Long-Acting Psychotraumatic Properties of a Cardiac Arrest Experience
Abstract
OBJECTIVE: Progress in resuscitation medicine allows an increasing proportion of patients to survive an out-of-hospital cardiac arrest. However, little is known about long-term adaptation to the vital breakdown. The present study assessed the long-term prevalence and severity of emotional disability of cardiac arrest survivors and ascertained whether survivors suffer from recurrent and intrusive recollections of the cardiac arrest. METHOD: Follow-up analysis was performed on all cardiac arrest survivors discharged from the hospital over a 5-year interval (1990–1994) in a defined inner city and suburban area. From 118 initially hospitalized cardiac arrest survivors, 45 patients were discharged alive from the hospital. After a mean follow-up period of 39 months (range=22–64), 25 patients exhibited sufficient cerebral performance for psychodiagnostic assessment; 21 patients were assessed. RESULTS: Despite an impaired ability to concentrate, cardiac arrest survivors had levels of psychological adjustment at follow-up that were similar to those of 35 cardiac patients whose clinical course was not complicated by cardiac arrest. However, the diagnosis of psychotraumatic symptoms in cardiac arrest survivors led to a sharp separation between favorable and nonfavorable outcome in affective regulation and level of functioning. Of the cardiac arrest patients, those with high scores of intrusion and avoidance (N=8) reported an enduring sense of demoralization with significantly more somatic complaints, depression, anxiety, lack of confidence in the future, and narrowing of social activities than those with low scores (N=11). Long-acting sedation at illness onset significantly predicted a favorable outcome. CONCLUSIONS: This study provides the first empirical evidence that the application of the posttraumatic stress disorder paradigm in the long-term evaluation of cardiac arrest survivors significantly contributes to defining a patient population at high risk for serious emotional disability.
Since the first report concerning the termination of ventricular fibrillation in man by transthoracic electrical countershock in 1956 (1) and its practical application to rescue cardiac arrest victims outside the hospital 10 years later (2), the progress in resuscitation technique allows an increasing proportion of patients suffering from cardiac arrest to survive. This accomplishment raises issues regarding medical responsibility toward these patients.
Studies to date suggest high levels of psychological distress in cardiac arrest survivors, particularly phobic anxiety and panic symptoms, in the early stages of adaptation (3–6). Investigators have also found that survivors use strong defense mechanisms to protect themselves from the overwhelming anxiety engendered by the implications of the event. Whether these coping strategies remain effective over time or lead to maladjustment is less clear. Data on long-term adaptation are rare and provide conflicting evidence about the amount and severity of emotional disability (7–9). The first aim of the present study, therefore, was to compare the long-term prevalence and severity of emotional disability in cardiac arrest survivors with that of cardiac patients whose course was not complicated by cardiac arrest.
To date, it is unclear where the limits should be set for the use of trauma as a paradigm for the explanation of comorbid psychopathology in internal medicine. Cardiac arrest survivors have, however, faced a breakdown event that is far outside the range of usual human experience and thus meet one core criterion for the development of a traumatic stress disorder. Nevertheless, the consideration of psychotraumatic symptoms that may occur in response to a cardiac arrest (10, 11) or to an acute myocardial infarction (12–15) is a relatively unexplored area. The second aim of the present study was therefore to ascertain to what extent the aforementioned patients continued to suffer from recurrent and intrusive recollections of the vital breakdown situation and whether they presented altered behavior patterns in terms of persistent avoidance of stimuli associated with the event and an increased arousal pattern.
Cardiac arrest survivors manifesting symptoms of intrusion, avoidance, and hyperreactivity may have failed to integrate and resolve the trauma (16). On the other hand, suffering from involuntary intrusive memories of the cardiac arrest event may have become part of the individual biography of the patients without leading to a clinically relevant dysregulation. Patients who manifest avoidance behavior (when exposed to stimuli that remind them of the resuscitation) may also be able to go on with their lives on an acceptable basis (17). The third aim of the present study was to ascertain whether patients who suffer from traumatic symptoms, mainly intrusion and avoidance, exhibit higher degrees of affect dysregulation and maladaptation than those who are not haunted by the memories of what happened to them.
Experiencing cardiac arrest outside the hospital is a recognizable trauma for survivors that fulfills the core criterion for a diagnosis of posttraumatic stress disorder (PTSD) according to DSM-IV and ICD-10, but it may not evoke symptoms of posttraumatic distress in every patient. PTSD research has identified a network of internal and external factors heralding subsequent development of maladaptation in the aftermath of a traumatic event (18). The data set of the present study allowed the formulation of a fourth aim: to analyze the contribution of specific stress factors around the breakdown situation to the development of PTSD symptoms in these patients.
