Low Levels of Transthyretin in the CSF of Depressed Patients
Abstract
OBJECTIVE: Transthyretin plays an important role in the transport and distribution of thyroid hormone in the central nervous system (CNS). This study replicated and extended to patients with nonrefractory depressive illness a pilot study indicating that patients with refractory major depression have significantly lower levels of CSF transthyretin than do healthy comparison subjects. METHOD: Lumbar punctures were performed in drug-free subjects with DSM-III-R major depression (N=18), DSM-III-R bipolar disorder, depressed phase (N=1), and healthy comparison subjects (N=24). CSF concentrations of transthyretin, determined by a quantitative dot-immunobinding assay, of the depressed patients and comparison subjects were compared by analysis of covariance (ANCOVA). The relationship between CSF transthyretin levels and Hamilton Depression Rating Scale scores was determined in a subset of the depressed patients. RESULTS: CSF concentrations of transthyretin were significantly lower in the depressed patients than in the comparison subjects by ANCOVA. Within the depressed group there was no significant overall correlation between CSF transthyretin levels and Hamilton depression scale scores, but there was a significant inverse correlation in male depressed patients (N=8) between CSF transthyretin concentrations and Hamilton depression scores. CONCLUSIONS: Lower CSF transthyretin concentrations in depressed patients may reflect either a stable trait in this population or a state change secondary to depression or other factors. Lower CSF transthyretin concentrations may result in altered CNS thyroid hormone homeostasis. Such alteration could account for certain mood and neurovegetative symptoms of depression and might contribute to failure of standard antidepressant treatment.
A link between depression and disturbances in thyroid function has often been proposed. Many symptoms of depression, such as low mood, lethargy, cognitive impairment, low appetite, constipation, and psychomotor retardation, resemble those of hypothyroidism. Although a substantial number of patients with hypothyroidism suffer from depression (1), changes in peripheral thyroid hormone levels are infrequently found in depressed patients (2, 3). The thyrotropin-releasing hormone (TRH) stimulation test has been used extensively to study subtle abnormalities in the hypothalamic-pituitary-thyroid (HPT) axis in depression. A consistent finding has been a blunted thyrotropin (TSH) response to TRH stimulation in about 25% of depressed patients (4, 5), particularly those with endogenous symptoms (6–8). Increased CSF levels of TRH have been reported in depression (9). In addition, up to 10% of depressed patients appear to have an exaggerated TSH response to the TRH stimulation test (10, 11). It has been suggested that low nocturnal TSH secretion may be a more sensitive measure of HPT axis dysfunction in depression than the TRH stimulation test (12). Low basal and TRH-stimulated levels of TSH as well as low peripheral thyroid hormone levels, while generally considered to be in the “subclinical” hypothyroid range, have been associated with poor response to antidepressant therapies (13–16). In addition, a recent meta-analysis (17) indicates that triiodothyronine (T3) supplementation of antidepressant medication can improve the response rate and can also convert patients with refractory depression to responders. Others (18, 19) have also reported a beneficial effect of thyroid hormone supplementation in patients with rapid-cycling and treatment-refractory bipolar illness. Bauer et al. (20) have speculated that a “restricted central hypothyroidism” may exist in such patients, in which CNS thyroid function, but not peripheral thyroid function, may be abnormal.
Transthyretin is a thyroid hormone-binding protein that is produced in abundance by the choroid plexus and is secreted into CSF. Transthyretin is also produced by the liver and is secreted into blood, where it appears to play a lesser role in peripheral blood thyroid transport than does thyroid-binding globulin. In humans, transthyretin is known to make up 10%–25% of total ventricular protein and is the sole thyroid hormone-binding protein found at a substantial level in CSF (21). Therefore, reduced levels of transthyretin in the brain might disrupt delivery of thyroid hormone to regions inside the blood-brain barrier, despite adequate thyroid hormone feedback to the hypothalamus and anterior pituitary to maintain euthyroid peripheral hormone levels.
