Clinical and Neurobiological Correlates of Cytogenetic Abnormalities in Childhood-Onset Schizophrenia
Abstract
OBJECTIVE: Cytogenetic abnormalities are increased in schizophrenia, suggesting a possible etiologic contribution. However, their clinical and pathophysiologic roles in the disorder are unknown. To investigate this, a group of children and adolescents participating in a comprehensive study of childhood-onset schizophrenia were screened for chromosomal abnormalities, and their clinical and neurobiological correlates were examined. METHOD: Cytogenetic screening with the use of high-resolution banding, fluorescent in situ hybridization for chromosome 22q11 deletions, and molecular fragile X testing was undertaken in a group of 47 children and adolescents with very early onset of schizophrenia. Clinical, neurobiological (including brain morphometry), and risk factor measures of the subjects with cytogenetic abnormalities were compared with those of the remaining patients without cytogenetic anomalies. RESULTS: Five patients had previously undiagnosed cytogenetic abnormalities. Lower performance IQ and more pronounced premorbid developmental impairments were seen in this subgroup. Rates of obstetric complications, familial schizophrenia spectrum disorders, and familial eye tracking dysfunction were similar for the patients with and without cytogenetic abnormalities. CONCLUSIONS: Cytogenetic abnormalities appear to be increased in childhood-onset schizophrenia, suggesting an association with a very early age at onset. The data from the subgroup of patients with cytogenetic anomalies are consistent with a model in which a childhood onset of schizophrenia is due to a greater impairment of neurodevelopment secondary to the interaction of a number of factors, particularly genetic ones.
Family, twin, and adoption studies have all demonstrated that genetic factors play a prominent role in the etiology of schizophrenia (1). However, the nature of this contribution remains unclear, perhaps because of etiologic heterogeneity (2, 3).
Contributing evidence for heterogeneity includes reports of a variety of cytogenetic abnormalities in adults with schizophrenia (2–5). Anomalies of the sex chromosomes (4) and the autosomes (3, 5) have been noted in patients with schizophrenia. The rate of velocardiofacial syndrome (deletion of chromosome 22q11) has been reported to be higher in schizophrenia (2%) (6) than in the general population (0.02%) (7). It is interesting that velocardiofacial syndrome has also been reported in patients with bipolar disorder (8). Despite this apparent excess, the role of these chromosomal abnormalities remains unclear.
Because patients with a very early onset may represent a more homogeneous subgroup of illness with more salient genetic effects (9), an ongoing study at the National Institute of Mental Health (NIMH) of childhood-onset schizophrenia (onset of psychosis by age 12) has examined a variety of risk factors in this group. Such patients are rare, but their clinical presentation and neurobiological measures are continuous with those of patients with an adult onset of schizophrenia (10). To date, 47 patients with childhood-onset schizophrenia have participated, all of whom were screened for cytogenetic abnormalities with the use of high-resolution banding, fluorescent in situ hybridization for deletions on chromosome 22q11, and molecular fragile X testing. No patient was referred with a known cytogenetic anomaly. Since these patients were participating in a systematic clinical and neurobiological study of very early-onset schizophrenia (11), there was a unique opportunity to examine the profiles of this group. This report describes the clinical, cognitive, brain morphometric, eye movement, and risk factor profiles of patients with childhood-onset schizophrenia with and without cytogenetic abnormalities. As it has been proposed that schizophrenia is etiologically heterogeneous (2), it was hypothesized that while patients with cytogenetic abnormalities would appear clinically similar to the larger patient group, they would have more deviant measures of brain function and lower rates of other risk factors, reflecting a greater impact of cytogenetic anomalies on early neurodevelopment and a lower salience of other genetic indicators of risk in these patients.
METHOD
Since 1991, through review of over 1,000 charts and in-person screening of over 200 subjects, 51 patients seen at NIMH have been diagnosed with schizophrenia according to the DSM-III-R criteria, with onset of psychosis by age 12. The diagnosis of childhood-onset schizophrenia was made with good reliability (kappa=0.77) (12) by two child psychiatrists using clinical and structured interviews, including the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (13). To date, 47 patients (28 male and 19 female), all referred without prior evidence of cytogenetic abnormalities, have participated in a comprehensive study of childhood-onset schizophrenia (11). At the time of initial assessment, the mean age of the patients was 14.3 years (SD=2.3), and they had developed psychotic symptoms at a mean age of 10.3 years (SD=1.8).
This study was approved by the NIMH institutional review board. Parents or legal guardians of all participants provided written consent, and all patients gave written assent for their participation.
All patients underwent genetic testing with high-resolution analysis of at least 20 Giemsa-trypsin banded metaphases from peripheral blood samples, fluorescent in situ hybridization (with the use of the cosmid probe D22S75) for the deletion on chromosome 22q11.2 associated with velocardiofacial syndrome (14), and molecular fragile X testing.