METHOD
Study and Comparison Groups
Patients were included in the study if they had been successfully resuscitated from ventricular fibrillation or pulseless tachycardia and they were 20 years of age or older. Patients sustaining cardiac arrest from a noncardiac cause (e.g., somatic trauma, intoxication or drug overdose, hypovolemia, hypothermia) were excluded. Patients were drawn from the Munich Early Defibrillation Feasibility Study (19), in which resuscitation was initiated by emergency technicians operating semiautomatic defibrillators, and were followed by emergency physicians on the scene. The study was carried out in a defined inner city and suburban area of Munich, Germany (see reference 19 for more details about the catchment area), and covered the 5-year period from Jan. 1, 1990, to Dec. 31, 1994. The follow-up investigation started July 1, 1996.
Data collection was done according to the Utstein Style (20). Data on the circumstances of the arrest were taken from the patients’ records, the emergency physician protocol, and automated documentation in the dispatching center. The data were documented in a standardized manner. Sedation was defined as the use of midazolam at the scene of the event. It was used at doses of 0.1 mg/kg of body weight or at a loading dose of 0.5 mg followed by doses in relation to the effect achieved.
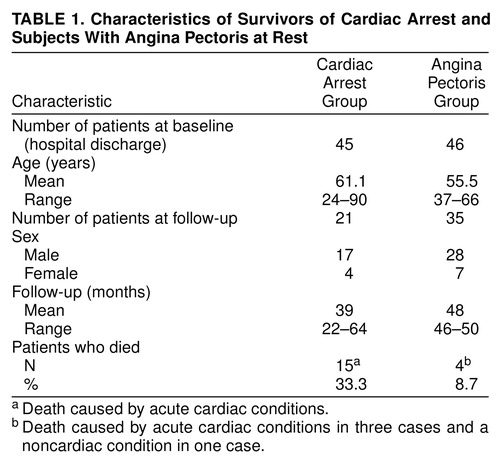
Of 118 initially hospitalized cardiac arrest survivors, 45 patients were discharged from the hospital after successful out-of-hospital resuscitation. There were 36 men and nine women. The mean age of the 45 patients at study entry was 61.1 years (range=24–90). Mean follow-up covered a period of 39 months (range=22–64, median=35). In all cases but one, the Glasgow-Pittsburgh cerebral performance categories (21) were assessed by telephone interview with the physician in charge of the patient and were subsequently confirmed by telephone interview with the patient (or a patient’s relative). Fifteen patients had died (cerebral performance category 5), and one was in a vegetative state (cerebral performance category 4). Three patients were in a state of severe cerebral disability (category 3). Four patients showed symptoms of moderate cerebral disability (category 2), and 21 patients (46%) exhibited good cerebral performance (category 1). The 25 patients in cerebral performance categories 1 and 2 were regarded as eligible for psychodiagnostic assessment. Three patients did not speak the German language sufficiently, and one patient had moved abroad.
Thus, the index group comprised 21 patients in whom the psychodiagnostic assessment was carried out. The 17 men and four women had a mean age of 59.7 years (range=35–78) at study entry. After consent to participate was given by telephone, a self-administered questionnaire was delivered by mail to the patients; the questionnaire contained general information about the aims and procedures of the study (including anonymous data processing and instructions concerning the handling of the psychodiagnostic instruments). Patients were told that nonparticipation would not have any disadvantage for them. Remailing the questionnaires was considered to constitute written consent.
The comparison group comprised 46 patients with a mean age of 55.5 years (range=37–66) with severe symptoms of angina pectoris at rest. The patients were consecutively assessed in the university outpatient clinic, and the cardiac origin of the chest pain was confirmed by a coronary arteriogram. The patients had no proven history of coronary artery disease before the invasive evaluation at study entry. After a follow-up of 4 years, the clinical status of 35 comparison patients was assessed by a structured telephone interview with the patients and by a self-administered questionnaire that was delivered by mail to the patients after instruction by telephone. The clinical characteristics of the index and comparison groups are shown in Table 1.
Psychodiagnostic Assessment
Anxiety and depression were evaluated with the 14-item Hospital Anxiety and Depression Scale (22); published norms (23) were obtained from a German standard population with coronary artery disease (N=5,496). The Hospital Anxiety and Depression Scale has four options for each item, with a scoring range of 0 to 3; seven items cover anxiety and seven cover depressive symptoms. The responses were scored on a Likert scale, giving a potential global scoring range of 0–21 for each subscale. A subscale range of 0–8 defines minor reactions; a score of 9 or 10, moderate expressions of anxiety or depression; and a score of 11 or higher, severe expressions of the affective states.
Illness-related aspects of quality of life were measured with the abridged version of an inventory (24) covering personal resilience and social activities on two four-item subscales. Self-estimates of the actual overall somatic and mental condition were assessed with two items (score=0–4 for each item). Sleeping disorders were measured with three items (score=0–3). Somatic complaints were measured with a modified binary 10-item version of the Zerssen symptom list (25).
For the assessment of symptoms according to the current operational diagnosis of PTSD in DSM-IV and ICD-10, data were obtained to estimate the patients’ degree of being troubled by reexperiencing the traumatic event (intrusion), the persistent avoidance of stimuli associated with the trauma, and persistent symptoms of increased arousal.