In our pilot study examining levels of CSF transthyretin in a psychiatric population (22), we found that eight patients with refractory depression had significantly lower levels of transthyretin than nine neurological patients serving as control subjects. The present study involved a new group of depressed patients, including treatment-responsive patients, who were compared with healthy subjects. In contrast to the pilot study, patients with a history of thyroid disease or treatment with thyroid hormones were excluded.
METHOD
The patients with depression (N=19) were from two sites. Eight were recruited for an inpatient protocol on transthyretin at the New York State Psychiatric Institute, New York. The other 11 were patients who had been admitted to the Western Psychiatric Institute and Clinic, Pittsburgh, for participation in a study of the risk of attempted suicide in patients with depression (23). Patients at both sites gave written informed consent for participation in the studies.
The eight patients admitted to the New York State Psychiatric Institute met the DSM-III-R criteria (pp. 218–224) for unipolar major depressive episode without psychotic features on the basis of a clinical interview. Scores on the 17-item Hamilton Depression Rating Scale (24) were determined at baseline. Patients were excluded if they had a history of thyroid disease or recent treatment with thyroid hormones or lithium. Patients were maintained free of psychotropic medications for 2 weeks before lumbar puncture, with the exception of lorazepam for extreme anxiety and insomnia.
Of the 11 patients admitted to Western Psychiatric Institute and Clinic, 10 met the DSM-III-R criteria for unipolar major depressive episode and one for bipolar disorder, depressed phase, on the basis of the Structured Clinical Interview for DSM-III-R (SCID) (25). Scores on the 17-item Hamilton depression scale were available for nine of the 11 patients. All of these patients were medication free for 2 weeks before lumbar puncture, and none had a history of thyroid disease or recent treatment with thyroid hormones or lithium.
All depressed patients from both institutions had full physical examinations and laboratory testing including blood chemistries, CBC, urine toxicology, and ECG.
The comparison subjects (N=24) were also from two sites. Five subjects were recruited at New York State Psychiatric Institute through the normal control component of the Mental Health Clinical Research Center (26) and had no history of psychiatric illness or current psychiatric illness on the basis of the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (SADS-L) (27) and clinical interview. All were physically healthy by history and physical examination, with no clinical or laboratory thyroid abnormalities. These subjects had been on a monoamine oxidase inhibitor (MAOI) diet for 24 hours before lumbar puncture and had been in a medication-free state for at least 1 week.
Nineteen comparison subjects were recruited by the National Institute of Mental Health (NIMH) (laboratory of Dr. Robert M. Post) through local announcement and had no history of psychiatric illness or current psychiatric illness on the basis of the SADS-L. None had a history of or a current major medical illness, including thyroid disease, and all had normal results on physical examinations. These subjects had been on an MAOI diet for at least 3 days before lumbar puncture and had been medication free for at least 1 week.
All depressed patients and comparison subjects fasted after midnight the night before lumbar puncture. CSF samples for transthyretin assay were obtained by lumbar puncture between 8:00 a.m. and 9:00 a.m., under sterile conditions with local anesthesia, with patients in the lateral decubitus position. CSF samples were frozen immediately and stored at –70°C until assay in the laboratory of one member of the research team (J.H.), who was blind to diagnosis and subject identity. The CSF samples at each institution were collected, stored, and shipped in a uniform manner, and only aliquots from the first 10 ml of the lumbar puncture CSF from each subject were used for the transthyretin assay.
