Double-Blind Controlled Investigation of Transcranial Magnetic Stimulation for the Treatment of Resistant Major Depression
Abstract
OBJECTIVE: The efficacy and safety of left prefrontal repetitive transcranial magnetic stimulation (rTMS) for treating resistant major depression were examined in a double-blind, controlled study. METHOD: Eighteen medication-resistant depressed subjects were randomly assigned to 2 weeks of real or sham rTMS, then permitted up to 4 weeks of real rTMS. Effects on mood, neuropsychological function, EEG, and hearing were assessed. RESULTS: The groups receiving real and sham rTMS improved in mood significantly over the 2-week double-blind period, but there was no significant difference between groups. CONCLUSIONS: Repetitive transcranial magnetic stimulation did not provide significantly greater improvement than did sham treatment. A 4-week course of rTMS, as administered in this study, was safe.
Repetitive transcranial magnetic stimulation (rTMS) has been investigated in a number of studies (1–4) as a subconvulsive alternative to ECT for depression. Sham-controlled studies (3, 4) have shown positive results with high-frequency (10–20-Hz) stimulation to the left dorsolateral prefrontal cortex. For example, Pascual-Leone et al. (4) reported improvement of over 50% in 11 of 17 psychotically depressed patients after 5 days of 10-Hz rTMS in a crossover study. However, George et al. (5) found a 25% improvement in only four of 12 patients after 10 days of 20-Hz rTMS. Further controlled studies of rTMS are warranted to study efficacy and optimal treatment characteristics. Safety has been investigated in normal volunteers receiving rTMS, but not over a 4-week course.
METHOD
We recruited 18 subjects (nine male; eight inpatients) with a DSM-IV major depressive episode; eight had melancholic features, three had a history of bipolar disorder, and one had psychotic features. All of the subjects scored 25 or higher on the Montgomery-Åsberg Depression Rating Scale (6). Those with major physical or neurological abnormalities and those treated with ECT during the current episode were excluded. Power analysis indicated that this sample size had 95% power (alpha=0.05) to detect the effect found by Pascual-Leone et al. (4).
After complete description of the study, written informed consent was obtained. For the first 2 weeks, during which both the investigators and patients were blind to treatment type, the subjects were randomly assigned to real or sham treatment; the patients were then allowed to continue real treatment to a maximum of 4 weeks. None withdrew within the first 2 weeks, and 14 patients requested 4 weeks of real treatment; two of the 14 withdrew after 3 weeks of this.
At baseline the groups receiving real and sham treatment did not differ significantly in gender, inpatient status, or type or severity of depression. Their mean ages were 45.7 years (SD=14.7) and 50.9 years (SD=14.7), respectively. The mean duration of the current episode was 7.3 months (SD=2.6) for the group receiving real rTMS and 12.6 months (SD=7.7) for the group receiving sham treatment, and the total durations of illness (i.e., number of lifetime episodes multiplied by average duration of episodes) were 21.9 months (SD=21.3) and 45.4 months (SD=45.6), respectively. The mean numbers of ineffective antidepressants taken during the current episode were 1.9 (SD=0.9) and 2.4 (SD=1.3), respectively, and the numbers taken during the total illness were 4.9 (SD=1.6) and 4.3 (SD=1.2). There were no significant differences in any of the preceding values.
Antidepressants were withdrawn 5 days before rTMS for five patients. For antidepressants that had failed to show an effect, steady doses were maintained for 2 weeks before and throughout the study; venlafaxine was administered to nine patients, and nefazadone was given to four patients. There were no significant differences in antidepressant treatment between the groups receiving real and sham rTMS.
Transcranial magnetic stimulation was administered with a 70-mm figure-8-shaped coil (Magstim Co., U.K.) to the left dorsolateral prefrontal cortex (measured as 5 cm anterior to the optimal site for activating the right first dorsal interosseus) with the following characteristics: 10 Hz, 110% of motor threshold, 30 trains of 5 seconds each, 30 seconds apart. The threshold was determined at rest with surface electromyography by using the method of limits (as described, for instance, in reference 4). The coil was tangential to the scalp for real treatment and at 45˚ (both wings touching) for sham treatment, with the coil extensions perpendicular to a line running from the stimulation site to the subject’s nose. Earplugs were worn.
