Dopamine D4 Gene 7-Repeat Allele and Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: Family, twin, and adoption studies show attention deficit hyperactivity disorder (ADHD) to have a substantial genetic component, and some studies have reported an association between ADHD and the dopamine D4 (DRD4) gene. METHOD: The authors recruited 27 triads that comprised an ADHD adult, his or her spouse, and their ADHD child. These triads were assessed for ADHD, and their DNA was genotyped for DRD4 alleles. RESULTS: A multiallelic transmission disequilibrium test suggested an association between ADHD and the DRD4 7-repeat allele. Among family members, the number of 7-repeat alleles predicted the diagnosis of ADHD. CONCLUSIONS: Prior reports of an association between ADHD and DRD4 generalize to families recruited through clinically referred ADHD adults. However, because there are some conflicting studies, further work is needed to clarify the role of DRD4 in the etiology of the disorder.
It is now well established that the biological relatives of children with attention deficit hyperactivity disorder (ADHD) are at increased risk for ADHD and other psychiatric disorders (1). Additional lines of evidence from twin, adoption, and segregation analysis studies suggest that the familial aggregation of ADHD has a substantial genetic component (1).
There have been few molecular genetic studies of ADHD. These have relied on either case-control or family-based association studies. Both studies capitalize on linkage disequilibrium, the fact that very small stretches of DNA will tend to be transmitted together through the generations. This means that if a DNA marker is either within a disease gene or very close to it, one version of that marker will be more common among diseased subjects, even if the marker itself has no functional significance. Case-control studies can produce spurious associations if the case and control groups are not well matched for ethnicity. In contrast, because family-based studies use parental alleles as a “control group,” this confounding does not occur.
Conflicting data have emerged from studies of the dopamine D4 (DRD4) gene in ADHD. Ebstein et al. (2) reported a case-control study in which the 7-repeat allele of DRD4 was associated with novelty-seeking. People with high levels of this personality trait are impulsive, exploratory, excitable, and quick-tempered—well-known features of ADHD. The association between DRD4 and novelty-seeking was replicated in a study that combined both case-control and family-based measures (3) but not in other case-control studies (4–7).
LaHoste et al. (8) noted several reasons why the DRD4 7-repeat allele has functional implications that are relevant for ADHD. This variant of DRD4 mediates a blunted response to dopamine. Moreover, the distribution of DRD4 mRNA in the brain suggests that it plays a role in cognitive and emotional functioning. LaHoste et al. found higher rates of the 7-repeat allele among ADHD children than among control children. This rate was maintained when the sample was increased by 50% (9).
The same group reported a trend association between the 7-repeat allele and ADHD in a small (N=19) family-based association study and a significant association in a case-control study of ADHD adults (10). Two family-based studies (11, 12) found excess transmission of the 7-repeat allele to ADHD children, but in one of these studies (11), this was limited to families where no other relative had ADHD. Moreover, a case-control study failed to replicate the ADHD-DRD4 association (13).
The present study sought to assess the association between ADHD and DRD4 in a group of families ascertained through a clinically referred ADHD adult. We used this strategy because the two available family studies of adult ADHD suggest that it is a highly familial disorder (14, 15).
METHOD
The families reported in this study were spouses and ADHD children of 27 ADHD adult patients from our psychopharmacology clinic (one spouse was also found to have ADHD). These adults had received a clinical diagnosis of ADHD and met DSM-IV criteria for ADHD when evaluated with the ADHD module from the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (16), modified to assess DSM-IV criteria. The spouses and children were similarly evaluated, and all family members provided blood for subsequent DNA extraction. The parents provided written informed consent for themselves and their minor children, who also provided oral assent.
DNA strands were isolated from whole blood as described by Schumm et al. (17). The DRD4 gene contains a varying number of 48 base pair repeats within the gene. We used a method described by Lichter et al. (18) and modified it by adding 7-deazaglutamyl transpeptidase (0.2 mM final concentration) and 10% dimethyl sulfoxide to the polymerase chain reaction cocktail. The polymerase chain reaction products were separated by electrophoresis on 3.5% NuSieve (FMC Bioproducts, Rockland, Me.) and stained with ethidium bromide. Laboratory procedures were blind to the diagnoses of subjects.
We analyzed the data with the exact multiallele transmission disequilibrium test of Cleves et al. (19), as implemented in the program SYMMETRY. Because this is an exact test, it is accurate when cell sizes are small or even zero. To determine which alleles accounted for the significant result, we computed a chi-square statistic contrasting the cell numbers for each pair of nondiagonal cells in the transmission disequilibrium test table. Because we had an a priori reason to suspect that the 7-repeat allele would account for the association between ADHD and DRD4, we did a confirmatory assessment of the role of the 7-repeat allele by assessing the chi-square contribution of each of the five pairs of cells in which the 7-repeat allele was transmitted and another allele was not (and vice versa). For these analyses, we used a Bonferroni-corrected p value of 0.01.