To assess the dimensions of intrusion and avoidance in a standardized manner, the Impact of Event Scale (26), German version (27), was applied. The instrument is a 16-item ordinal scale with four options for each item (rated 1–4) and has two subscales measuring intrusion and avoidance. The responses were scored on a Likert scale, giving a potential global scoring range of 16–64 and 8–32 for each subscale, respectively. Furthermore, patients were asked whether the event had markedly changed their lives and whether they could actually recall the event. To assess the extent of retrograde amnesia, patients were asked whether they could remember details immediately before and some hours before the onset of the event or whether they suffered from a patchy recall loss even 1 or more days before the event.
To achieve an estimate of increased arousal in cardiac arrest survivors, the following items (all with a scoring range of 0–4) were combined: three items on sleeping disorders and one item each on difficulty concentrating, hypervigilance, and being easily frightened. The total scoring range was 0–24.
Because the Impact of Event Scale does not have norms in the German version, it was difficult to state what represents a clinically pathological state. To overcome this problem, we decided to define as a psychotraumatic patient group (index group) those patients who scored 50% or higher on the Impact of Event Scale and who labeled themselves as still troubled by the event (one item). Patients with high scores on the Impact of Event Scale were considered to have PTSD. For validation of the criterion, we used a diagnostic checklist recommended by the World Health Organization (28) of all symptoms necessary for the current operational ICD-10 diagnosis of PTSD and then counted the prevalence in the group with high scores on the Impact of Event Scale. In all patients in this subgroup, multiple symptoms of intrusion, avoidance, and hyperarousal were affirmed on the checklist. Thus, the patient group satisfied the criteria for a PTSD diagnosis (not reported).
Statistical Analysis
Differences between continuous variables were analyzed by the t test for two independent groups. Categorical variables were tested for equal distribution with the Pearson test (chi-square). Differences with a probability value of less than 0.05 were considered significant. We used stepwise logistic regression analysis as a multivariate model to identify the independent contribution of resuscitation variables to the prediction of traumatic conditions in patients after survival of a cardiac arrest. Statistical analysis was carried out with SPSS (version 6.22). All values are presented as means and standard deviations.
RESULTS
Twenty-one survivors of cardiac arrest outside the hospital were assessed within a mean interval of 39 months (range=22–64) after the life-threatening event. The study population achieved high levels of emotional stability and well-being: 15 (75%) of the patients were found to be in the normal range of both the depression and anxiety subscales of the Hospital Anxiety and Depression Scale (score of 9 or less). The index population’s mean depression score (5.3, SD=4.5) exceeded only slightly that of the German standard cardiac population (5.0, SD=3.7). The mean anxiety score was lower in the cardiac arrest group (5.5, SD=4.4) than in the reference population (6.8, SD=4.1).
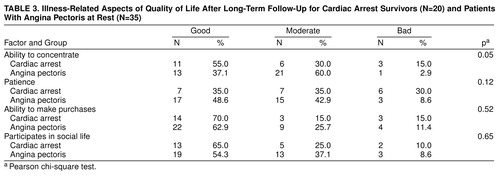
We compared the follow-up status of the index patients and 35 coronary artery patients with severe symptoms of angina pectoris at illness onset and found that the cardiac arrest group exhibited no significant differences in self-estimates of their overall mental and somatic condition (Table 2) or in illness-related aspects of quality of life (Table 3). In addition, no significant differences concerning various aspects of sleeping disorders could be detected. Of all the factors that were measured in the cardiac arrest survivors and the coronary artery comparison group, only the ability to concentrate reached a significant difference. It was impaired in the index group in comparison to the coronary artery group (χ2=5.8, df=2, p<0.05).
Despite this apparently favorable overall adaptation in the long-term course after the breakdown situation, more than 50% of the cardiac arrest survivors (N=11) claimed that the event had significantly changed their lives. With the help of the Impact of Event Scale, patients who had high scores (N=8) and low scores (N=13) on intrusive thoughts and avoidant states were separated in the cardiac arrest group. Table 4 shows that the procedure to define the PTSD group led to a significant difference in the global Impact of Event Scale score (t=–7.07, df=17, p<0.0001), as well as in the avoidance subscale score (t=–3.19, df=17, p<0.001) and the intrusion subscale score (t=–7.34, df=17, p<0.0001). Furthermore, patients with PTSD had a higher mean score on increased arousal (9.88, SD=3.56) than patients without PTSD (5.82, SD=5.82). The difference was statistically significant (t=–2.33, df=17, p<0.03).
Patients with PTSD had significantly higher mean scores on depression (t=–4.46, df=18, p<0.003) and anxiety (t=–3.48, df=18, p<0.003) than their counterparts (Table 4). Self-estimates of the patients’ mental and somatic condition were also significantly impaired in the PTSD group compared to the group without PTSD (Table 2), as were beliefs concerning an optimistic future course of the disease condition (negative in six [75%] of the PTSD patients and three [27%] of the non-PTSD patients, χ2=4.23, df=2, p<0.04). Patients with PTSD had a significantly lower score than non-PTSD patients in ability to concentrate (mean=2.75, SD=1.03, versus mean=3.91, SD=0.67; t=3.08, df=18, p<0.006). Accordingly, preoccupation with somatic complaints was significantly more pronounced in the PTSD group (t=3.00, df=18, p<0.008). Figure 1 displays the difference between the two groups as a sum percent curve for the 10 items of the complaint scale.