Psychiatrists (P.M., P.S.) blind to the subjects’ treatment groups assessed depression severity weekly during rTMS and 1 month after completion. The Hamilton Rating Scale for Depression (7), the Montgomery-Åsberg Depression Rating Scale (6), the CORE scale for measuring objective features of psychomotor disturbance (8), the self-rated Beck Depression Inventory (9), and the self-rated AUSSI (Affect Underpinned by Severity and Social Impairment) scale (10) were used. Auditory thresholds and performance on the following neuropsychological tasks were assessed at baseline and fortnightly during treatment: Mini-Mental State examination, digit span, simple and complex reaction time, Luria hand sequences, visual paired associates learning, verbal fluency, Tower of London, Rey Auditory Verbal Learning Test, and Autobiographical Memory Interview. EEGs were performed at baseline and after 2 weeks. Effects of a single rTMS session on orientation, reaction time, finger-tapping speed, and the stand-walk test were assessed on days 2 and 3 of treatment.
The depression ratings and neuropsychological test scores for all patients were analyzed for change over the first 2 weeks by using a repeated measures design. For the mood ratings, contrast analyses tested for linear and quadratic trends over time and for treatment-by-trend interactions. Neuropsychological data were examined by using Bonferroni-adjusted t tests. A second repeated measures, intention-to-treat analysis was applied to the 14 patients who embarked on 4 weeks of real rTMS (either initially or after 2 weeks of sham treatment), to test for mood changes over the period of real treatment only.
RESULTS
There was significant linear improvement over the 2-week double-blind period on the Hamilton depression scale (F=24.1, df=1, 16, p<0.001), the Montgomery-Åsberg Depression Rating Scale (F=27.1, df=1, 16, p<0.001), and the CORE scale (F=6.9, df=1, 16, p<0.05), but there was no significant difference between the groups receiving real and sham treatment (Figure 1).
Over 4 weeks of real treatment, significant linear trends indicating continuing improvement were also seen in the scores on the Hamilton scale (F=30.3, df=1, 12, p<0.001), the Montgomery-Åsberg scale (F=29.4, df=1, 12, p<0.001), the CORE scale (F=13.0, df=1, 12, p<0.005), the AUSSI scale (F=26.0, df=1, 12, p<0.001), and the Beck Depression Inventory (F=15.5, df=1, 12, p<0.005).
Most patients found rTMS uncomfortable (three reported mild headaches), but some noted no side effects. There were no seizures or neurological complications. No significant neuropsychological changes were found after a single session of rTMS, 2 weeks of real or sham treatment, or 4 weeks of real rTMS. One patient showed a transient rise in auditory threshold (3–4-kHz range) after 6 weeks of rTMS. The EEGs were qualitatively reported by neurologists as unchanged except for the appearance of left anterior temporal theta activity in one patient.
DISCUSSION
Unlike previous studies, this investigation showed no difference between high-frequency left prefrontal rTMS and a sham treatment in effect on depression. It is difficult to reconcile our findings with those of Pascual-Leone et al. (4), who reported substantial response after 1 week with a similar technique and similar treatment characteristics but whose patients and study design differed from ours. It may be that rTMS is particularly effective in psychotic depression or that its efficacy was selectively enhanced by the specific concurrent medications permitted by Pascual-Leone et al. (e.g., tricyclic antidepressants). It is also possible that in the crossover design of that study, each treatment phase affected subsequent phases, although the results suggest otherwise.
The modest response we found after 2 weeks of rTMS is similar to that of George et al. (5), although the response to real rTMS in their study differed significantly from the minimal response to sham treatment. The sham response seen in our study may be due to the considerable clinical contact and activity involved, although it is surprising given the refractory nature of the depression. Alternatively, our sham (which delivered a much weaker stimulus) may have been modestly effective.