All subjects underwent extensive clinical assessment with the Brief Psychiatric Rating Scale (BPRS) (15), the Scale for the Assessment of Positive Symptoms (SAPS) (16), and the Scale for the Assessment of Negative Symptoms (SANS) (17). A psychological test battery, including the Wechsler Intelligence Scale for Children (WISC-III) (18), was administered at the time of first admission to the study. Five patients, all without cytogenetic abnormalities, were unable to complete the WISC-III because of their poor clinical condition.
Eye tracking and brain morphometry were determined for all patients in order to examine the neurobiological correlates of cytogenetic abnormalities. Thirty-six probands performed a smooth pursuit eye movement task (19); the remainder, none of whom had cytogenetic abnormalities, were unable to complete the task because of difficulties with compliance. Each record was scored qualitatively by one of us (R.N.), blind to patient identity, using a scale of 1 (best) to 5 (worst) (20). It was decided before the records were scored that subjects with a score of 2.5 and greater would be classified as abnormal trackers, while those with a score less than 2.5 would be classified as normal trackers. Two of us (R.N., G.K.T.) rated a subset of this group (N=10) and demonstrated high reliability (intraclass correlation coefficient=0.98).
All subjects (apart from three with dental braces) had a brain magnetic resonance imaging (MRI) scan on a 1.5-T Signa scanner. The three structures most consistently found to be abnormal (in comparison with healthy control subjects) in MRI studies of schizophrenia—total cerebral volume, lateral ventricular volume, and hippocampal volume (21)—were compared in patients with and without cytogenetic anomalies with the use of measures described previously (22).
In the risk factor assessment, original birth records of 34 probands (the remainder were unavailable) were assessed blind to patient identity by two of us (J.N.G., D.M.) to determine the presence of obstetric complications as defined by Lewis and colleagues (23). Reliability was high (kappa=0.87), and any discrepancies were resolved by consensus of the raters.
With the use of a method suggested by Hollis (24), original case notes, including pediatric, psychiatric, psychological, and educational reports, with supplementation by parental recall when necessary, were examined blind to cytogenetic status to determine the presence of premorbid impairments of speech and language, motor functioning, and social interactions.
To assess the presence of schizophrenia spectrum disorders (schizophrenia, schizoaffective disorder, schizotypal personality disorder, and paranoid personality disorder) in the patients’ families, all available first-degree relatives 18 years of age and over (N=105) were interviewed with the use of the Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (25) and the Structured Interview for DSM-IV Personality (26). Siblings under the age of 18 years (N=20) were assessed with the Diagnostic Interview for Children and Adolescents (27). All interviews were done blind to cytogenetic status by a clinical social worker trained in the administration of such interviews (M.L.) who has demonstrated good reliability with a psychiatrist (R.N.) in the use of these interviews (kappa=0.83 for schizophrenia spectrum disorders). In addition, all available relatives over the age of 13 years (N=78) completed a smooth pursuit eye movement task (19), which was scored qualitatively as described earlier.
In the statistical analysis, the patients with and without cytogenetic anomalies were compared by means of Fisher’s exact chi-square test and Student’s t test. A significance level of 0.05 (two-tailed) was used.
RESULTS
Of the 47 patients tested, five (two male and three female) (10.6%; 95% confidence interval=1.8–19.4) were found to have cytogenetic abnormalities. These included three with velocardiofacial syndrome (14), one with Turner’s syndrome (deletion of part of the long arm of one X chromosome—46,X,i[Xq24]) (28), and one with a balanced translocation of chromosomes 1 and 7 (t[1;7]) (29). None of the patients showed evidence of the fragile X mutation.
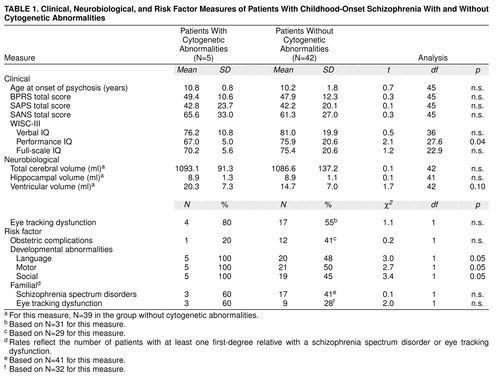
The patients with cytogenetic abnormalities had lower performance IQs but not verbal or full-scale IQs than those without such anomalies (Table 1). There were no significant differences, however, in age at onset or symptom profile (including total psychopathology level and positive and negative symptoms) between the subjects with and without cytogenetic abnormalities.
Rates of smooth pursuit eye movement abnormalities are also shown in Table 1. There were no significant differences between the two groups in rates of eye tracking dysfunction.