We analyzed whether age, sex, and selected factors of the resuscitation and procedures on the scene of the collapse contributed to the risk of developing PTSD symptoms. The following factors were rejected by the multivariate model: onset of event during daytime/nighttime, call response time more than 8 minutes, more than four shocks delivered, and no return of spontaneous circulation before arrival of the emergency physician. We found that sedation at illness onset significantly predicted a favorable outcome (Table 5). In these patients, the risk of developing PTSD symptoms was fivefold lower than that in their counterparts.
DISCUSSION
The present study reveals that the prevalence of emotional disability and impairment in quality of life in cardiac arrest survivors is similar to that of cardiac patients whose clinical course was not complicated by cardiac arrest. Five patients (25%) in the present study qualified for moderate to severe forms of depression. Severe forms of depression in cardiac patients, as assessed with various instruments, have been reported to range from 15% to about 25% (29–31). Data on the maintenance of depressive symptoms in cardiac arrest survivors over more than a 1-year observation period have not been available in the literature until now. The present 3-year follow-up results are in line with the study of Roine et al. (32), which revealed initially high degrees of depression in hospitalized cardiac arrest survivors and confirmed a steep decline in prevalence rates over 1 year. Bedell et al. (33) followed in-hospital cardiac arrest survivors for 6 months and also found that they were severely depressed at the time of discharge from acute hospital care. Six months later, the patients’ mean depression score had fallen significantly and was within the range of scores for a normal community population.
Unexpectedly, the prevalence of patients who were incapacitated by fear was also low: the mean anxiety score was even lower in the cardiac arrest group (5.5, SD=4.4.) than in the reference population (6.8, SD=4.1). There were only a few patients in whom the fear of another cardiac arrest led them to regulate their daily lives and limit their activities to ensure immediate access to medical care. Considering that the fatality rate is far higher in cardiac arrest survivors than in patients with angina pectoris at rest (a fatality rate after hospital discharge of 33% in the present cardiac arrest group versus 8.7% in the comparison group), this is a remarkable finding. It proves that the majority of patients have successfully maintained or have rebuilt effective coping resources. This finding is shared by other investigations (34–37). Hillis et al. (35) did not find any behavioral or emotional differences between cardiac arrest survivors and patients with coronary artery disease. Miranda (38) and Sunnerhagen et al. (9), however, did not confirm these favorable results. They revealed a diminished personal interest in the environment and an increased sense of disorientation in cardiac arrest survivors. Sauvé (39) reported elevated scores for psychological distress in 61 cardiac arrest survivors 6 months to 4 years after cardiac arrest.
Low levels of impairment and the lack of significant differences between the index and comparison groups also hold true for all further affective, biobehavioral, and cognitive patient variables that were assessed in the present study (sleeping disorders, social activities, health beliefs). Among quality-of-life variables, the ability to concentrate was the only factor that was scored significantly lower in cardiac arrest survivors than in the comparison group. This finding confirms earlier investigations by Hillis et al. (35), Sauvé (39), Bertini et al. (40), and Grubb et al. (41), who also found more difficulties with memory and concentration in cardiac arrest patients than in patients with acute myocardial infarction. Beuret et al. (42) also found that persistent mild memory disturbances were common in this patient group but did not preclude adequate professional activity and positive life perception. Those results are encouraging because they confirm the beneficial consequences of resuscitation efforts for the majority of discharge-to-hospital survivors of a cardiac arrest.
The favorable outcome in psychodiagnostic assessment that has been shown in previous studies(8, 9, , 33, 42) may be still somewhat surprising in the light of what these patients experienced. Undoubtedly, patients who survive cardiac arrest outside a hospital face an event that is outside the range of usual human experience, and the aftermath of this event would be distressing to almost anyone. Such a characterization meets the key criterion for definition of a traumatic event (in ICD-10 and DSM-IV) that may provoke PTSD. Surprisingly, there are no data available that have considered the experience of surviving a sudden cardiac arrest as a traumatic event. This study is, to our knowledge, the first one to show that evidence of the breakdown is present in a considerable proportion of survivors in the long-term course after the cardiac arrest experience and leads to recurrent and intrusive recollections of the event and to avoidance behavior in a clinically relevant number of cases.
Psychic fixation on the traumatic event may not be surprising, considering the severity of the arrest experience. The event may have become part of the patient’s individual biography without harming the subject any further. The present study revealed, however, that patients who maintained high scores of intrusion and avoidance behavior concerning the cardiac arrest experience over a long time period apparently failed to integrate the memory of the event into their preexisting schemata (12, 13) and suffered from a serious affective dysregulation and a pronounced impairment in quality of life. Patients with PTSD symptoms exhibited significantly higher degrees of depression and anxiety and a diminished capacity to resist emotional burden (43). They reported an enduring sense of demoralization with lack of confidence in the future and low estimates of their mental and somatic condition.
