phase 2 medication. Adjunctive and concomitant medications were permitted throughout the trial, except for additional antipsychotics. The patients were seen at least monthly. The drug package insert for quetiapine specifies that it is to be given twice a day, whereas olanzapine and risperidone may be given once a day. To protect the blinding of treatment with the newer atypical antipsychotics, half of the patients randomly assigned to olanzapine and risperidone were assigned to twice a day and half to once a day dosing. To minimize initial side effects, the patients assigned to quetiapine began treatment by receiving one 100-mg capsule on days 1 and 2, one twice a day on day 3, and one for the first dose on day 4. All patients assigned to twice-a-day dosing received five identical capsules to begin treatment.
Objectives and Outcomes
We hypothesized that there would be significant differences in the overall effectiveness of clozapine, olanzapine, quetiapine, and risperidone, in particular, and that treatment with clozapine would be significantly more effective than treatment with some or all of the newer atypical antipsychotics.
The primary outcome measure, time until treatment discontinuation for any reason, represents a synthesis of clinician and patient judgments that an assigned treatment was sufficiently efficacious and sufficiently tolerable to continue from visit to visit. Secondary outcomes included time to discontinuation for inadequate therapeutic benefit, intolerable side effects, or patient decision.
Raters for psychopathology and adverse event assessments were aware of the patients’ assignment to clozapine versus a newer atypical antipsychotic, but they were blind to which newer antipsychotic was used. Assessments and rater training are described in Swartz et al. (12) . Because of the small size for this group, only limited and exploratory examinations of psychopathology measures and adverse event measures were undertaken.
Statistical Methods
Randomly assigned patients who received at least one dose of study medication comprised the intent-to-treat group. The main objective was the evaluation of clozapine versus olanzapine, quetiapine, and risperidone. Time from the beginning of phase 2 until treatment discontinuation was estimated by Kaplan-Meier survival curves. Treatment groups were compared with Cox proportional hazards regression models (11) , with adjustment for whether the patient had an exacerbation in the 3 months before entering the study, tardive dyskinesia status, and whether the patient was initially randomly assigned to perphenazine (and thus had an additional treatment phase before entering phase 2). The overall difference between the four treatments was evaluated with a three degrees of freedom (df) test. If significant at p≤0.05, clozapine was then compared with each of the other atypical antipsychotics with a Hochberg adjustment for multiple comparisons (12) , in which the largest p value was compared to 0.05 and the smallest p value was compared to 0.05/3=0.017. In addition, the three atypical drugs were compared to each other relative to p≤0.05 by means of step-down testing: pairwise comparisons were evaluated only if the p value from the test with df=2 was ≤0.05. Similar analyses were conducted for time until phase 2 discontinuation because of lack of efficacy, intolerability, and patient decision. For these analyses, the patients discontinuing for any other reason were censored at the time of discontinuation.
Treatment groups were compared for change from phase 2 baseline score on the Positive and Negative Syndrome Scale (PANSS) (13) and the Clinical Global Impression (CGI) Scale severity score at months 3 and 6 by using an analysis of covariance (ANCOVA) with adjustment for whether the patient had an exacerbation in the 3 months before entering the study and baseline value. Time was classified into quarterly intervals of phase 2 treatment, represented by months 3, 6, 9, and 12. End-of-phase assessments were assigned to the next interval. Months 9–12 were excluded from statistical testing because of small group sizes.
Treatment groups were compared for baseline characteristics with an analysis of variance (ANOVA), a chi-square test, or Fisher’s exact test. Overall treatment comparisons for safety outcomes are presented for descriptive purposes without correction for multiple comparisons. p values are based on Poisson regression or ANCOVA, both of which were adjusted for differential duration of phase 2 study drug. Fisher’s exact test was used in cases of small group size. For laboratory parameters, exposure-adjusted ANCOVA least-squares means are presented, but because of skewed distributions, p values are from a rank ANCOVA.
Results
Baseline Characteristics and Disposition
Figure 1 depicts the enrollment, allocation, and follow-up of study patients; 1,493 patients were enrolled in the study and randomly assigned to treatment in phase 1. Of the 1,052 patients who were eligible for phase 2, 99 patients (9%) entered the “efficacy pathway” described in this article, 444 patients (42%) entered the “tolerability pathway,” and 509 patients (48%) did not enter phase 2.