We used logistic regression to determine if, among family members, the number of 7-repeat alleles predicted the diagnosis of ADHD. Because the nonindependence of siblings from the same family leads to inaccurate estimates of statistical significance, we adjusted our analyses by using Huber’s formula (20) as implemented in the Stata Reference Manual (21) to produce accurate statistical tests.
RESULTS
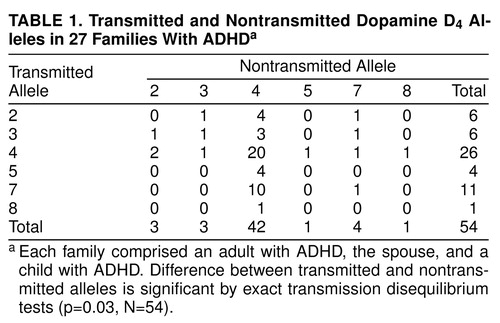
Each parent in the 27 families has two DRD4 alleles; one is transmitted to the ADHD child, the other is not. Table 1 shows the 54 pairs of transmitted and nontransmitted alleles. The exact multiallelic transmission disequilibrium test for the table was significant (p=0.03, N=54), indicating that one or more alleles were preferentially transmitted to the ADHD child.
The follow-up chi-square tests of cells containing the 7-repeat allele found that only one pair significantly contributed to the overall significance: the number of times the 7-repeat allele was transmitted and the 4-repeat allele was not (N=10) was greater than the number of times the 4-repeat allele was transmitted and the 7-repeat allele was not (χ2=7.4, df=1, N=1, p=0.007). This met our Bonferroni criterion for statistical significance (0.01). No other pair of cells even approached statistical significance (all chi-square values ≥2, df=1, all p values ≥0.39).
Consistent with the transmission disequilibrium test results, logistic regression showed that the number of 7-repeat alleles was a significant predictor of the diagnosis of ADHD among family members (z=2.5, N=81, p=0.01). The probability of having ADHD among subjects with zero, one, and two 7-repeat alleles was 0.58, 0.73, and 1.00, respectively.
DISCUSSION
Our results suggest that there may be an association between ADHD and the 7-repeat allele of the dopamine D4 gene in families ascertained through ADHD adults. The DRD4 results are encouraging because positive associations with the 7-repeat allele have been reported from ADHD samples (8–11). Notably, the prevalence of the 7-repeat allele is low in Asian populations (22), as is the prevalence of ADHD (23).
Although we view our results as suggestive, especially in light of prior work, several considerations call for caution in declaring DRD4 to be an ADHD gene. Only 13 of the parents provided information about the transmission of the 7-repeat allele. A larger group would be needed to draw definitive inferences. There has been one failure to replicate the DRD4 association in a case-controlled study (13), and our finding of a positive association in nuclear families containing an ADHD child and an ADHD adult conflicts with the report by Bailey et al. (11) that the association was limited to families in which no other relative had ADHD. Even if the DRD4-ADHD association is real, it could be caused by an unknown gene very close to DRD4.
Notably, 58% of subjects without the 7-repeat allele had ADHD. This prevalence is higher than one would expect in the population, given that our method of ascertainment called for both an ADHD parent and an ADHD child. Nevertheless, it suggests that the 7-repeat allele cannot be viewed as a necessary cause of ADHD. These challenges to a DRD4 hypothesis for ADHD call for further work to clarify the relevance of the association that we have reported.
Received May 13, 1998; revision received Sept. 16, 1998; accepted Oct. 26, 1998. From the Pediatric Psychopharmacology Unit of the Child Psychiatry Service, Massachusetts General Hospital; Harvard Medical School, Boston; the Massachusetts Mental Health Center, Harvard Institute of Psychiatric Epidemiology and Genetics, Boston; Osaka Medical College, Japan; Genome Therapeutics, Inc., Waltham, Mass.; and the Stata Corporation, College Station, Tex. Address reprint requests to Dr. Biederman, Pediatric Psychopharmacology Unit (ACC 725), MGH, 15 Parkman St., Boston, MA 02114. Supported in part by NIMH grants MH-57934 (to Dr. Faraone) and MH-41314 (to Dr. Biederman).
