Panic Disorder Subtypes: Differential Responses to CO2 Challenge
Abstract
OBJECTIVE: The purpose of this study was to investigate the possibility of a differential sensitivity to CO2 in patients diagnosed with panic disorder subtypes that were defined by the presence of prominent respiratory symptoms. METHOD: The authors used a 35% CO2 and 65% O2 mixture as a challenge agent. Fifty-one unmedicated subjects with DSM-III-R panic disorder, who were divided into respiratory (N=28) and nonrespiratory (N=23) subtypes by their symptom profiles, underwent a CO2 challenge procedure. Patients in the two groups were compared with regard to physiological and psychological measures, pulmonary function tests, panic rates, and smoking habits. RESULTS: The patients in the respiratory group were significantly more sensitive to CO2 than were the patients in the nonrespiratory group. The respiratory group also had higher scores on the Panic and Agoraphobia Scale and had a longer duration of illness; both of these factors can be indicators of illness severity. In addition, the respiratory group’s higher cigarette consumption (mean=12.46 package-years, SD=2.49) may have been a contributory factor not only for illness severity but also for the pathogenesis of panic disorder. CONCLUSIONS: The CO2 challenge procedure appears to be a good dissection tool in the understanding of different subtypes of panic disorder. Moreover, there may be a more specific association with prominent respiratory symptom subtype and CO2 hypersensitivity.
Currently, there is substantial research interest in hyperventilation, respiratory distress, and pulmonary physiology among patients with panic disorder. Enhanced sensitivity to CO2 among patients with panic disorder was first reported by Gorman et al. (1), which prompted research on producing panic attacks in the laboratory. Since then, numerous studies using different concentrations of CO2 (5%, 7%, and 35%) repeatedly showed that patients with panic disorder experienced more panic attacks than both normal subjects and subjects with other psychiatric disorders (2–8). In addition, some studies suggested that CO2 hypersensitivity might be related to a familial vulnerability to panic disorder and might be a disease-specific trait marker (9–11). Regarding the lactate and CO2 literature and the clinical observation that patients with panic disorder often complain of dyspnea and hyperventilation, Klein (12) proposed that panic attacks were related to the hypersensitivity of brainstem chemoreceptors. Such a dysfunction would make a person vulnerable to “false suffocation alarms,” namely, panic attacks. According to this hypothesis, CO2 hypersensitivity is directly involved in the pathophysiology of panic disorder.
A wide range of physical and cognitive symptoms characterize the clinical picture of panic disorder with substantial variability in symptom profile, severity of symptoms, and phobic avoidance among panic patients. On the basis of clinical observations some reports have suggested that panic disorder could be divided into subtypes (13–16). Although these studies have described different symptom clusters as subtypes of panic disorder, all agree that a group of patients with “prominent respiratory symptoms” emerged as a distinct subtype. Briggs et al. (15) further noted that the group of patients whose panic attacks are characterized by prominent respiratory symptoms suffered more spontaneous panic attacks and responded to imipramine, whereas patients with the nonrespiratory subtype suffered more situational panic attacks and responded more to alprazolam. These data suggest that there might be a subgroup of panic patients with prominent respiratory symptoms who are also more apt to experience panic attacks, according to the false suffocation alarm hypothesis.
We further investigated the subtyping of Briggs et al. (15) by examining the CO2 sensitivity rates of patients with the proposed panic disorder subtypes. Our hypothesis was that in patients with prominent respiratory symptoms, CO2 challenge would precipitate panic attacks more easily; therefore, patients with the prominent respiratory subtype would show greater CO2 sensitivity than patients with the nonrespiratory subtype.