Moreover, PTSD sufferers were preoccupied by negative health beliefs, and they exhibited more somatic complaints than patients without PTSD. This finding confirms a number of studies that have noted an increased reporting of physical symptoms in patients with PTSD (44, 45). It is unlikely that the underlying disease condition is responsible for the difference in symptom reporting because all patients had experienced the same life-threatening coronary event. McFarlane et al. (45) assumed that the physical symptoms may be related to the high levels of arousal that form part of the symptom complex of PTSD. The pronounced awareness of endogenous somatic signals may be due to an impaired ability to process and differentiate relevant and irrelevant information.
PTSD symptoms may interact with the clinical conditions of cardiac arrest survivors in several ways. First, persistently impaired memory and concentration are widely confirmed as core symptoms in cardiac arrest patients (35, 39–42). More recently, alterations in memory functions have also been shown in PTSD patients (46, 47). Cardiac arrest patients with PTSD symptoms in the present study had significantly lower concentration scores than cardiac arrest patients without PTSD symptoms. PTSD may thus amplify disturbances in cognitive functions. We used stepwise logistic regression analysis in the present study to identify the contribution of major resuscitation variables to the prediction of PTSD symptoms and could not find a significant association, indicating that it is not likely that the PTSD patients were exposed to more ischemia than non-PTSD patients. It cannot be ruled out completely that patients with PTSD symptoms suffer from more severe brain damage than their counterparts. Moreover, brain damage may be subtle and may preferentially hit areas of the brain that relate to emotional regulation. It has, in fact, been shown that ischemic damage causes selective vulnerability to the brain, with a preponderance of neural loss in region CA1 of the hippocampus (48).
Second, PTSD is strongly associated with symptoms of alterations in sympathetic functions (49). A growing body of evidence indicates that PTSD is associated with increased noradrenergic responsiveness in cardiovascular measures such as heart rate, blood pressure, and norepinephrine response to traumatic reminders (50). It is well established that a markedly elevated baseline level of sympathetic neural outflow is a risk factor for derangements in heart rhythm (51) and heart failure (52) and a key trigger of myocardial ischemia during daily life (53). Thus, PTSD may act as a major coronary risk factor in long-term course after cardiac arrest survival.
Not all patients suffered from PTSD-like symptoms. Obviously, the cardiac arrest event did not evoke symptoms of distress in all patients, or stress responses were not maintained over the long-term course. This phenomenon underlines the fact that PTSD is not simply a function of the experience itself but may be attributed either to factors associated with the emergency situation and/or to adaptive capabilities of the cardiac arrest victim. It is likely that a premorbid history of affect dysregulation amplifies the risk for developing PTSD (54). In the present study, no data were available concerning personal coping abilities and personality factors of the affected patients. However, the data set allowed the estimation of the contribution of specific stress factors around the breakdown situation and revealed that prolonged sedation in the initial phase of resuscitation was a significant protective factor. It may be speculated that prolonged sedation helped to inhibit imprinting the actively troublesome and painful dimension of the event (16, 47). There was, indeed, a marked difference in the duration of unconsciousness in the group that received sedatives (N=7) and the group that did not (N=13) (mean=618.2 minutes, SD=2205, and mean=92.1 minutes, SD=84, respectively) (p=0.01, t test for equality of means, two independent groups). Conscious processing of overwhelming adverse stimuli was inhibited during the most vulnerable stage of the disease onset. Patients were thus provided with a considerable time delay to deal with all the implications of what had happened to them, awakening within surroundings that apparently generated positive outcome expectancies.
The present study shows that the amount of emotional disability in patients surviving an out-of-hospital cardiac arrest is comparable to that in cardiac patients whose illness onset was not complicated by a comparable vital threat. However, when patients were grouped according to persistent intrusion and avoidance behavior concerning the traumatic event, it became evident that the prevalence of affective disability and behavioral maladaptation was significantly concentrated in the group suffering from PTSD-like symptoms. The application of the PTSD paradigm contributed convincingly to defining the patient population at risk for a persistent serious affective disturbance.
A major limitation of the present study is the small size of the patient population, which may provoke false negative results because of type II errors. Moreover, there are no norms and no defined cutoff points for a strict definition of PTSD. Pathologic autonomic nervous system arousal is considered to be a major key clinical feature of PTSD and is only phenotypically registered in the present study (sleeping disorders, irritability, difficulties to concentrate). Future studies should assess the degree of functional hypersensitivity and ergotropic tonus.
The encouraging results of the present study need to be confirmed in larger patient groups and may also stimulate prospective studies that address the time-dependent course of maladaptation and affective disability (including issues of critical onset during the adaptation process) and predictors for maintenance of psychic trauma in cardiac arrest survivors. The possible interaction between discrete cognitive abnormalities, which may be part of the PTSD syndrome, and emotional disability in cardiac arrest survivors also requires scientific attention before conceptualizing treatment regimes for these patients.