 |
1. Faraone S, Biederman J: Genetics of attention-deficit hyperactivity disorder. Child Adolesc Psychiatr Clin North Am 1994; 3:285–302Crossref, Google Scholar
2. Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH: Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet 1996; 12:78–80Crossref, Medline, Google Scholar
3. Benjamin J, Patterson C, Greenberg BD, Murphy DL, Hamer DH: Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet 1996; 12:81–84Crossref, Medline, Google Scholar
4. Malhotra AK, Virkkunen M, Rooney W, Eggert M, Linnoila M, Goldman D: The association between the dopamine D4 receptor (D4DR) 16 amino acid repeat polymorphism and novelty seeking. Mol Psychiatry 1996; 1:388–391Medline, Google Scholar
5. Jönsson EG, Nöthen MM, Gustavsson JP, Neidt H, Brené S, Tylec A, Propping P, Sedvall GC: Lack of evidence for allelic association between personality traits and the dopamine D4 receptor gene polymorphisms. Am J Psychiatry 1997; 154:697–699Link, Google Scholar
6. Gelernter J, Kranzler H, Coccaro E, Siever L, New A, Mulgrew CL: D4 dopamine-receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am J Hum Genet 1997; 61:1144–1152Google Scholar
7. Sullivan PF, Fifield WJ, Kennedy MA, Mulder RT, Sellman JD, Joyce PR: No association between novelty seeking and the type 4 dopamine receptor gene (DRD4) in two New Zealand samples. Am J Psychiatry 1998; 155:98–101Link, Google Scholar
8. LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy JL: Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry 1996; 1:128–131Google Scholar
9. Kennedy JL, Richter P, Swanson JM, Wigal SB, LaHoste GJ, Sunohara G: Association of dopamine D4 receptor gene and ADHD in 1997 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, APA, 1997, pp 129–130Google Scholar
10. Sunohara G, Barr C, Jain U, Schachar R, Roberts W, Tannock R, Malone M, Kennedy JL: Association of the D4 receptor gene in individuals with ADHD:1) a family-based control study of children with ADHD; 2) a case-control of adults with ADHD (abstract). Am J Hum Genet 1997; 61:A296Google Scholar
11. Bailey JN, Palmer CGS, Ramsey C, Cantwell D, Kim K, Woodward JA, McGough J, Asarnow RF, Nelson S, Smalley SL: DRD4 gene and susceptibility to attention deficit hyperactivity disorder: differences in familial and sporadic cases (abstract). Am J Med Genet Neuropsychiatr Genet 1997; 74:623Google Scholar
12. Swanson JM, Sunohara GA, Kennedy JL, Regino R, Fineberg E, Wigal T, Lerner M, Williams L, LaHoste GJ, Wigal S: Association of the dopamine receptor D4 (DRD4) gene with a refined phenotype of attention deficit hyperactivity disorder (ADHD): a family-based approach. Mol Psychiatry 1998; 3:38–41Crossref, Medline, Google Scholar
13. Castellanos FX, Lau E, Tayebi N, Lee P, Long RE, Giedd JN, Sharp W, Marsh WL, Ginns EI, Rapoport JL, Sidransky E: Evidence that a D4DR*7R dopamine receptor polymorphism is not correlated with attention deficit/hyperactivity disorder (abstract). Am J Med Genet Neuropsychiatr Genet 1997; 74:623Google Scholar
14. Manshadi M, Lippmann S, O’Daniel RG, Blackman A: Alcohol abuse and attention deficit disorder. J Clin Psychiatry 1983; 44:379–380Medline, Google Scholar
15. Biederman J, Faraone SV, Mick E, Spencer T, Wilens T, Kiely K, Guite J, Ablon JS, Reed E, Warburton R: High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: a pilot study. Am J Psychiatry 1995; 152:431–435Link, Google Scholar
16. Orvaschel H: Psychiatric interviews suitable for use in research with children and adolescents. Psychopharmacology (Berl) 1985; 21:737–745Google Scholar
17. Schumm JW, Knowlton RG, Braman JC, Barker DF, Botstein D, Akots G, Brown VA, Gravius TC, Helms C, Hsiao K: Identification of more than 500 RFLPs by screening random genomic clones. Am J Hum Genet 1988; 42:143–159Medline, Google Scholar
18. Lichter J, Barr C, Kennedy J, van Tol H, Kidd K, Livak K: A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Genet 1993; 2:767–773Crossref, Medline, Google Scholar
19. Cleves MA, Olson JM, Jacobs KB: Exact transmission-disequilibrium tests with multiallelic markers. Genet Epidemiol 1997; 14:337–347Crossref, Medline, Google Scholar
20. Huber PJ: The behavior of maximum likelihood estimates under non-standard conditions, in Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, vol 1. Berkeley, Calif, UCB, 1967, pp 221–233Google Scholar
21. Stata Reference Manual, release 5. College Station, Tex, Stata Corp, 1997Google Scholar
22. Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK: The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet 1996; 98:91–101Crossref, Medline, Google Scholar
23. Leung PWL, Luk SL, How TP, Taylor E, Mak FL, Bacon-Shone J: The diagnosis and prevalence of hyperactivity in Chinese schoolboys. Br J Psychiatry 1996; 168:486–496Crossref, Medline, Google Scholar