METHOD
Subjects
The subjects were 51 outpatients (34 women, 17 men; mean age=33.8 years, SD=9.4, range=18–58) with panic disorder with or without agoraphobia. The diagnosis of panic disorder was made by using the Structured Clinical Interview for DSM-III-R (SCID) (17). Patients with past or current major depression, obsessive-compulsive disorder, schizophrenia, bipolar disorder, or substance abuse or dependence and patients with significant medical illnesses such as cardiac, circulatory, and respiratory disorders, epilepsy, or pregnancy were excluded. All patients underwent a complete physical examination and laboratory tests (hemogram, blood biochemistry, thyroid function tests, ECG, and chest X-ray) before entry into the study. Patients were either never medicated or drug free for at least 2 weeks before the study. However, low-dose, short-acting benzodiazepines were permitted until 36 hours before testing. Patients were required to refrain from alcohol for 36 hours and food or smoking for 2 hours before the challenge. All subjects were assessed with the Panic and Agoraphobia Scale (18) and the Hamilton Depression Rating Scale (19) before the testing. The Panic and Agoraphobia Scale is a 13-item questionnaire that covers the past week and assesses the degree of the severity of panic disorder by gathering information on phenomenology, frequency, and severity of panic attacks and the existence and severity of agoraphobia and phobic avoidance, anticipatory anxiety, disability, and worries about health. All of the Panic and Agoraphobia Scale items were 5-point (scored 0–4), and the total score range was 0–52.
Procedure
The study was approved by the institution’s ethics committee, and all patients provided written informed consent. Subjects were tested in a pulmonary physiology laboratory. The State-Trait Anxiety Inventory (20) was used to evaluate baseline anxiety before the procedure. After administration of the State-Trait Anxiety Inventory, a baseline cardiopulmonary assessment was performed that included heart rate, blood pressure, respiratory rate, and respiratory function tests such as forced vital capacity (FVC), forced expiratory volume at first second (FEV1), peak expiratory flow (PEF), and forced expiratory flow during the middle of FVC (FEF25%–75%). After a 15-minute resting interval, subjects were informed that they would be asked to inhale a harmless gas mixture, following which they might experience some discomfort or symptoms of anxiety; there was no mention of the possibility of a panic attack.
The procedure was performed by a trained senior research assistant (B.B.) who was blind to the patients’ subtype status but not to the panic disorder diagnosis. In the challenge procedure, subjects were first asked to exhale as much air as possible, then to take a very deep breath of a mixture of 35% CO2 and 65% O2 through a face mask and hold their breaths for 5 seconds. The flow of the gas mixture was set at 14 liters/minute. Measurements of blood pressure, heart rate, and respiratory rate were repeated during the first minute after the inhalation and 15 minutes after the procedure. Subjects completed the 17-item Acute Panic Inventory (21) between the two measurements. CO2-induced panic attack was determined as defined by Sanderson et al. (22). The criteria for postinhalation panic attack were 1) sensation of fear or panic, 2) at least four DSM-III-R panic attack symptoms, and 3) at least one cognitive symptom (i.e., fear of dying, losing control, or going crazy).
Subtyping the natural panic attacks of the subjects on the basis of symptom profile was made as described by Briggs et al. (15). These symptoms were derived from a large group of panic disorder patients by using cluster and principal component analysis. Although the symptoms of fear of dying and numbness/tingling were not related primarily to respiratory function, they emerged as a part of the respiratory subtype in the study by Briggs et al.(15). Accordingly, subjects reporting at least four of these five symptoms (shortness of breath, choking/smothering sensations, fear of dying, chest pain/discomfort, and tingling/numbness) were classified as the prominent respiratory symptom group (N=28). Subjects reporting three or fewer of the five symptoms were subtyped as the nonrespiratory symptom group (N=23). Thus, each subject was grouped in only one panic disorder subtype. Subtype determinations were made by the other investigator (T.A.), who was blind as to whether or not the subject experienced a CO2-inducedpanic attack.
Data Analyses
Differences among the panic disorder subtypes in sex and age distribution; marital, educational, and employment status; and panic rates were tested with chi-square analyses. Baseline vital signs; results of baseline pulmonary function tests; scores on the Panic and Agoraphobia Scale, State-Trait Anxiety Inventory, and Hamilton depression scale; and lifetime smoking habits were assessed with t tests for the independent samples statistics. Baseline vital sign measurements and first and 15th minute response measurements (heart rate, systolic and diastolic pressure, respiratory rate) were used in 2×2×2×3 analysis of variance (ANOVA) for repeated measures. The between-group comparisons included the prominent respiratory symptom subtype and nonrespiratory symptom subtype, agoraphobic and nonagoraphobic patients, and smokers and nonsmokers.
The clinical predictors of CO2-induced panic response were identified by a forward stepwise logistic regression analysis by entering subtype status, duration of illness, age at onset, history of smoking, Panic and Agoraphobia Scale score, and State and Trait scores on the State-Trait Anxiety Inventory as variables.