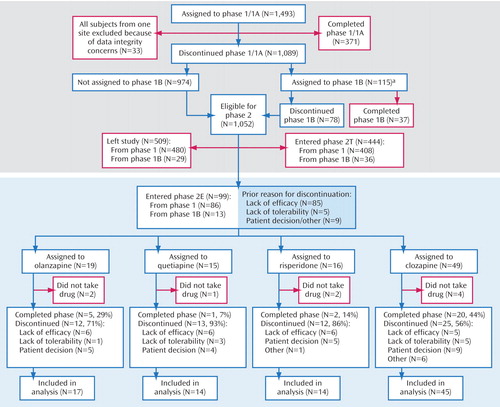
a Phase 1B: double-blind treatment with olanzapine, quetiapine, or risperidone for those patients first assigned to perphazine.
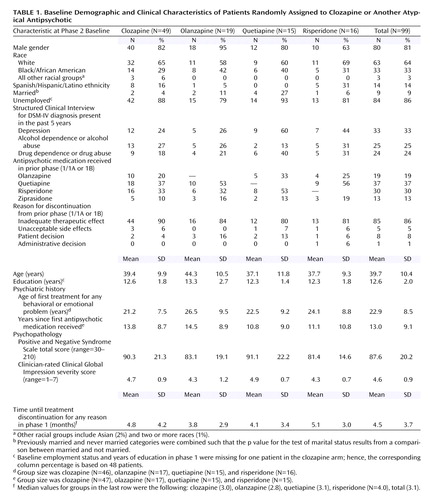
Table 1 displays the demographic and clinical characteristics of the 99 patients who were randomly assigned to treatment in phase 2. Their mean age was 39.7 years, and 81% were men; 64% were white, and 14% were Hispanic/Latino. They had had, on average, 12.6 years of education; 86% were unemployed, and 74% had never married. There were no significant differences across the treatment groups on these measures.
In the preceding phase, 19% had received olanzapine, 37% quetiapine, 30% risperidone, and 13% ziprasidone; they had been treated with these medications, on average, for 4.5 months (SD=3.7) (median time to discontinuation=3.1 months). In the preceding phase, 86% had discontinued treatment because of an inadequate therapeutic benefit, 5% because of unacceptable side effects, 8% based on patient decision, and 1% based on administrative decision. There were no significant differences across the treatment groups on these measures.
The mean phase 2 baseline PANSS total score was 87.6, and the mean CGI severity item score was 4.6, i.e., in the moderately to markedly severe range of illness for the group. There were no significant differences across the treatment groups on these measures. The 99 patients who entered the phase 2 efficacy trial were, on average, sicker than the other patients (N=1,361) who entered the CATIE investigation, even at phase 1 baseline (PANSS total scores: mean=80.6, SD=17.5, versus mean=75.3, SD=17.5) (t=2.9, df=1447, p=0.004). Over the course of their participation in phase 1/1B, these 99 patients’ conditions worsened, as demonstrated by a 7.0 (SD=18.5) point increase in their PANSS total scores (within-sample t test of change: p<0.001).
In comparison to the 444 patients who entered the phase 2 tolerability trial, the 99 patients who entered the phase 2 clozapine trial were less likely to be women (19% versus 31%) (χ 2 =5.2, df=1, p<0.02) and more likely to have had four or more prior hospitalizations for schizophrenia (58% versus 48%) (χ 2 =4.8, df=1, p<0.03). The phase 2 baseline PANSS total scores were higher for the patients entering this efficacy trial than for the patients who entered the tolerability trial (mean=87.6, SD=20.2, versus mean=77.0, SD=18.6) (t=5.0, df=534, p<0.001).
Of the 318 patients who discontinued treatment with a newer atypical antipsychotic in phase 1 or 1B because of inadequate therapeutic benefit, 85 entered this phase 2 efficacy trial, 184 entered the phase 2 tolerability trial, and 49 did not continue in the main CATIE pathways. These three groups did not differ on age or on PANSS total score at the phase 1 baseline.