CSF concentrations of transthyretin were measured in duplicate by a dot-blot immunoassay. Two hundred milliliters of 1:50 diluted CSF samples in phosphate-buffered saline (136 mM NaCl, 13.4 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl; pH=7.4) were dot-blotted onto nitrocellulose membrane with use of the Bio-Dot manifold (Bio-Rad, Hercules, Calif.). The membrane was first blocked with 3% bovine serum albumin (Sigma, St. Louis) and then incubated with 1:500 dilution of antihuman prealbumin (transthyretin) antibody (Boehringer Mannheim, Indianapolis) for 2 hours at room temperature. After washing three times with phosphate-buffered saline containing 0.05% Tween-20, the membrane was incubated with 1:1000 dilution of peroxidase conjugated anti-rabbit IgG (Sigma) and developed with 3,3"-diaminobenzidine as substrate. The membrane was scanned immediately with the OneScanner (Apple Computer), and the optical density of each dot was analyzed with the National Institutes of Health image program. Twofold serially diluted human prealbumin (transthyretin; Sigma) was used for the standard curve. By plotting the logarithm of transthyretin concentration on the y axis and the optical density on the x axis, the curve was found to be linear within the limited range of 2–96 ng per dot, with correlation coefficients of more than 0.95. Both standards and samples were analyzed in duplicate, and the results were calculated as the mean value. Random samples were assayed repetitively on several occasions, and the coefficients of variation for between-day analyses ranged from 4.7% to 10.3% (the latter value is an exceptionally high coefficient of variation in this range).
We performed preliminary analyses of CSF transthyretin levels, age, sex distribution, and Hamilton depression scale scores because the depressed patients and comparison subjects were from three different sites as outlined above (also, see table 1 for the site breakdown of demographic characteristics). CSF transthyretin levels were not normally distributed; therefore, we performed a natural log transformation of this variable to normalize its distribution. The depressed patients differed in age and Hamilton depression scale scores between the New York State Psychiatric Institute and Western Psychiatric Institute and Clinic sites: the patients from New York State Psychiatric Institute were older and presented with higher Hamilton depression scale scores. The sex distribution between the sites could not be adequately assessed. Given the differences just mentioned, we decided to perform a two-way analysis of covariance (ANCOVA) assessing main effects of diagnosis and sex, with age included as a covariate. A two-tailed t test and a chi-square test were used to compare age and sex distribution, respectively, in the depressed and comparison groups. The relationship between CSF transthyretin concentration and Hamilton depression score was assessed with Pearson correlation coefficients.
RESULTS
Demographic characteristics, Hamilton depression scale scores, and CSF transthyretin data, presented by recruitment site, are displayed in table 1. The results of the ANCOVA show that CSF transthyretin concentrations were significantly lower in the depressed patients than in the comparison subjects (mean=4.41 mg/liter, SD=1.61, versus mean=7.28 mg/liter, SD=4.68). A scattergram of the raw CSF transthyretin values is displayed in Figure 1. The range of CSF transthyretin levels for the depressed patients was 1.93–7.22 mg/liter, and for the comparison subjects 2.16–19.67 mg/liter. There was no significant main effect of sex (F=0.37, df=1, 38, p=0.55) nor a sex-by-diagnosis interaction (F=1.42, df=1, 38, p=0.24). Age as the covariate was also not significant (F=0.12, df=1, 38, p=0.73). Age and sex were not significantly different (t=1.18, df=41, p=0.25, and χ2=0.98, df=1, p=0.32, respectively) between the pooled normal comparison groups and the pooled depressed groups. There was a significant difference in Hamilton depression scale scores between the patients at the two sites, and there was a marginal difference in CSF transthyretin levels between the normal comparison subjects at their two sites (table 1). The correlation between CSF transthyretin concentrations and Hamilton depression scale scores within the groups of depressed subjects was not significant, nor was there such a correlation within the female depressed subgroup. However, within the male depressed subgroup (N=8), there was a significant inverse correlation between CSF transthyretin levels and Hamilton depression scale scores (Figure 2).
DISCUSSION
We found mean CSF transthyretin levels to be significantly lower in a group of 19 patients with depression than in a group of 24 normal comparison subjects. This indicates possible CNS hypothyroidism in depressed patients despite apparent euthyroidism indicated by routine peripheral blood thyroid hormone measures.
Transthyretin, synthesized and secreted into CSF by the choroid plexus, is a specific thyroxine (T4) transport protein responsible for the uptake of T4 across the blood-brain barrier and for its distribution throughout the CNS (28–33). Because transthyretin is a polypeptide tetramer that does not readily cross the blood-brain barrier, the transthyretin found abundantly in peripheral circulation, synthesized and secreted by the liver, is entirely distinct from CNS transthyretin. There is much evidence indicating that CNS thyroid homeostasis is regulated at least semi-independently from that in other organ systems (34); therefore, CNS thyroid dysfunction can be expected not always to correlate fully with peripheral thyroid status. Alteration in CNS thyroid hormone levels has major effects on serotonergic, adrenergic, and GABA-ergic systems (35–38).
