Effectiveness of Switching Antipsychotic Medications
Abstract
Objective: Changing antipsychotics is common despite the dearth of information on risks and benefits associated with medication changes. The authors examined phase 1 findings from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study to explore whether it was more advantageous to continue taking the medication being received at baseline or to switch to a different antipsychotic. Method: First, for patients randomly assigned to treatment with olanzapine (N=314) or risperidone (N=321), the authors assessed the impact of being assigned to stay with the medication they were receiving at entry into the study versus being assigned to switch to these medications from a different antipsychotic. Second, for patients whose baseline antipsychotic was olanzapine (N=319), risperidone (N=271), or quetiapine (N=94), the authors examined the impact of being randomly assigned to stay with the same antipsychotic versus switch. Finally, the authors assessed the impact of removing the data of 209 patients whose random assignment was to stay with their baseline antipsychotic. The authors followed analysis strategies for CATIE; primary outcome was time until all-cause treatment discontinuation. Results: Individuals randomly assigned to olanzapine and risperidone who were continuing with their baseline medication had significantly longer times until discontinuation than did those assigned to switch antipsychotics. When these “stayers” were removed, differences seen in the original CATIE phase 1 analyses were attenuated, although the original pattern of results remained. Conclusions: Comparisons of medication effectiveness should take into account whether medications being compared were each newly initiated. Further, unless the clinical situation requires a medication change, prescribers may want to take steps to optimize current medication regimens (e.g., dosage adjustments, behavioral or psychosocial interventions) before switching medications.
Changing medications is a common feature of psychiatric practice, and medication changes often are part of life for people with schizophrenia. Rates of switching medication vary by setting and by provider within setting, suggesting that the decision to “stay or switch” is influenced by providers’ prescribing practices as well as by patient factors (1 , 2) . Few randomized trials speak to this stay-versus-switch question, so it is fortunate that data from phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study (3) afford a rare opportunity to examine experimentally the effectiveness of staying with a given antipsychotic versus switching to another one (4) .
In the phase 1 results from CATIE, 23% of the participants randomly assigned to olanzapine already were on a regimen of olanzapine monotherapy at the time of study entry, 18% of participants randomly assigned to risperidone were already receiving risperidone monotherapy, and 5% of participants assigned to quetiapine were already receiving quetiapine monotherapy (3) . The phase 1 report also noted that individuals who were receiving olanzapine or risperidone treatment at the time of study enrollment had longer times-to-all-cause-discontinuation than did those who entered the trial receiving different or no antipsychotic medications. The purpose of this report is to compare all-cause treatment discontinuation for patients whose random assignment resulted in staying with the same antipsychotic they were already receiving at baseline versus switching to a different one, and to examine the extent to which excluding patients randomly assigned to continue taking their baseline medication would affect the discontinuation rates observed in the CATIE trial.
Method
Study Participants
As previously reported, individuals were eligible for participation in the CATIE study if they were ages 18 to 65, had received a diagnosis of schizophrenia, and were judged appropriate for treatment with oral antipsychotic medications (see Stroup and colleagues [5] for more detailed inclusion criteria and Lieberman and colleagues [3] for more detailed information about study methods; additional information is also available in the supplement that accompanies the online version of this article). We examined subsets of the 1,432 study participants used in the CATIE phase 1 analyses.
Question 1: Stay Versus Switch?
The first group of analyses addressed the question, “Did individuals who were randomly assigned to stay with the same antipsychotic medication they were already taking at study entry discontinue their assigned treatment at a different rate than individuals assigned to switch?”
We addressed this question in two ways:
1. Staying with versus switching to treatment: For those participants randomly assigned to treatment with olanzapine or risperidone, what was the impact of having been randomly assigned to stay with the same medication versus switching to these treatments from a different antipsychotic?
2. Staying with versus switching from treatment: For those participants who entered the study already receiving olanzapine, risperidone, or quetiapine monotherapy, what was the impact of being randomly assigned to stay with the same medication versus switching from the medication to one of the other four antipsychotics?