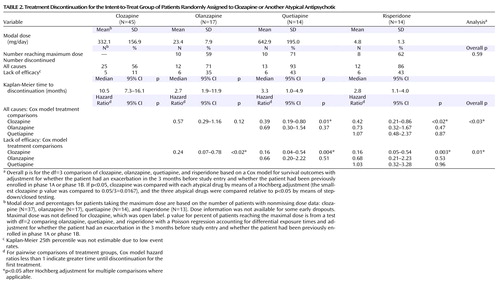
Mean modal doses prescribed during the trial were 332.1 mg/day for clozapine, 23.4 mg/day for olanzapine, 642.9 mg/day for quetiapine, and 4.8 mg/day for risperidone. Fifty-nine percent of olanzapine-treated patients, 71% of quetiapine-treated patients, and 62% of risperidone-treated patients reached the maximum dose of four capsules a day.
Treatment Discontinuation
Discontinuation outcomes are presented in Table 2 and Figure 2 . Following random assignment in the phase 2 efficacy pathway, 69% (N=62) of the intent-to-treat patients discontinued treatment before completion of the study (median treatment duration=5 months).
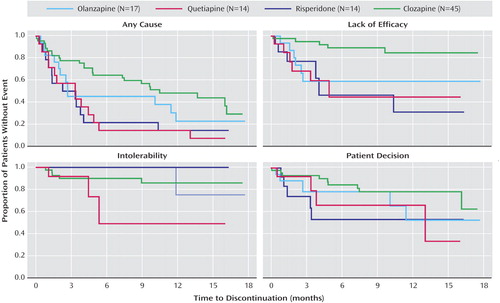
Forty-four percent (N=20) of the clozapine-treated patients, 29% (N=5) of the olanzapine-treated patients, 7% (N=1) of the quetiapine-treated patients, and 14% (N=2) of the risperidone-treated patients continued taking their phase 2 medication for the duration of the trial. Median time until treatment discontinuation for any reason was 10.5 months for the clozapine-treated patients, 2.7 months for the olanzapine-treated patients, 3.3 months for the quetiapine-treated patients, and 2.8 months for the risperidone-treated patients ( Figure 2 ). Clozapine was significantly superior to quetiapine (hazard ratio=0.39, p=0.01) and risperidone (hazard ratio=0.42, p<0.02) but not olanzapine. We repeated this analysis including only the intent-to-treat patients who discontinued phase 1/1B because of an inadequate therapeutic response (N=78). The results are similar, with clozapine (median time to discontinuation=13.7 months) significantly superior to quetiapine (3.4 months, hazard ratio=0.37, p<0.02) and risperidone (2.3 months, hazard ratio=0.20, p<0.001) but not olanzapine.
Treatment discontinuation due to lack of efficacy
Eleven percent (N=5) of the clozapine-treated patients, 35% (N=6) of the olanzapine-treated patients, and 43% (N=6) of both the quetiapine- and the risperidone-treated patients discontinued treatment because of lack of efficacy ( Figure 2 ). Clozapine was significantly superior to olanzapine (hazard ratio=0.24, p<0.02), quetiapine (hazard ratio=0.16, p=0.004), and risperidone (hazard ratio=0.16, p=0.003).
Other reasons for treatment discontinuation
There were no significant differences between the treatments in time to discontinuation because of intolerable side effects or patient decision ( Figure 2 ).
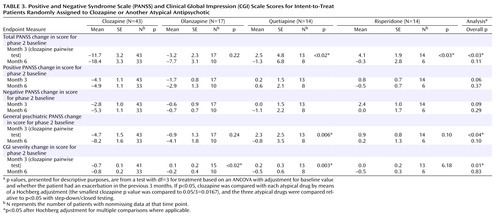
Psychopathology
At the 3-month assessment, the patients assigned to clozapine had greater reductions in the PANSS total score (mean=–11.7, SE=3.2) than the patients assigned to quetiapine (mean=2.5, SE=4.8, p=0.02) or risperidone (mean=4.1, SE=1.9, p<0.03) but not olanzapine ( Table 3 ). A similar pattern was seen on the PANSS general psychopathology subscale, although clozapine was only substantially better than quetiapine. The patients assigned to clozapine had greater reductions on the Clinical Global Impression Scale for severity at 3 months (mean=–0.7, SE=0.1) compared to the patients assigned to olanzapine (mean=0.1, SE=0.2, p<0.02) and quetiapine (mean=0.2, SE=0.3, p=0.003).