The present study replicated our earlier finding that depressed patients have significantly lower levels of CSF transthyretin than comparison subjects. It is important to note the major differences between these two studies (which had no patients in common). The pilot study included eight patients with treatment-refractory depression, whereas not all patients in the present study had refractory depression. In the earlier study four patients had a history of hypothyroidism, two of whom were receiving thyroid replacement therapy at the time of the study, whereas the current study excluded any patients with a history, laboratory evidence, or treatment of thyroid disease. The comparison group used in this study consisted of normal volunteers rather than patients with neurological disease. Unlike the previous study, in this study the comparison group was not significantly different in age and sex from the depressed group, thereby reducing concerns about possible influences of these variables on transthyretin levels.
A substantial drawback of the current study was the pooling of patients and comparison subjects from three sources. Importantly, all CSF samples were collected, stored, and shipped in a uniform manner, and all were analyzed for CSF transthyretin level in the same laboratory by the same investigator, who was blind to diagnosis and subject identity. It should be noted that there was a significant (p<0.02) between-site difference in Hamilton depression scale scores; the eight New York State Psychiatric Institute patients had a mean score that was almost 8 points higher than that of the 11 Western Psychiatric Institute and Clinic patients. This difference statistically equaled the difference in the experimental variable, CSF transthyretin level, between depressed patients and comparison subjects (p<0.02). Certainly, this laboratory measure obtained in a single laboratory was less liable to between-site sampling error than the on-site scoring of the Hamilton depression scale, but there is also the possibility that the depressed patients were different at the two sites. Indeed, there was also a significant difference in age between the patients at the two sites. Yet despite these differences, the CSF transthyretin levels were equally low at the two sites, supporting the notion that there was institutional bias in the scoring of the Hamilton depression scale. For these reasons, we believe that pooling of the patient groups was justified, and our analysis included age as a covariate. There was a strong trend for the CSF transthyretin levels to be different between the two comparison subject sites, perhaps arguing against pooling of these groups. However, the small number of comparison subjects (N=5) from the New York State Psychiatric Institute site in fact reduced the significance of the overall finding of higher levels of CSF transthyretin in the comparison subjects; therefore, omitting these comparison subjects from the final data analysis was considered less justifiable.
The possibility exists that diet and weight changes due to depression could result in unmeasured confounding metabolic factors responsible for the difference in CSF transthyretin levels between groups, although this would not necessarily diminish the potential importance of the finding. Another concern is whether the 2-week medication-free period before lumbar puncture was adequate for psychotropics with long active metabolite half-lives. In addition, it should be considered that psychotropic medications might have effects on transthyretin level that remain longer than 2 weeks.
It is notable that the standard deviation for CSF transthyretin levels was much smaller for the depressed patients in this study than for the comparison subjects. While the comparison subjects had a wide range of CSF transthyretin values, the highest value in the depressed group was lower than the mean for the comparison group. This raises the question of whether people without depression have a capacity to enhance or up-regulate CSF transthyretin levels, in contrast to a “ceiling” for such a capacity in people with a depressive disorder. The additional linear inverse correlation between CSF transthyretin level and Hamilton depression score that was identified in the male depressed patients is intriguing and deserves follow-up, although this finding is quite tentative given the small pooled sample (N=8).
Future clinical studies should include correlation of CSF transthyretin levels with CSF TRH, CSF free and total T3 and T4, results of a TRH stimulation test, and possibly also measures of nocturnal TSH secretion. In addition, measurement of CSF transthyretin in individuals during depression and after recovery from depression might discern whether a low transthyretin level is a state or a trait. Finally, clinical study of thyroid hormone augmentation of standard antidepressant treatment should be carried out with pretreatment and posttreatment measurements of central and peripheral thyroid parameters, including CSF transthyretin levels.