The subset of study participants for the “staying with versus switching to” analysis consisted of those participants in the CATIE phase 1 intent-to-treat analyses who were assigned to take either olanzapine or risperidone, since these groups included enough study participants to allow statistical comparisons (only 5% or fewer of study participants assigned to take quetiapine, perphenazine, or ziprasidone were already taking these medications as monotherapy at baseline). We grouped these individuals into two groups: “stayers” versus “switchers.” “Stayers” were individuals assigned to the same medication they were receiving at study entry (with what ever dosage retitration was permissible within the study protocol of 1, 2, 3, or 4 blinded capsules). “Switchers” were individuals assigned to either a different medication or who were not receiving an antipsychotic at study entry (additional information on the stayers and switchers are included in the supplement that accompanies the online version of this article). Within the olanzapine double-blind treatment group, 77 (25%) of 314 participants were stayers and the remaining 237 (75%) were switchers. Within the risperidone double-blind treatment group, 62 (19%) of 321 participants were stayers and the remaining 259 (81%) were switchers. We examined time to all-cause discontinuation and time to discontinuation due to inefficacy, intolerability, and patient decision.
The subset of study participants for the “staying with versus switching from” analysis consisted of the 319 individuals receiving olanzapine monotherapy, the 271 individuals receiving risperidone monotherapy, and the 94 individuals receiving quetiapine monotherapy at study entry who were included in the intent-to-treat analysis of the original findings (3) . We examined time to all-cause discontinuation for this subset of individuals. Using those receiving olanzapine monotherapy at baseline as an example, we compared those assigned to stay with olanzapine versus those assigned to switch from olanzapine to risperidone, quetiapine, ziprasidone, or perphenazine.
Question 2: Same Results If Stayers Omitted?
If individuals whose random assignment resulted in staying with the antipsychotic medication they were taking at study entry were excluded, would the CATIE phase 1 results be different?
For these analyses, we excluded those individuals who were randomly assigned to continue taking an antipsychotic medication they were receiving at baseline either as monotherapy or with another antipsychotic. Specifically, we excluded 209 (15%) study participants: 93 assigned to olanzapine (28% of the group), 74 assigned to risperidone (22% of the group), 31 assigned to quetiapine (9% of the group), four assigned to perphenazine (2% of the group), and seven assigned to ziprasidone (4% of the group).
Statistical Analyses
All of the statistical tests were 2-tailed. For evaluation of the “staying with versus switching to” question, we paralleled the original analysis strategies for the phase 1 CATIE findings (3) using Kaplan-Meier survival curves to estimate the time to discontinuation of treatment and Cox proportional-hazards regression models (6) to compare differences in discontinuation rates for the double-blind treatments (olanzapine versus risperidone) and the prior medication groups (switched from study entry medication versus stayed with study entry medication). The Cox model was stratified according to site and adjusted for tardive dyskinesia status and whether the individual had had an exacerbation of schizophrenia in the preceding 3 months. Additional Cox models included a test of the interaction between double-blind medication assignment (risperidone, olanzapine) and group (stayer, switcher), as well as the effect of potential baseline covariates and the moderating effect (interaction) of covariates on staying versus switching. Also paralleling the original analyses for the phase 1 findings (3) , we examined the effect of staying versus switching on changes in total score on the Positive and Negative Syndrome Scale (PANSS) through time, applying a mixed model that included the same fixed covariates as for the time to discontinuation and added baseline PANSS score, time (classified into months), and interactions between double-blind treatment and time, baseline PANSS score and time, stay versus switch status and treatment, and baseline PANSS score and stay versus switch status. An additional model tested for a 3-way interaction between double-blind treatment, stay versus switch status, and time.