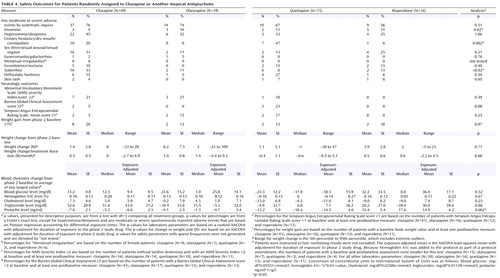
Adverse Events
Adverse events and side effects are listed in Table 4 . Because of small groups, outcomes were highly variable. All patients who entered this trial were treated with another newer antipsychotic at baseline; this may have decreased the likelihood that we would detect “new” occurrences of adverse events that have been associated, to a greater or lesser degree, with all of the antipsychotics used (e.g., weight gain). Insomnia was most common with risperidone (31%) and least common with clozapine (4%). Anticholinergic symptoms (urinary hesitancy, dry mouth, constipation) were most common with quetiapine (47%) and somewhat common with clozapine (20%). Sialorrhea was most common with clozapine (33%). There were no noteworthy differences across the treatment groups in metabolic measures or the rate of use of hypoglycemic or lipid-lowering treatments. Prolactin levels rose in patients treated with risperidone and fell in patients in the other three treatment groups. In the clozapine group, one patient had a serious adverse event of eosinophilia, and one patient developed agranulocytosis. Both events led to discontinuation of treatment.
Discussion
This study is the first to compare clozapine to the newer atypical antipsychotics in a population of patients prospectively determined to have not improved during treatment with another newer antipsychotic drug. In this group of patients who had just discontinued a course of treatment with a newer atypical antipsychotic, treatment with clozapine was significantly more effective than switching to another of the newer atypical antipsychotics. In particular, patients receiving clozapine were significantly less likely to discontinue treatment for any reason than patients receiving quetiapine or risperidone. In addition, patients receiving clozapine were less likely to discontinue treatment because of inadequate therapeutic response than were patients receiving any of the newer atypical antipsychotics. These advantages for clozapine were strong enough to achieve statistical significance despite small groups.
The results of this study are consistent with previous studies finding clozapine more effective than conventional antipsychotics. Essock et al. (6) randomly assigned 227 severely ill patients with schizophrenia or schizoaffective disorder in Connecticut state hospitals to up to 2 years of open-label treatment with either clozapine or usual care with conventional neuroleptics. Clozapine-treated patients had fewer extrapyramidal side effects and disruptiveness than patients treated with usual care, but the groups did not differ on severity of psychopathology or quality of life. Clozapine-treated patients were not more likely to be discharged, but once they were, they were less likely to be readmitted. Of the 136 patients who began treatment with clozapine, 74% were still receiving clozapine at 1 year, and 66% were still receiving clozapine at 2 years. Of note, by the end of 2 years, 66% of the patients assigned to usual care had begun a trial of clozapine. Rosenheck et al. (14) randomly assigned 423 patients with treatment-resistant schizophrenia to up to 1 year of double-blind treatment with either clozapine or haloperidol. Fifty-seven percent of the clozapine-treated patients but only 28% of the haloperidol-treated patients completed the year of treatment. Clozapine-treated patients had slightly but significantly lower psychopathology scores and better quality-of-life scores than haloperidol-treated patients. Clozapine-treated patients had significantly fewer days in the hospital over the year than haloperidol-treated patients (143.8 days versus 168.1). Agranulocytosis developed in three clozapine-treated patients; all recovered after clozapine was discontinued.