Presented in part at the 27th annual meeting of the Society for Neuroscience, New Orleans, Oct. 25–30, 1997. Received Jan. 22, 1998; revision received Nov. 5, 1998; accepted Nov. 16, 1998. From the Departments of Psychiatry and Neurology, College of Physicians and Surgeons of Columbia University; the Departments of Clinical Psychobiology, Clinical Psychopharmacology, and Neuroscience, New York State Psychiatric Institute, New York; and the Department of Neurology, Hospital for Joint Diseases, New York University, New York. Address reprint requests to Dr. Gorman, Department of Psychiatry, College of Physicians and Surgeons of Columbia University, 722 West 168th St., New York, NY 10032. Supported by grant MH-15144 to Dr. Sullivan, grant MH-49718 to Dr. Hatterer, Senior Scientist Award MH-00416 to Dr. Gorman, and grant MH-46745 to Dr. Mann from NIMH and a Young Investigator Ward to Dr. Marangell from the National Alliance for Research on Schizophrenia and Depression. The authors thank Mark George and Mark Frye for collecting and/or maintaining CSF samples from the normal comparison subjects at NIMH.
 |
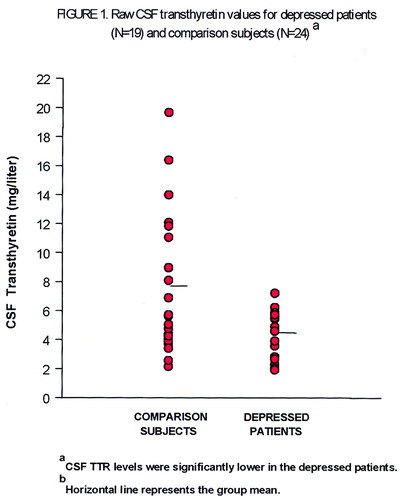
FIGURE 1. Raw CSF Transthyretin Values for Depressed Patients (N=19) and Comparison Subjects (N=24)a
aHorizontal lines represent the group means. CSF transthyretin levels were significantly lower in the depressed patients (F=5.98, df=1, 38, p<0.02).
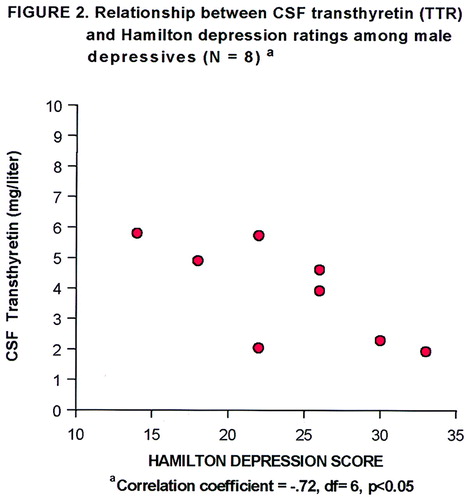
FIGURE 2. Relationship Between CSF Transthyretin Levels and Hamilton Depression Ratings Among Male Depressed Patients (N=8)a
aCorrelation coefficient=–0.72, df=6, p<0.05.