All reported p values are for two-tailed tests of significance.
RESULTS
Demographic and Baseline Variables
The mean age at onset of panic disorder was 30.0 years (SD=10.0). The mean duration of illness was 38.2 months (SD=50.2). There were 28 patients (19 women, nine men) in the prominent respiratory symptom subtype group and 23 (15 women, eight men) in the nonrespiratory symptom subtype group. Chi-squareanalyses among the two subtypes showed no significant differences in sex distribution, education, or marital and occupational status. According to t tests, there were no significant differences between the two subtypes in mean number of panic symptoms, mean age, and mean years of education.
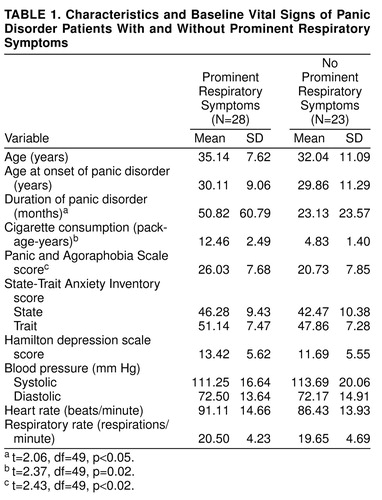
Thirty-four patients (67%) met the DSM-III-R criteria for panic disorder with agoraphobia, whereas 17 patients (33%) met the criteria for panic disorder without agoraphobia. Concomitant SCID diagnoses were generalized anxiety disorder in six patients (12%), social phobia in one patient (2%), simple phobia in one patient (2%), and conversion disorder in one patient (2%). Patients with or without comorbid psychiatric diagnoses, patients subtyped as having panic disorder with or without agoraphobia, patients who did or did not panic in response to the CO2 challenge, and patients in the respiratory or nonrespiratory subtype groups did not show any differences in the comparisons on the baseline mean values for pulmonary function tests and other baseline vital signs. Data on the baseline measurements of vital signs and some other clinical variables are presented in table 1.
According to repeated measures ANOVA with the Bonferroni correction, two between-subjects effects emerged for heart rate measurements. There were significant interactions of smoking status (smokers versus nonsmokers) by subtypes (respiratory versus nonrespiratory) (F=4.53, df=1, 43, p=0.04) and subtypes by agoraphobia (with or without agoraphobia) (F=6.43, df=1, 43, p=0.02). No other between-subjects interactions were observed for any measurements.
Regarding within-subjects effects, a significant time effect (F=3.20, df=2, 86, p=0.05) and significant subtypes-by-time interaction (F=3.47, df=2, 86, p=0.04) for heart rate were found. The mean pre- and two postintervention measurements of heart rate were 91.11 (SD=14.66), 88.78 (SD=11.25), and 85.96 (SD=9.97) for the prominent respiratory subtype group and 86.43 (SD=13.93), 92.00 (SD=15.38), and 84.69 (SD=11.16) for the nonrespiratory subtype group, respectively, indicating that the heart rate of patients in the nonprominent respiratory subtype group was more responsive to CO2. In addition, baseline heart rate was higher in patients in the respiratory subtype group as a reflection of high level of baseline anxiety.
Time effect (F=6.41, df=2, 86, p=0.003) and agoraphobia-by-time interaction (F=3.84, df=2, 86, p=0.03) for systolic pressure were also significant. The systolic pressure after the first minute of challenge for patients without agoraphobia (mean=121.92 mm Hg, SD=21.68) was significantly higher than that for patients with agoraphobia (mean=113.20 mm Hg, SD=19.67). A significant time effect was found for respiratory rate (F=3.69, df=2, 86, p=0.03), indicating that the patients had a higher respiratory rate right after inhalation (mean=21.47 respirations/minute, SD=4.49) than they had before inhalation (mean=20.11 respirations/minute, SD=4.42).