The major limitation of this study was that clozapine was administered open label. This could have led to bias in treatment discontinuation decisions by clinicians who viewed clozapine as the patients’ “last best shot” at recovery and who, therefore, kept patients treated with clozapine longer. On the other hand, clozapine’s burden of life-threatening side effects could also lead clinicians to discontinue patients who were not showing an early response. In addition, clozapine-treated patients had more frequent contact with clinical staff because of weekly laboratory monitoring and prescription renewals. In keeping with the effectiveness model of the CATIE investigation, we wished to preserve the ecological validity of all treatments; the blinding of treatment with clozapine would have required monitoring of all treatment groups for safety issues specific to clozapine. In addition, our group was small and did not offer adequate power for reasonable comparisons across the treatment groups on all adverse events.
Only 85 of 318 (27%) of the patients who discontinued a newer atypical antipsychotic in phase 1 because of inadequate therapeutic effects entered the phase 2 clozapine trial. Despite its therapeutic advantages, clozapine has been underused (15 , 16) , perhaps because of the array of serious side effects it may cause; these include agranulocytosis, myocarditis, other inflammatory reactions, seizures, obesity, diabetes mellitus, and other metabolic abnormalities. Extensive monitoring is required to avoid the consequences of these side effects (17 , 18) . An argument can be made to establish specialized “clozapine clinics” within systems of care, which have standardized monitoring in place to detect these side effects early and experienced clinicians who can intervene rapidly to limit their deleterious effects. Given the superior effectiveness of clozapine relative to all other antipsychotics, efforts to develop models of service delivery that would encourage its greater use are warranted.
1. Claghorn J, Honigfeld G, Abuzzahab FS, Wang R, Steinbook R, Tuason V, Klerman G: The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol 1987; 7:377–384Google Scholar
2. Kane JM, Honigfeld G, Singer J, Meltzer HY, the Clozaril Collaborative Study Group: Clozapine for the treatment–resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Google Scholar
3. Pickar D, Owen RR, Litman RE, Konicki E, Guiterrez R, Rapaport M: Clinical and biological response to clozapine in patients with schizophrenia: crossover comparison with fluphenazine. Arch Gen Psychiatry 1992; 49:345–353Google Scholar
4. Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT: Clozapine in schizophrenic outpatients: effects on positive and negative symptoms. Arch Gen Psychiatry 1994; 151:20–26Google Scholar
5. Carpenter WT Jr, Conley RR, Buchanan RW, Breier A, Tamminga CA: Patient response and resource management: another view of clozapine treatment of schizophrenia. Am J Psychiatry 1995; 152:827–832Google Scholar
6. Essock SM, Hargreaves WA, Covell NH, Goethe J: Clozapine’s effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacol Bull 1996; 32:683–697Google Scholar
7. Volavka J, Czobor P, Sheitman B, Lindenmeyer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002; 159:255–262; erratum, 159:2132Google Scholar
8. Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B: Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry 2001; 158:518–526Google Scholar
9. Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J, American Psychiatric Association Steering Committee on Practice Guidelines: Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004; 161(2 suppl)1–56Google Scholar
10. Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens ML, Lieberman JA: The national institute of mental health clinical antipsychotic trials of intervention effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull 2003; 29:15–31Google Scholar
11. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, the CATIE Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353: 1209–1223Google Scholar
12. Swartz MS, Perkins DO, Stroup TS, McEvoy JP, Nieri JM, Haak DC: Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial. Schizophr Bull 2003; 29:33–43Google Scholar
13. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Google Scholar
14. Rosenheck R, Cramer J, Xu W, Thomas J, Henderson W, Frisman L, Fye C, Charney D, Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia: A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. N Engl J Med 1997; 337:809–815Google Scholar
15. Taylor DM, Young C, Paton C: Prior antipsychotic prescribing in patients currently receiving clozapine: a case note review. J Clin Psychiatry 2003; 64:30–34Google Scholar
16. Weissman EM: Antipsychotic prescribing practices in the veterans healthcare administration—New York metropolitan region. Schizophr Bull 2002; 28:31–42Google Scholar
17. Henderson DC, Nguyen DD, Copeland PM, Hayden DL, Borba CP, Louie PM, Freudenreich O, Evins E, Cather C, Goff DC: Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry 2005; 66:1116–1121Google Scholar
18. Wehmeier PM, Heiser P, Remschmidt H: Myocarditis, pericarditis and cardiomyopathy in patients treated with clozapine. J Clin Pharm Ther 2005; 30:91–96Google Scholar