For evaluation of the “staying with versus switching from” question, we used Kaplan-Meier survival curves for each subset of individuals based on monotherapy medication at study entry to estimate the time-to-discontinuation of treatment for each of the five double-blind medications. If the curves appeared to be substantially different across the double-blind treatments, we applied a post hoc Cox proportional-hazards regression model (6) , with adjustment for whether the individual had had an exacerbation of schizophrenia in the preceding 3 months and whether the person had tardive dyskinesia at study entry, to quantify the strength of double-blind treatment group differences in discontinuation rates. We also applied ANOVA and chi-square analyses to examine differences in background characteristics among baseline medication groups (olanzapine monotherapy; risperidone monotherapy; quetiapine monotherapy; no medication; more than one antipsychotic including olanzapine, risperidone, or quetiapine; and monotherapy or polypharmacy with agents other than olanzapine, risperidone, or quetiapine).
To reproduce the primary CATIE results excluding the “stayers,” we used the same methods as in the main paper (3) first to compare olanzapine, risperidone, quetiapine, and perphenazine for all individuals without tardive dyskinesia at baseline, and if an overall difference was found, subsequently compare perphenazine versus each of the three atypical antipsychotics using a Hochberg adjustment for multiple comparisons (7) and compare olanzapine, quetiapine, and risperidone for all individuals. Secondary comparisons involving ziprasidone, which was added to the study after 40% of patients had enrolled, were limited to the cohort of patients enrolled after ziprasidone was added, and also received a Hochberg adjustment. Comparisons of ziprasidone with perphenazine excluded tardive dyskinesia patients. We used Kaplan-Meier survival curves to estimate the time to discontinuation of treatment. We used Cox proportional-hazards regression models (6) , stratified according to site with adjustment for whether the individual had had an exacerbation of schizophrenia in the preceding 3 months and tardive dyskinesia status where applicable, to test the significance of double-blind treatment group differences in discontinuation rates.
To evaluate how the exclusion of individuals who had been randomly assigned to stay with their baseline medication affected power or enhanced or diminished the primary findings as originally reported (3) , we compared the power of the two sets of analyses (original analyses versus this subset) and, for each pairwise comparison of double-blind treatments, we computed the ratio of the original hazard ratio to the hazard ratio produced in the analysis of this subset.
Results
Question 1: Stay Versus Switch?
Figure 1 summarizes the overall discontinuation rates among phase 1 CATIE participants whose random assignment to olanzapine or risperidone meant staying with or switching from their baseline treatment. Figure 2 displays the results from Kaplan-Meier survival curves for all-cause discontinuation (Kaplan-Meier survival curves and Cox proportional hazards models examining discontinuation due to lack of efficacy, intolerability, and patient decision are presented in the supplement that accompanies the online version of this article). The discontinuation rates were lowest for those who were assigned to stay on their study entry medication relative to those who were assigned to switch to either medication ( Figures 1 and 2 ). Results of the Cox proportional hazards model for all-cause discontinuation revealed a significant effect for stay versus switch status (hazard ratio=0.69, p=0.007) and for double-blind treatment (olanzapine versus risperidone: hazard ratio=0.76, p=0.006). The interaction between double-blind treatment and stay versus switch status was not significant, meaning that although both staying with one’s baseline medication and being randomly assigned to double-blind olanzapine treatment led to longer times to discontinuation, there was no extra synergistic effect of staying with olanzapine. Results were consistent across the baseline medications that comprised the switch group, and were unchanged when adjusting for significant baseline covariates (PANSS score, age). No significant interactions with stay versus switch status were found. Results for stay versus switch were similar for discontinuation due to lack of efficacy, intolerability, and patient decision (data presented in the supplement that accompanies the online version of this article). The advantage for stayers was more pronounced in those with less severe baseline PANSS scores when examining discontinuation due to efficacy (p=0.03).