Panic Rates
Thirty-three (65%) of 51 patients had a panic attack as a response to the CO2 procedure. One patient (2%) had a panic attack with limited symptoms, but we did not accept this response as a CO2-induced panic attack. Patients who experienced a CO2-induced panic attack had significantly higher baseline scores on the Panic and Agoraphobia Scale (mean=25.42, SD=8.34) than did patients without panic attacks (mean=20.38, SD=6.78) (t=–2.19, df=49, p<0.03). They also had significantly higher scores on the State-Trait Anxiety Inventory—State Form (mean=47.36, SD=10.13) than did patients without panic attacks (mean=39.44, SD=7.46) (t=–2.91, df=49, p<0.01). There were no statistically significant differences between panicking and nonpanicking patients on any other sociodemographic or clinical variables or on any baseline vital sign measurements. As expected, patients who had panicked in response to CO2 scored significantly higher on the Acute Panic Inventory (mean=10.57, SD=7.35) than did patients who had not panicked (mean=1.27, SD=1.44) (t=–5.29, df=49, p<0.001).
Stepwise logistic regression analysis revealed that higher baseline scores on the State-Trait Anxiety Inventory—State Form (Wald=5.76, df=1, p<0.02) and subtype status (Wald=5.03, df=1, p<0.05) predicted the CO2-induced panic response for the entire patient group.
Subtypes
Twenty-two (79%) of 28 patients in the prominent respiratory symptom subtype group and 11 (48%) of 23 patients in the nonrespiratory symptom subtype group experienced a panic attack. The panic rate in response to CO2 among the panic disorder subtypes was significantly different (χ2=3.97, df=1, p<0.05, with Yates’s correction). In addition, patients with prominent respiratory symptoms had significantly higher scores on the Panic and Agoraphobia Scale and had a longer duration of illness than patients with nonrespiratory symptoms (table 1). Neither subtype differed significantly on any other baseline clinical variable.
In the entire study group, 37 patients (73%) had a history of smoking, and 14 patients (27%) reported that they had never smoked. The mean amount of cigarette consumption was 8.39 package-years (package of cigarettes consumed daily multiplied by years) (SD=8.92) for the study group. Regarding the lifetime smoking habits of patients in the two groups, for 21 patients (75%) in the prominent respiratory symptom subtype group and 16 (70%) in the nonrespiratory symptom subtype group, smoking preceded the onset of panic disorder. Cigarette consumption for patients in the prominent respiratory subtype group (mean=12.46 package-years, SD=2.49) appeared to be greater than that for patients in the nonrespiratory subtype group (mean=4.83 package-years, SD=1.40). The difference was significant (t=2.37, df=49, p=0.02). Patients in the prominent respiratory subtype group had a longer history of smoking (mean=150.21 months, SD=120.39) than patients in the other group (mean=89.74 months, SD=98.74); however, this difference did not reach statistical significance (t=1.93, df=49, p=0.06).
DISCUSSION
We found, as hypothesized, that patients with panic disorder with prominent respiratory symptoms were more sensitive to the CO2 challenge than were patients with nonrespiratory symptoms. Our results are consistent with Klein’s false suffocation alarm hypothesis (12). In a substantial group of panic patients, heightened CO2 sensitivity may play an important role in the pathophysiology of panic disorder. Moreover, there may be a more specific association with prominent respiratory symptom subtype and CO2 hypersensitivity.
Although implicating a biologically mediated pathophysiological process, treatment response data alone might be insufficient to create etiologically homogeneous patient subgroups. Both imipramine and alprazolam are beneficial in treating heterogeneous panic patient groups (23–25). It is therefore clear that additional biological markers are needed to establish a more precise subtyping. Although imipramine and alprazolam both seem to block CO2-induced panic (26–29), CO2 hypersensitivity can still be a valuable tool for delineating subtypes. In this study, only respiratory subtype and high baseline anxiety scores on the State-Trait Anxiety Inventory—State predicted a CO2-induced panic attack. Taken together with the differential response to drugs, as reported by Briggs et al. (15), our finding of differential CO2 hypersensitivity of patients in these subtype groups strengthens the possibility of an accurate subtyping of panic disorder. A recent study (30) reported that there are no response differences to either hyperventilation or 35% CO2 challenges among medicated panic patients when they are classified on the basis of severity of dyspnea symptoms and low or high baseline partial CO2 pressure levels. However, there is still far from complete agreement on the exact symptom profile of a respiratory subtype (12–16). In addition, an unusually low rate of panic response was observed in this study. Therefore, conflicting differences in results among studies may be attributable to consideration of merely dyspnea symptoms and disregard of some relevant panic symptoms for subtyping and the criteria used to define a panic response.