Further investigation of optimal stimulation characteristics is needed, but our results argue against uncritical acceptance of rTMS as an effective antidepressant treatment. We found that 4 weeks of rTMS yielded progressive improvement (although some of the real rTMS was administered on an open basis) and was safe in terms of cognitive functioning, EEG, and auditory threshold.
Presented in part at the 53rd annual meeting of the Society of Biological Psychiatry, Toronto, May 28–30, 1998. Received July 14, 1998; revision received Dec. 7, 1998; accepted Jan. 20, 1999. From the Mood Disorders Unit, Neuropsychiatric Institute and Prince of Wales Medical Research Institute, Prince of Wales Hospital; and the School of Psychiatry, University of New South Wales, Sydney, Australia. Address reprint requests to Dr. Loo, Psychiatry Unit, Prince of Wales Hospital, High and Avoca Streets, Randwick, N.S.W. 2031, Australia; [email protected] (e-mail). Supported by Australian National Health and Medical Research Council Mood Disorders Unit Program Grant 953208, private donations from Mr. Peter Joseph (Director, Bankers Trust, Australia) and Mr. William Loewenthal (Company Director), and a Research Fellowship from the New South Wales Institute of Psychiatry (Dr. Loo). The authors thank Mr. Dusan Hadzi-Pavlovic for help on statistical analyses, staff members of the Prince of Wales Hospital audiology and clinical neurophysiology departments for their contributions, and Dr. Janet Taylor for advice on transcranial magnetic stimulation.
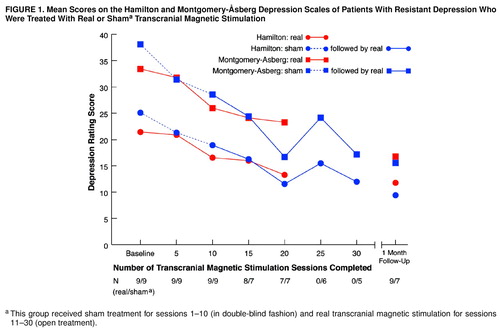
FIGURE 1. Mean Scores on the Hamilton and Montgomery-Åsberg Depression Scales of Patients With Resistant Depression Who Were Treated With Real or Shama Transcranial Magnetic Stimulation
aThis group received sham treatment for sessions 1–10 (in double-blind fashion) and real transcranial magnetic stimulation for sessions 11–30 (open treatment).
1. Hoflich G, Kasper S, Hufnagel A, Ruhrmann S, Moller H-J: Application of transcranial magnetic stimulation in treatment of drug-resistant major depression—a report of two cases. Hum Psychopharmacol 1993; 8:361–365Crossref, Google Scholar
2. Kolbinger H, Hoflich G, Hufnagel A, Moller H-J, Kasper S: Transcranial magnetic stimulation (TMS) of major depression—a pilot study. Hum Psychopharmacol 1995; 10:305–310Crossref, Google Scholar
3. George M, Wassermann E, Williams W, Callahan A, Ketter T, Basser P, Hallett M, Post R: Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6:1853–1856Google Scholar
4. Pascual-Leone A, Rubio B, Pallardo F, Catala M: Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996; 348:233–237Crossref, Medline, Google Scholar
5. George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM: Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997; 154:1752–1756 Google Scholar
6. Montgomery S, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
7. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
8. Parker G, Hadzi-Pavlovic D, Boyce P, Wilhelm K, Brodaty H, Mitchell P, Hickie I, Eyers K: Classifying depression by mental state signs. Br J Psychiatry 1990; 157:55–65Crossref, Medline, Google Scholar
9. Beck A, Ward C, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4561–4585Google Scholar
10. Parker G, Hadzi-Pavlovic D, Sengoz A, Boyce P, Mitchell P, Wilhelm K, Hickie I, Brodaty H: A brief self-report depression measure assessing mood state and social impairment. J Affect Disord 1994; 30:133–142Crossref, Medline, Google Scholar