ACKNOWLEDGMENTS
The following hospitals in the city of Munich took part in the study: Stiftsklinik Augustinum (Prof. Dr. v. Essen, OA Dr. Reimer), Krankenhaus Dritter Orden (OA Dr. Schwarzfischer), Klinik Josephinum (Dr. Wildfeuer), Krankenhaus Neuwittelsbach (Prof. Dr. Scherer), Kreiskrankenhaus Pasing (Prof. Dr. Luther, Dr. Gutsch), Kreiskrankenhaus Perlach (Dr. Burghardt), Städtisches Krankenhaus Bogenhausen (Prof. Dr. Delius), Städtisches Krankenhaus Harlaching (Prof. Dr. Lindlbauer, OA Dr. Scheinpflug), Städtisches Krankenhaus Neuperlach (Prof. Dr. Henselmann, OA Dr. Fischer), Städtisches Krankenhaus Schwabing (Prof. Dr. Döring, OA Dr. Ohly), Medizinische Klinik Innenstadt der Ludwig-Maximilians-Universität (Prof. Dr. Theisen, OA Dr. Scheininger), Chirurgische Klinik der Ludwig-Maximilians-Universität (Prof. Dr. Schweiberer, OA Dr. Höcherl), Klinikum Großhadern der Ludwig-Maximilians-Universität (OA Dr. Jänicke), Klinikum Rechts der Isar der Technischen Universität München (Prof. Dr. Schömig).
Received Dec. 4, 1997; revisions received April 29 and Sept. 17, 1998; accepted Nov. 20, 1998. From the Institut und Poliklinik für Psychosomatische Medizin, Med. Psychologie und Psychotherapie, Institut für Anästhesiologie, and I. Medizinische Klinik des Klinikums Rechts der Isar der Technischen Universität München; and MEDIS-Institut der GSF–Forschungszentrum für Umwelt und Gesundheit, München-Neuherberg. Address reprint requests to Dr. Ladwig, Priv. Doz., Institut u. Poliklinik für Psychosomatische, Medizin, Psychotherapie u. Med. Psychologie, Klinikum Rechts der Isar der TUM, Langerstraße 3, 81675 München, Germany; [email protected] (e-mail). The authors thank Birgitt Marten-Mittag, Dipl. Soz., for assisting with the statistical analysis and Sandra Thomas, M.D., M.S., for her advice in the preparation of the manuscript. They also thank the men and women of the Munich emergency services.
 |
 |
 |
 |
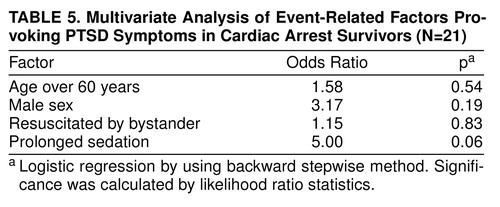 |
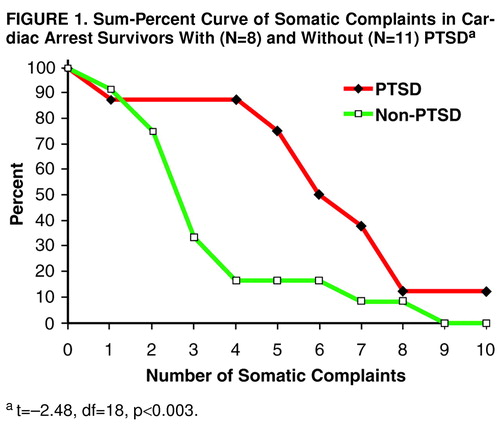
FIGURE 1. Sum-Percent Curve of Somatic Complaints in Cardiac Arrest Survivors With (N=8) and Without (N=11) PTSDa
at=–2.48, df=18, p<0.003.