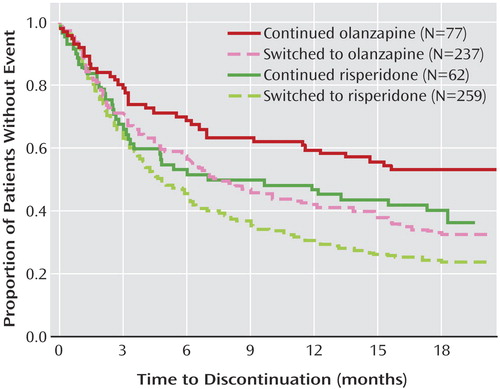
a Median months to all-cause discontinuation: continued risperidone=8.4, switched to olanzapine=7.7, switched to risperidone=4.7. The median time to discontinuation for those who continued olanzapine was not estimable because of low event rates but was above 11.6 weeks with 95% certainty.
Similar to overall findings (3) , there were improvements in total PANSS scores through time, with a treatment-by-time interaction (p=0.001) indicating that individuals assigned to olanzapine had initially larger improvements relative to those assigned to risperidone, with diminishing differences over time. However, there were no significant differences between people who started a new medication versus staying on their old one, nor for the interaction of stay versus switch status with double-blind treatment group, time, or baseline PANSS score.
Given the advantages conferred to stayers in the “staying with versus switching to” analyses, we examined the second part of question 1, the impact of entering the study on regimens of olanzapine, risperidone, or quetiapine monotherapy and staying with that medication versus switching to a different one (i.e., the “staying with versus switching from” question). For the 319 individuals who were taking olanzapine at baseline, the advantage of staying with olanzapine relative to switching to any of the other four double-blind treatments was pronounced. In contrast, for the 271 individuals who were taking risperidone monotherapy at baseline, all five treatment groups were similar with respect to time to all-cause discontinuation. For the 94 individuals who were taking quetiapine monotherapy at baseline, those who were assigned to stay with quetiapine appeared to discontinue their phase 1 medication more quickly than did those who were assigned to switch to olanzapine or risperidone. Kaplan-Meier survival curves and Cox proportional hazards models are presented in the supplement that accompanies the online version of this article, along with differences between baseline medication groups with respect to baseline characteristics.
Question 2: Same Results If Stayers Omitted?
Figure 3 shows the outcome measures for time to all-cause discontinuation from CATIE phase 1 and the same measures with stayers removed. The ordering of the curves in Figure 3 looks much the same whether stayers are included or excluded. Removing the stayers attenuated the magnitude of the gap between the top-most curve (olanzapine) and the other curves. Once stayers were removed, the all-cause discontinuation rates and median time to all-cause discontinuation were 68% and 7.7 months, respectively, for those assigned to olanzapine, 75% and 5.6 months for those assigned to perphenazine, 76% and 4.7 months for those assigned to risperidone, 82% and 4.7 months for those assigned to quetiapine, and 80% and 3.5 months for those assigned to ziprasidone. Comparisons examining discontinuation due to lack of efficacy, intolerability, and patient decision are presented in the supplement that accompanies the online version of this article. Results of the primary Cox proportional hazards model excluding tardive dyskinesia individuals revealed no significant overall treatment group differences (p=0.09), so pairwise comparisons were not evaluated for statistical significance. The removal of the stayers resulted in about a 10% drop in power for pairwise comparisons involving olanzapine (the group with most individuals removed) compared with the analyses originally reported. Additionally, the hazard ratios for olanzapine versus risperidone, quetiapine, and perphenazine when tardive dyskinesia patients were excluded were 90%–94% of those obtained in the original analyses. Hence, statistical significance was diminished both because of a loss of power and because removing the individuals who were randomly assigned to continue taking the medication they had been taking at study entry (stayers) attenuated the originally reported findings.
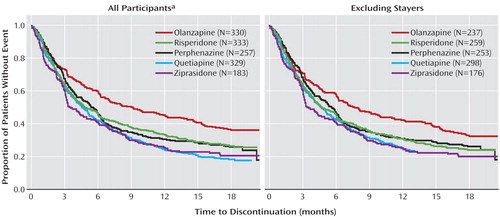
a Findings as originally reported by Lieberman et al. (3). Figure reproduced with permission from The New England Journal of Medicine .