1. Sachar EJ: Psychiatric disturbances associated with endocrine disorders, in American Handbook of Psychiatry, 2nd ed, vol 4. Edited by Reiser MF; Arieti S, editor-in-chief. New York, Basic Books, 1975, pp 299–313Google Scholar
2. Orsulak PJ, Crowley G, Schlesser MA, Giles D, Fairchild C, Rush AJ: Free triiodothyronine (T3) and thyroxine (T4) in a group of unipolar depressed patients and normal subjects. Biol Psychiatry 1985; 20:1047–1054Google Scholar
3. Fava M, Labbate LA, Abraham ME, Rosenbaum JF: Hypothyroidism and hyperthyroidism in major depression revisited. J Clin Psychiatry 1995; 56:186–192Medline, Google Scholar
4. Loosen PT, Prange AJ Jr: Serum thyrotropin response to thyrotropin-releasing hormone in psychiatric patients: a review. Am J Psychiatry 1982; 139:405–416Link, Google Scholar
5. Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Weissenburger JE, Fulton CL, Fairchild CJ, Roffwarg HP: Dexamethasone response, thyrotropin-releasing hormone stimulation, rapid eye movement latency, and subtypes of depression. Biol Psychiatry 1997; 41:915–928Crossref, Medline, Google Scholar
6. Kirkegaard C: The thyrotropin response to thyrotropin-releasing hormone in endogenous depression. Psychoneuroendocrinology 1981; 6:189–212Crossref, Medline, Google Scholar
7. Rush AJ, Schlesser MA, Roffwarg HP, Giles DE, Orsulak PJ, Fairchild CJ: Relationships among the TRH, REM latency, and dexamethasone suppression tests: preliminary findings. J Clin Psychiatry 1983; 44:23–29Medline, Google Scholar
8. Winokur A, Amsterdam JK, Osler J, Mendels J, Snyder PJ, Caroff SN, Brunswick DJ: Multiple hormonal responses to protirelin (TRH) in depressed patients. Arch Gen Psychiatry 1983; 40:525–531Crossref, Medline, Google Scholar
9. Banki CM, Bissette G, Arato M, Nemeroff CB: Elevation of immunoreactive CSF TRH in depressed patients. Am J Psychiatry 1988; 145:1526–1531Google Scholar
10. Targum SD, Greenberg RD, Harmon RL, Kessler K, Salerian AJ, Fram DH: Thyroid hormone and the TRH stimulation test in refractory depression. J Clin Psychiatry 1984; 45:345–346Medline, Google Scholar
11. Gitlin MJ, Weiner H, Fairbanks L, Hershman JM, Friedfeld N: Failure of T3 to potentiate tricyclic antidepressant response. J Affect Disord 1987; 13:267–272Crossref, Medline, Google Scholar
12. Bartalena L, Placidi GF, Martino E, Falcone M, Pellegrini L, Dell-Osso L, Pacchiarotti A, Pinchera A: Nocturnal serum thyrotropin (TSH) surge and the TSH response to TSH-releasing hormone: dissociated behavior in untreated depressives. J Clin Endocrinol Metab 1990; 71:650–655Crossref, Medline, Google Scholar
13. Duval F, Mokrani MC, Croq MA, Jautz M, Bailey P, Diep TS, Macher JP: Effect of antidepressant medication on morning and evening thyroid function tests during a major depressive episode. Arch Gen Psychiatry 1996; 53:833–840Crossref, Medline, Google Scholar
14. Joffe RT, Levitt AJ: Major depression and subclinical (grade 2) hypothyroidism. Psychoneuroendocrinology 1992; 17:215–221Crossref, Medline, Google Scholar
15. Ninan PT, Musselman DL, Knight BT, Kane CP, Bonsal RW, Nemeroff CB: Hypothalamic-pituitary-thyroid axis and antidepressant response in major depression, in Scientific Abstracts of the 35th Annual Meeting of the American College of Neuropsychopharmacology. Nashville, Tenn, ACN, 1996, p 235Google Scholar
16. Howland RH: Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993; 54:47–54Medline, Google Scholar
17. Aronson R, Offman HJ, Joffe RT, Naylor CD: Triiodothyronine augmentation in the treatment of refractory depression: a meta-analysis. Arch Gen Psychiatry 1996; 53:842–848Crossref, Medline, Google Scholar
18. Whybrow PC: The therapeutic use of triiodothyronine and high-dose thyroxine in psychiatric disorder. Acta Med Austriaca 1994; 21:47–52Medline, Google Scholar