1. Zoll PM, Linenthal A, Gibson W, Paul M, Norman L: Termination of ventricular fibrillation in man by externally applied electric countershock. N Engl J Med 1956; 254:727–732Crossref, Medline, Google Scholar
2. Pantridge JF, Geddes JS: A mobile intensive-care unit in the management of myocardial infarction. Lancet 1967; 2:271–273Crossref, Medline, Google Scholar
3. Dlin BM: The experience of surviving almost certain death. Adv Psychosom Med 1980; 10:111–118Crossref, Medline, Google Scholar
4. Klapp BF, Wecke G, Kunde-Hoffmann S, Köppel LC: Psychische Belastungsreaktionen auf Intensivstationen. Intensivmed 1992; 29(suppl 1):23–29Google Scholar
5. Druss RG, Kornfeld DS: The survivors of cardiac arrest: a psychiatric study. JAMA 1967; 201:291–296Crossref, Medline, Google Scholar
6. Holland J, Sgroi SM, Marwit SJ, Solkoff N: The ICU-syndrome: fact or fancy? Psychiatr Med 1973; 4:241–249Google Scholar
7. Dobson M, Tattersfield AE, Adler MW, McNicol MW: Attitudes and long-term adjustment of patients surviving cardiac arrest. Br Med J 1971; 3:207–212Crossref, Medline, Google Scholar
8. Bergner L, Hallstorm AP, Bergner M, Eisenberg M, Cobb LA: Health status of survivors of cardiac arrest and of myocardial infarction controls. Am J Public Health 1985; 75:1321–1323Google Scholar
9. Sunnerhagen KS, Johansson O, Herlitz J, Grimby G: Life after cardiac arrest: a retrospective study. Resuscitation 1996; 31:135–140Crossref, Medline, Google Scholar
10. Ladwig KH, Schoefinius A, Danner R, Gürtler R: Psychosomatic factors in the evaluation of long-term courses of cardiac arrest survivors: is it worthwhile (abstract)? Resuscitation 1996; 31(suppl):S11Google Scholar
11. Ladwig KH, Dammann G: Psychological adaptation after successful out-of-hospital resuscitation (editorial). J Psychosom Res 1997; 43:559–564Crossref, Medline, Google Scholar
12. Horowitz MJ, Hulley S, Alvarez W, Billings J, Benfari R, Blair S, Borhani N, Simon N: News of risk for early heart disease as a stressful event. Psychosom Med 1980; 42:37–46Crossref, Medline, Google Scholar
13. Kutz I, Shabtai H, Solomon Z, Neumann M, David D: Post-traumatic stress disorder in myocardial infarction patients: prevalence study. Isr J Psychiatry Relat Sci 1994; 31:48–56Medline, Google Scholar
14. Doerfler LA, Pbert L, DeCosimo D: Symptoms of posttraumatic stress disorder following myocardial infarction and coronary artery bypass surgery. Gen Hosp Psychiatry 1994; 16:193–199Crossref, Medline, Google Scholar
15. Van Driel RC, Op den Velde W: Myocardial infarction and post-traumatic stress disorder. J Trauma Stress 1995; 8:151–159Crossref, Medline, Google Scholar
16. Van der Kolk BA, van der Hart O, Marmar CR: Dissociation and information processing in posttraumatic stress disorder, in Traumatic Stress: The Effects of Overwhelming Experience on Mind, Body, and Society. Edited by van der Kolk BA, McFarlane AC, Weisaeth L. New York, Guilford Press, 1996, pp 303–327Google Scholar
17. Van der Kolk BA, McFarlane AC: The black hole of trauma. Ibid, pp 3–23Google Scholar
18. Resnick HS, Kilpatrick DG, Best CL, Kramer TL: Vulnerability-stress factors in development of posttraumatic stress disorder. J Nerv Ment Dis 1992; 180:424–430Crossref, Medline, Google Scholar
19. Ladwig KH, Schoefinius A, Danner R, Gürtler R, Herman R, Koeppel A, Hauber P: Effects of early defibrillation by ambulance personnel on short and long-term outcome of cardiac arrest survival: the Munich Experiment. Chest 1997; 112:1584–1591Google Scholar
20. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest (new abridged version): the “Utstein Style.” Br Heart J 1992; 67:325–333Crossref, Medline, Google Scholar
21. Jennett B, Bond M: Assessment of outcome after severe brain damage: a practical scale. Lancet 1975; 1:480–484Crossref, Medline, Google Scholar
22. Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
23. Herrmann CH, Buss U, Snaith RP: Psychologisches Screening von Patienten einer kardiologischen Akutklinik mit einer deutschen Fassung der “Hospital Anxiety and Depression” (HAD) Scala. Psychother Psychosom Med Psychol 1991; 41:83–92Medline, Google Scholar
24. Siegrist J, Broer M, Junge A: Profil der Lebensqualität chronisch Kranker (PLC): Testhandbuch. Göttingen, Germany, Hogrefe, 1995Google Scholar
25. von Zerssen D: Die Beschwerden-Liste: Depressivitäts-Skala (D-S). Weinheim, Germany, Beltz, 1976Google Scholar
26. Horowitz MJ, Wilner N, Alvarez W: Impact of Event Scale: a measure of subjective stress. Psychosom Med 1979; 41:209–218Crossref, Medline, Google Scholar
27. Ferring D, Filipp SH: Teststatistische Überprüfung der Impact of Event-Skala: Befunde zu Reliabilität und Stabilität. Diagnostika 1994; 40:344–362Google Scholar
28. Hiller W, Zaudig M, Mombour W: International Diagnostic Checklists for ICD-10 and DSM-IV (Handbook). Seattle, Hogrefe & Huber, 1996Google Scholar
29. Schleifer SJ, Macari-Hinson MM, Coyle DA, Slater WR, Kahn M, Gorlin R, Zucker HD: The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789Google Scholar