Discussion
In clinical practice, people with schizophrenia sometimes change medication in hope that the next medication will work better than the current one. The analyses of the CATIE phase 1 findings presented here suggest that the likelihood of finding a medication that fits better than the current medication (as measured by time to all-cause discontinuation) is a function both of the medication being switched from and the medication being switched to, and that such switches have only modest success rates among the medications used in CATIE phase 1. The subgroup analysis of CATIE patients entering the study on a regimen of olanzapine monotherapy indicated that, on average, it may be better to stay with olanzapine than to switch to one of the other antipsychotics used in phase 1. Similarly, analyses of patients receiving double-blind olanzapine and risperidone treatment indicated that stayers, on average, fared somewhat better than patients newly switched to these two medications (about half of stayers versus over 70% of switchers discontinued their assigned medication).
When individuals who did not switch medications at study entry were excluded from the analyses, the core findings of CATIE were supported ( Figure 3 ), although statistical significance was attenuated both because of loss of power with the reduced sample size and because removing stayers removed a number of individuals receiving olanzapine who tolerated that medication relatively well. As shown in Figure 3 , the separation between the olanzapine curve and the other curves is still apparent when stayers are excluded, but the separation is narrower than when all participants are included. Under the CATIE study conditions where side effects were monitored, individuals newly treated with olanzapine (olanzapine switchers) were more likely to discontinue their assigned medication due to intolerability than were individuals assigned to olanzapine who were already receiving it at study entry (olanzapine stayers). Olanzapine switchers also were more likely to discontinue their assigned medication due to intolerability than were individuals receiving risperidone at study entry who were assigned to risperidone (risperidone stayers) and individuals who were newly treated with risperidone (risperidone switchers) (data in online supplement). These limitations in olanzapine’s tolerability acted to offset some of its superior efficacy as originally reported (3) .
The finding that risperidone or olanzapine stayers fared better than switchers raises the more general question of the extent to which switching from any nonclozapine oral antipsychotic monotherapy to another is a hopeful treatment strategy. These findings suggest that, unless the clinical situation requires a medication change, prescribers should take steps to optimize current medication regimens (e.g., via dosage changes, behavioral or psychosocial interventions, adjunctive medications) before switching medications. Certainly, individuals’ desires to try a different medication should be respected. What we are stressing is the clinical appropriateness of working diligently with patients to maximize the effectiveness of the current antipsychotic before taking on the risks associated with a medication change in hopes that the next one will be more satisfactory (i.e., taken for a longer period of time). Short of a compelling clinical indication to switch medication, different individuals (whether patient or prescriber) have different thresholds for being willing to continue a medication given limitations in effectiveness or side effects. For example, one individual might be willing to accept an increase in a particular side effect in exchange for symptom improvement, while another individual may choose to change to a medication with less symptom control in order to ameliorate particular side effects. On the one hand, changing medications offers the possibility of further symptom improvement or reduction of side effects experienced with the current antipsychotic medication. On the other hand, changing medications risks destabilizing a formerly stable person (8) as well as increases the burden on already stretched clinical resources from both increased monitoring following a medication change and increased clinical attention if symptom exacerbations occur. The field has very few randomized trial studies examining the relative effectiveness of staying with one’s current antipsychotic treatment versus switching to another (8 , 9) , hence this opportunity to examine the phase 1 CATIE data from this perspective is particularly timely.
In cases where medication changes are clinically warranted or where individuals prefer to try something new, the analyses of CATIE phase 1 presented here along with the results of CATIE phase 2 may help inform medication choice. Specifically, in phase 2 of CATIE, some of the people who discontinued their phase 1 medication stayed with their new phase 2 medication for the remainder of the study, indicating a successful antipsychotic medication switch. When clozapine was a treatment arm, such successful switches were significantly more likely, when considering discontinuation for any reason, among individuals who switched to clozapine relative to quetiapine and risperidone but not to olanzapine (10) . In addition, individuals receiving clozapine were significantly less likely to discontinue treatment for reasons of inefficacy than were individuals receiving any of the other atypicals (10). When clozapine was not a treatment arm and when considering those who discontinued the previous medication for reasons of inefficacy, olanzapine was superior to quetiapine and ziprasidone, and risperidone was superior to quetiapine (11) .