19. Baumgartner A, Bauer M, Hellweg R: Treatment of intractable nonrapid cycling bipolar affective disorder with high-dose thyroxine: an open clinical trial. Neuropsychopharmacology 1994; 10:183–189Crossref, Medline, Google Scholar
20. Bauer MS, Whybrow PC, Winokur A: Rapid cycling bipolar affective disorder, I: association with grade I hypothyroidism. Arch Gen Psychiatry 1990; 47:427–432Crossref, Medline, Google Scholar
21. Herbert J, Wilcox JN, Pham KC, Fremeau RT, Zeviani M, Dwork A, Soprano DR, Makover A, Goodman DS, Zimmerman EA, Roberts JL, Schon EA: Transthyretin: a choroid plexus-specific transport protein in human brain. Neurology 1986; 36:900–911Crossref, Medline, Google Scholar
22. Hatterer JA, Herbert J, Hidaka C, Roose SP, Gorman JM: CSF transthyretin in patients with depression. Am J Psychiatry 1993; 150:813–815Link, Google Scholar
23. Malone KM, Haas GL, Sweeney JA, Mann JJ: Major depression and the risk of attempted suicide. J Affect Disord 1995; 34:173–185Crossref, Medline, Google Scholar
24. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
25. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
26. Schechter D, Strasser TJ, Santangelo C, Kim E, Endicott J: “Normal” control subjects are hard to find: a model for centralized recruitment. Psychiatry Res 1994; 53:301–311Crossref, Medline, Google Scholar
27. Endicott J, Spitzer RL: A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
28. Dickson PW, Aldred AR, Menting JGT, Marley PD, Sawyer WH, Schreiber G: Thyroxine transport in choroid plexus. J Biol Chem 1987; 262:13907–13915Google Scholar
29. Schreiber G, Aldred AR, Jaworowski A, Nilsson C, Achen MG, Segal MB: Thyroxine transport from blood to brain via transthyretin synthesis in choroid plexus. Am J Physiol 1990; 258:R338–R345Google Scholar
30. Chanoine JP, Alex S, Fang SL, Stone S, Leonard JL, Korhle J, Braverman LE: Role of transthyretin in the transport of thyroxine from the blood to the choroid plexus, the cerebrospinal fluid, and the brain. Endocrinology 1992; 130:933–938Medline, Google Scholar
31. Blay P, Nilsson C, Owman C, Aldred A, Schreiber G: Transthyretin expression in the rat brain: effect of thyroid functional state and role in thyroxine transport. Brain Res 1993; 632:114–120Crossref, Medline, Google Scholar
32. Southwell BR, Duan W, Alcorn D, Brack C, Richardson SJ, Kohrle J, Schreiber G: Thyroxine transport to the brain: role of protein synthesis by the choroid plexus. Endocrinology 1993; 133:2116–2126Google Scholar
33. Schreiber G, Southwell BR, Richardson SJ: Hormone delivery systems to the brain—transthyretin. Exp Clin Endocrinol Diabetes 1995; 103:75–80Crossref, Medline, Google Scholar
34. Baumgartner A, Hiedra L, Pinna G, Eravci M, Prengel H, Meinhold H: Rat brain type II 5"-iodothyronine deiodinase activity is extremely sensitive to stress. J Neurochem 1998; 71:817–826Crossref, Medline, Google Scholar
35. Sandrini M, Marrama D, Vergoni AV, Bertolini A: Effects of thyroid status on the characteristics of alpha 1-, alpha 2-, beta, imipramine and GABA receptors in the rat brain. Life Sci 1991; 48:659–666Crossref, Medline, Google Scholar
36. Heal DJ, Smith SL: The effects of acute and repeated administration of T3 to mice on 5-HT-1 and 5-HT-2 function in the brain and its influence on the actions of repeated electroconvulsive shock. Neuropharmacology 1988; 27:1239–1248Google Scholar
37. Tejani-Butt SM, Yang J, Kaviani A: Time course of altered thyroid states on 5-HT-1a receptors and 5-HT uptake sites in rat brain: an autoradiographic analysis. Neuroendocrinology 1993; 57:1011–1018Google Scholar
38. Mason GA, Bondy SC, Nemeroff CB, Walker CH, Prange AJ Jr: The effects of thyroid state on beta-adrenergic and serotonergic receptors in rat brain. Psychoneuroendocrinology 1987; 12:261–270Crossref, Medline, Google Scholar