Our rather simple methods of assessing the baseline respiratory functions did not reveal any overt pathology or differences between panic disorder subtypes. However, it is still possible that abnormalities in respiratory physiology and disordered breathing in panic patients, as shown by others(4, 31–35), may yield differences between subtypes. Patients in the prominent respiratory subtype group have a significantly longer duration of illness, more severe panic and phobic symptoms, and greater disability, as reflected by higher scores on the Panic and Agoraphobia Scale, than patients in the nonrespiratory subtype group. The relevance of these findings to the subtype status was supported indirectly with treatment response studies, in which factors such as increased social disability and longer duration of illness predicted poorer response to alprazolam, whereas milder illness at baseline predicted a better response (36, 37). Subtype and severity determinations of panic disorder in studies of treatment and respiratory psychophysiology will be more informative and should be considered in future research.
Furthermore, patients in the prominent respiratory subtype group are more likely to be heavier smokers than are patients in the nonrespiratory subtype group. Higher smoking prevalence in anxiety patients has been reported (38). It is possible that panic patients with prominent respiratory symptoms may have a high level of anxiety that is reduced by smoking. Nevertheless, smoking itself could create the symptom of breathlessness and even dyspnea by inducing airway obstruction. Our finding of a high prevalence of smokers and a high amount of cigarette use among the panic patients, especially among those with prominent respiratory symptoms, suggests a possible predisposing agent for respiratory diseases, as well as for panic disorder. Although Verburg et al. (39) studied only two respiratory panic symptoms in the determination of subtype, they reported a higher prevalence of bronchitis in panic patients with prominent respiratory symptoms. On the basis of other data on respiratory disease and panic disorder comorbidity (40–42), it is likely that respiratory diseases may predispose people to panic disorder and may account, at least in part, for the respiratory symptoms of panic disorder. However, without prospective studies relating smoking and bronchitis with panic disorder and subtypes, these suggestions will remain speculative.
A history of traumatic suffocation experiences and nocturnal panic attacks associated with the predominantly respiratory subtype have been recently reported (43). Along with the literature and our data, observations suggest that it is worthwhile considering some noticeable clinical features that are helpful in distinguishing proposed subtypes. It appears that panic patients with prominent respiratory symptoms also have more spontaneous and nocturnal panic attacks, past traumatic suffocation experiences, past respiratory diseases, heavier smoking history, longer duration of illness, more sensitivity to CO2 challenge, and more responsiveness to tricyclic treatment and that this subtype might be related to a more severe and disabling type of panic disorder (15, 39, 43).
A number of methodological limitations of this study deserve mention. First, focusing on only two subtypes of panic disorder was the major limitation of this study. There are studies implicating other subtypes such as vestibular, gastrointestinal, cardiac, and mixed (13–16, 44, 45), in which research on CO2-induced panic is relevant. Second, baseline arterial blood gas measures, tidal volume,and end-tidal CO2 pressure measurements were not available in the present study. These measurements may be important for identifying the respiratory abnormalities that could also predict the subtype differences. Third, an unknown bias might have prevailed because the researchers were not blind to the panic disorder diagnosis. Despite these shortcomings, we believe that our findings still have convincing implications in panic research.
In conclusion, differential responses to CO2 challenge have provided a stronger biological basis to a preliminary subtyping. This underscores the need for further biological and phenomenological research to determine the exact symptom profile of the subtypes. Precise subtyping of panic patients in turn, by lowering diagnostic heterogeneity, will reduce most of the contradictory findings across the panic disorder studies.
Received March 13, 1998; revision received Aug. 28, 1998; accepted Oct. 8, 1998. From the Department of Psychiatry, Faculty of Medicine, Dokuz Eylöl University. Address reprint requests to Dr. Alkin, Dokuz Eylöl Öniversitesi, Tip Faköltesi, Psikiyatri ABD, 35340 Balçova, Izmir, Turkey. The authors thank Vedat Pazarlioglu, who served as a consultant on the data analysis.