30. Ladwig KH, Lehmacher W, Roth R, Breithardt G, Budde T, Borggrefe M: Affective disorders and survival after acute myocardial infarction: results from the post-infarction late potential study (PILP). Eur Heart J 1991; 12:959–964Medline, Google Scholar
31. Frasure-Smith N, Lesperance F, Talajic M: Depression following myocardial infarction: impact of 6 month survival. JAMA 1993; 270:1819–1825Google Scholar
32. Roine RO, Kajaste S, Kaste M: Neuropsychological sequelae of cardiac arrest. JAMA 1993; 269:237–242Crossref, Medline, Google Scholar
33. Bedell SE, Delbanco TL, Cook EF, Epstein FH: Survival after cardiopulmonary resuscitation in the hospital. N Engl J Med 1983; 309:569–576Crossref, Medline, Google Scholar
34. Kimman GP, Ivens EMA, Hartman JAM, Hart HN, Simoons ML: Long-term survival after successful out-of-hospital resuscitation. Resuscitation 1994; 28:227–232Crossref, Medline, Google Scholar
35. Hillis M, Sinclair D, Butler G, Cain E: Prehospital cardiac arrest survival and neurologic recovery. J Emerg Med 1993; 11:245–252Crossref, Medline, Google Scholar
36. Perez N, Castro M, Guiroa C, Cabezas A, Munoz G, Vazquez M: Quality of life in cardiorespiratory arrest patients attended in an out-of-hospital setting (abstract). Resuscitation 1996; 31(suppl):S14Google Scholar
37. Hsu JW, Madsen CD, Callaham ML: Quality-of-life and formal functional testing of survivors of out-of-hospital cardiac arrest correlates poorly with traditional neurologic outcome scales. Ann Emerg Med 1996; 28:597–605Crossref, Medline, Google Scholar
38. Miranda DR: Quality of life after cardiopulmonary resuscitation. Chest 1994; 106:524–530Crossref, Medline, Google Scholar
39. Sauvé MJ: Long-term physical functioning and psychosocial adjustment in survivors of sudden cardiac death. Heart Lung 1995; 24:133–144Crossref, Medline, Google Scholar
40. Bertini G, Giglioli C, Giovannini F, Bartoletti A, Cricelli F, Margheri M, Russo L, Taddei T, Taiti A: Neuropsychological outcome of survivors of out-of-hospital cardiac arrest. J Emerg Med 1990; 8:407–412Crossref, Medline, Google Scholar
41. Grubb NR, O’Carroll R, Cobbe SM, Sirel J, Fox KA: Chronic memory impairment after cardiac arrest outside hospital. BMJ 1996; 313:143–146Crossref, Medline, Google Scholar
42. Beuret P, Feihl F, Vogt P, Perret A, Romand JA, Perret C: Cardiac arrest: prognostic factors and outcome at one year. Resuscitation 1993; 25:171–179Crossref, Medline, Google Scholar
43. Helzer JE, Robins LM, McEvoy L: Post-traumatic stress disorder in the general population: findings of the epidemiological catchment area survey. N Engl J Med 1987; 317:1630–1634Google Scholar
44. Solomon Z, Mikulincer M, Kotler M: A two year follow up of somatic complaints among Israeli combat stress reaction casualties. J Psychosom Res 1987; 31:463–469Crossref, Medline, Google Scholar
45. McFarlane AC, Atchison M, Rafalowicz E, Papay P: Physical symptoms in post-traumatic stress disorder. J Psychosom Res 1994; 38:715–726Crossref, Medline, Google Scholar
46. Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthey G, Charney DS: Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry 1993; 150:1015–1019Google Scholar
47. Van der Kolk B, Burbridge JA, Suzuki J: The psychobiology of traumatic memory: clinical implications of neuroimaging studies, in Psychobiology of Posttraumatic Stress Disorder. Edited by Yehuda R, McFarlane AC. New York, Annals of the New York Academy of Science, 1997, pp 99–113Google Scholar
48. Inoue T, Kato H, Araki T, Kogure K: Emphasized selective vulnerability after repeated nonlethal cerebral ischemic insults in rats. Stroke 1992; 23:739–745Crossref, Medline, Google Scholar
49. Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney DS: Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry 1997; 54:246–254Crossref, Medline, Google Scholar
50. Bremner JD, Krystal JH, Southwick SM, Charney DS: Noradrenergic mechanisms in stress and anxiety, II: clinical studies. Synapse 1996; 23:39–51Crossref, Medline, Google Scholar
51. Lown B, DeSilva RA, Lenson R: Roles of psychological stress and autonomic nervous system changes in provocation of ventricular premature complexes. Am J Cardiol 1978; 41:979–985Crossref, Medline, Google Scholar
52. Middlekauff HR, Hamilton MA, Stevenson LW, Mark AL: Independent control of skin and muscle sympathetic nerve activity in patients with heart failure. Circulation 1994; 90:1794–1798Google Scholar
53. Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, Stone P, Forman S, Knatterud G, Sheps DS, Kaufmann PG, PIMI Investigators: Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Circulation 1996; 94:2768–2777Google Scholar
54. Fauerbach JA, Lawrence J, Haythornthwaite J, Richter D, McGuire M, Schmidt C, Munster A: Preburn psychiatric history affects posttrauma morbidity. Psychosomatics 1997; 38:374–385Crossref, Medline, Google Scholar