This study has several limitations worth noting. First, the post hoc analyses were exploratory and, as such, carry concerns about spurious findings from multiple comparisons. Thus, the results should be interpreted as preliminary, and future randomized clinical trials should address the stay versus switch question directly. Second, sample sizes were only sufficient to evaluate with confidence the relative effectiveness of staying versus switching for two antipsychotic medications, olanzapine and risperidone. We recognize that other antipsychotic medications may behave differently under these stay or switch conditions. Indeed, randomized trial evidence indicates that individuals with treatment-resistant schizophrenia are better off switching to clozapine than staying with conventional antipsychotics, at least with respect to lowered likelihood of rehospitalizations (9) . Hence, future studies should examine this question for other agents and for larger numbers of stayers. Because so many individuals with chronic disorders gain some benefit from their current medication (so-called “partial responders”), the “stay versus switch” question has great day-to-day clinical significance and is not addressed by designs where individuals switching to one medication are compared with individuals switching to another medication. Third, dosages of the medications in the double-blind condition may have advantaged some medications (e.g., olanzapine) and not others (e.g., quetiapine and ziprasidone). Finally, we note that these results apply to individuals who were enrolled in the CATIE phase 1 trial, and these individuals varied widely in clinical severity. Because random assignment to the medication taken at baseline was possible for participants taking one of the study drugs, staying on this medication had to be considered a viable clinical option. Those who were completely satisfied with their medication would not have been expected to enter the study; thus, CATIE participants who were taking one of the study drugs at baseline were incompletely satisfied with this drug but did not absolutely require a change.
The results of these analyses are consistent with a critical finding in all previous reports from CATIE: the available antipsychotic medications have considerable limitations. We would expect that enhanced availability of evidence-based psychosocial treatments would improve the overall effectiveness of treatment and promote recovery.
Perhaps the most important finding from the present report is that, even in randomized clinical trials, there can be biases in estimates of treatment response because of differences in the study participants’ exposures to treatment prior to the trial, and that random assignment to treatment condition alone is not a sufficient safeguard to eliminate this bias. For example, as demonstrated in these analyses, comparisons of medication effectiveness need to take into account whether medications being compared were each newly initiated.
1. Covell NH, Jackson CT, Evans AC, Essock SM: Antipsychotic prescribing practices in Connecticut’s public mental health system: rates of changing medications and prescribing styles. Schizophr Bull 2002; 28:17–29Google Scholar
2. Weissman EM: Antipsychotic prescribing practices in the Veterans Healthcare Administration: New York Metropolitan Region. Schizophr Bull 2002; 28:31–42Google Scholar
3. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223Google Scholar
4. Essock SM, Covell NH, Jackson CT: Antipsychotic drugs and schizophrenia (letter). N Engl J Med 2005; 354:298–299Google Scholar
5. Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA: The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull 2003; 29:15–31Google Scholar
6. Cox DR: Regression models and life-tables. J Royal Stat Soc [B] 1972; 34:187–220Google Scholar
7. Hochberg Y: A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988; 75:800–802Google Scholar
8. Gardos G: Are antipsychotic drugs interchangeable? J Nerv Ment Dis 1974; 159:343–348Google Scholar
9. Essock SM, Hargreaves WA, Covell NH, Goethe J: Clozapine’s effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacol Bull 1996; 32:683–697Google Scholar
10. McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RSE, Davis CE, Severe J, Hsiao JK, for the CATIE Investigators: Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006; 163:600–610Google Scholar
11. Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Perkins DO, Keefe RSE, Davis CE, Severe J, Hsiao JK, for the CATIE Investigators: Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006; 163:611–622Google Scholar