 |
1. Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, Klein DF: Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry 1984; 141:857–861Link, Google Scholar
2. Griez E, Lousberg H, van den Hout MA, van der Molen MG: CO2 vulnerability in panic disorder. Psychiatry Res 1987; 20:87–95Crossref, Medline, Google Scholar
3. Gorman JM, Papp LA, Coplan CD, Martinez JM, Lennon S, Goetz RR, Ross D, Klein DF: Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am J Psychiatry 1994; 151:547–553Link, Google Scholar
4. Papp LA, Klein DF, Martinez J, Schneier F, Cole R, Liebowitz MR, Hollander E, Fyer AJ, Jordan F, Gorman JM: Diagnostic and substance specificity of carbon-dioxide-induced panic. Am J Psychiatry 1993; 150:250–257Link, Google Scholar
5. Verburg C, Griez E, Meijer J: A 35% carbon dioxide challenge in simple phobias. Acta Psychiatr Scand 1994; 90:420–423Crossref, Medline, Google Scholar
6. Perna G, Barbini B, Cocchi S, Bertani A, Gasperini M:35% CO2 challenge in panic and mood disorders. J Affect Disord 1995; 33:189–194Google Scholar
7. Perna G, Bertani A, Arancio C, Ronchi P, Bellodi L: Laboratory response of patients with panic and obsessive-compulsive disorders to 35% CO2 challenges. Am J Psychiatry 1995; 152:82–89Google Scholar
8. Verburg K, Griez E, Meijer J, Pols H: Discrimination between panic disorder and generalized anxiety disorder by 35% carbon dioxide challenge. Am J Psychiatry 1995; 152:1081–1083Google Scholar
9. Perna G, Cocchi S, Bertani A, Arancio C, Bellodi L: Sensitivity to 35% CO2 in healthy first-degree relatives of patients with panic disorder. Am J Psychiatry 1995; 152:623–625Link, Google Scholar
10. Perna G, Bertani A, Caldirola D, Bellodi L: Family history of panic disorder and hypersensitivity to CO2 in patients with panic disorder. Am J Psychiatry 1996; 153:1060–1064Google Scholar
11. Coryell W: Hypersensitivity to carbon dioxide as a disease-specific trait marker. Biol Psychiatry 1997; 41:259–263Crossref, Medline, Google Scholar
12. Klein DF: False suffocation alarms, spontaneous panic and related conditions. Arch Gen Psychiatry 1993; 49:282–288Google Scholar
13. Bass C, Kartsounis L, Lelliott P: Hyperventilation and its relationship to anxiety and panic. Integr Psychiatry 1987; 5:274–291Google Scholar
14. Aronson TA, Logue JM: Phenomenology of panic attacks: a descriptive study of panic disorder patients’ self reports. J Clin Psychiatry 1988; 49:8–13Medline, Google Scholar
15. Briggs AC, Stretch DD, Brandon S: Subtyping of panic disorder by symptom profile. Br J Psychiatry 1993; 163:201–209Crossref, Medline, Google Scholar
16. Shioiri T, Someya T, Murashita S, Takahashi S: The symptom structure of panic disorder: a trial using factor and cluster analysis. Acta Psychiatr Scand 1996; 93:80–86Crossref, Medline, Google Scholar
17. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
18. Bandelow B: Assessing the efficacy of treatments for panic disorder and agoraphobia, II: the Panic and Agoraphobia Scale. Int Clin Psychopharmacol 1995; 10:73–81Google Scholar
19. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
20. Spielberger CD, Gorsuch RL, Lushene RD: STAI Manual. Palo Alto, Calif, Consulting Psychologists Press, 1970Google Scholar
21. Dillon DJ, Gorman JM, Liebowitz MR, Fyer AJ, Klein DF: Measurement of lactate induced panic and anxiety. Psychiatry Res 1987; 20:97–105Crossref, Medline, Google Scholar
22. Sanderson WC, Rapee RM, Barlow DH: The influence of an illusion of control on panic attacks induced via inhalation of 5.5% carbon dioxide-enriched air. Arch Gen Psychiatry 1989; 46:157–162Crossref, Medline, Google Scholar
23. Klein DF: Delineation of two drug-responsive anxiety syndromes. Psychopharmacologia 1964; 5:397–408Crossref, Medline, Google Scholar
24. Zitrin CM, Klein DF, Woerner MG: Treatment of phobias, I: comparison of imipramine and placebo. Arch Gen Psychiatry 1983; 40:125–138Crossref, Medline, Google Scholar
25. Ballenger JC, Burrows GD, DuPont RL, Lesser IM, Noyes R, Pecknold JC, Rifkin A, Swinson RP: Alprazolam in panic disorder and agoraphobia: results from a multicenter trial. Arch Gen Psychiatry 1988; 45:413–422Crossref, Medline, Google Scholar
26. Woods SW, Charney DS, Loke WK, Goodman GR, Redmond DE Jr, Heninger GR: Carbon dioxide sensitivity in panic anxiety. Arch Gen Psychiatry 1986; 43:900–909Crossref, Medline, Google Scholar
27. Sanderson WC, Wetzler S, Asnis GM: Alprazolam blockade of CO2-provoked panic in patients with panic disorder. Am J Psychiatry 1994; 151:1220–1222Google Scholar
28. Woods SW, Charney DS, Delgado PL, Heninger GR: The effect of long-term imipramine treatment on carbon dioxide-induced anxiety in panic disorder patients. J Clin Psychiatry 1990; 51:505–507Medline, Google Scholar
29. Gorman JM, Browne ST, Papp LA, Martinez J, Welkowitz L, Coplan JD, Goetz RR, Kent J, Klein DF: Effect of antipanic treatment on response to carbon dioxide. Biol Psychiatry 1997; 42:982–991Crossref, Medline, Google Scholar
30. Schmidt NB, Telch MJ, Jaimez TL: Biological challenge manipulation of PCO2 levels: a test of Klein’s (1993) suffocation alarm theory of panic. J Abnorm Psychol 1996; 105:446–454Crossref, Medline, Google Scholar
31. Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, Kinney J, Klein DF: Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry 1988; 45:31–39Crossref, Medline, Google Scholar
32. Papp LA, Martinez JM, Klein DF, Coplan JD, Gorman JM: Rebreathing tests in panic disorder. Biol Psychiatry 1995; 38:240–245Crossref, Medline, Google Scholar
33. Stein MB, Millar TW, Larsen DK, Kryger MH: Irregular breathing during sleep in patients with panic disorder. Am J Psychiatry 1995; 152:1168–1173Google Scholar
34. Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, deJesus MJ, Ross D, Goetz R, Gorman JM: Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. Am J Psychiatry 1997; 154:1557–1565Google Scholar
35. Hegel MT, Ferguson RJ: Psychophysiological assessment of respiratory function in panic disorder: evidence for a hyperventilation subtype. Psychosom Med 1997, 59:224–230Google Scholar
36. Woodman CL, Noyes R Jr, Ballenger JC, Lydiard RB, Sievers G, Mihalko D: Predictors of response to alprazolam and placebo in patients with panic disorder. J Affect Disord 1994; 30:5–13Crossref, Medline, Google Scholar
37. Basoglu M, Marks IM, Swinson RP, Noshirvani H, O’Sullivan G, Kuch K: Pre-treatment predictors of treatment outcome in panic disorder and agoraphobia treated with alprazolam and exposure. J Affect Disord 1994; 30:123–132Crossref, Medline, Google Scholar
38. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA: Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 1986; 143:993–997Link, Google Scholar
39. Verburg K, Griez E, Meijer J, Pols H: Respiratory disorders as a possible predisposing factor for panic disorder. J Affect Disord 1995; 33:129–134Crossref, Medline, Google Scholar
40. Yellowes PM, Alpers JH, Bowden JJ, Bryant GD, Ruffin RE: Psychiatric morbidity in patients with chronic airflow obstruction. Med J Aust 1987; 146:305–307Crossref, Medline, Google Scholar
41. Karajgi B, Rifkin A, Doddi S, Kolli R: The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. Am J Psychiatry 1990; 147:200–201Link, Google Scholar
42. Perna G, Bertani A, Politi E, Colombo G, Bellodi L: Asthma and panic attacks. Biol Psychiatry 1997; 42:625–630Crossref, Medline, Google Scholar
43. Bouwer C, Stein DJ: Association of panic disorder with a history of traumatic suffocation. Am J Psychiatry 1997; 154:1566–1570Google Scholar
44. Lydiard RB, Laraia MT, Howell EF, Ballenger JC: Can panic disorder present as irritable bowel syndrome? J Clin Psychiatry 1986; 47:470–473Google Scholar
45. Stein MB, Asmundson GJG, Ireland D, Walker JR: Panic disorder in patients attending a clinic for vestibular disorders. Am J Psychiatry 1994; 151:1697–1700Google Scholar