Brain MRI data suggested that patients with cytogenetic abnormalities may have larger lateral ventricles than those without (Table 1). Neither total cerebral volume nor hippocampal volume differed significantly between the groups. It is of interest that all three patients with velocardiofacial syndrome had large midsagittal corpus callosum areas.
Developmental histories showed significantly more impairments in the patients with cytogenetic anomalies than in the patients without such abnormalities (Table 1). All five had premorbid language, motor, and social development abnormalities, as compared with approximately 50% of the larger patient group who had impairments in each of these areas.
Of the 125 relatives assessed by diagnostic interview, 26 were found to have a schizophrenia spectrum disorder (30). Using a hierarchical method to assign diagnoses (31), we found that three had schizophrenia or schizoaffective disorder, 13 had schizotypal personality disorder, and 10 had paranoid personality disorder. Fourteen of the 78 relatives who completed the smooth pursuit eye movement task had abnormal tracking (30), while 13 of the 34 patients for whom birth records were available had a history of obstetric complications (30). As shown in Table 1, the patients with cytogenetic abnormalities did not differ from those without them on any of these measures of risk for schizophrenia. The number of relatives with schizophrenia spectrum disorders (three of 14 versus 23 of 112; χ2=0.01, df=1, n.s.) and smooth pursuit eye tracking dysfunction (three of 13 versus 11 of 65; χ2=0.3, df=1, n.s.) did not differ between the two groups, and nor did the rates of obstetric complications (one of five versus 12 of 49; χ2=0.2, df=1, n.s.).
DISCUSSION
In a group of 47 patients with childhood-onset schizophrenia, five had previously undiagnosed cytogenetic abnormalities. These patients had lower performance IQs, more profound impairments of premorbid development, and some evidence suggesting that they may have larger lateral ventricles than those without these anomalies. They did not otherwise differ clinically or in their rates of risk factors for schizophrenia, including familial schizophrenia spectrum disorders, familial eye tracking dysfunction, and obstetric complications.
The rate of cytogenetic abnormalities in the patients with an onset of schizophrenia in childhood (10.6%) is clearly higher than that in the general population (0.85%) (32) and may also be higher than that in patients with an onset in adulthood (4–6), suggesting an association with earlier age at onset. This association is supported by the earlier age at onset in a group of adults with velocardiofacial syndrome and schizophrenia (33).
Aberrant neurodevelopment is a common sequela of cytogenetic abnormalities (34–37) and is also strongly implicated in the etiology of schizophrenia (38, 39). The poorer premorbid functioning of the patients in this group with cytogenetic abnormalities, their lower performance IQ, and the possibility that they may have larger lateral ventricles suggest that their early age at onset probably reflects a greater disruption of early neurodevelopment.
The role of the cytogenetic abnormalities in these patients is unclear. It is possible that abnormal gene “dosage” secondary to these disruptions directly results in the later development of schizophrenia. However, most patients with balanced translocations, Turner’s syndrome, and velocardiofacial syndrome do not develop schizophrenia, and since most genetic models implicate several major genes in the etiology of the disorder (40), other factors are probably involved as well. The similar rates of familial schizophrenia spectrum disorders and eye tracking dysfunction for the patients with and without cytogenetic anomalies further suggest that chromosomal disruptions are not likely to be a sufficient cause of schizophrenia. Rather, these data are compatible with a model in which a variety of defects in the control of brain development (including cytogenetic abnormalities) result in a series of structural changes that, in combination with more specific risk factors (indexed by familial schizophrenia spectrum disorders and eye tracking dysfunction), lead to the later development of schizophrenia (41).
These findings are preliminary because of the small size of the study group, necessitated by the rarity of childhood-onset schizophrenia, the prevalence of which is reported to be less than one-fiftieth of the prevalence of adult-onset schizophrenia (42), although our data suggest that it is even less common than this (12). The failure of several patients to complete different parts of the assessment battery may have further decreased our ability to find subtle differences between the groups. Although the patients with cytogenetic abnormalities did not differ from the other patients on the parameters assessed, ongoing studies of a larger group of these patients will address these problems. The inclusion criterion of a premorbid IQ over 70 may have reduced the number of patients with cytogenetic abnormalities, who are more likely to have mental retardation (43). In addition, the grouping together of different cytogenetic anomalies assumes a functional equivalence of these abnormalities, which is unlikely. The number of statistical comparisons made may also have adversely affected the results.
In conclusion, the clinical and neurobiological data from the subgroup of patients with cytogenetic anomalies and schizophrenia are consistent with the hypothesis that an earlier age at onset of schizophrenia may be due to a greater disruption of normal brain development secondary to the interaction of a number of factors, particularly genetic ones (30).
Presented in part at the 28th meeting of the Society of Biological Psychiatry, Toronto, May 27–31, 1998. Received Oct. 30, 1998; revisions received Feb. 3 and March 9, 1999; accepted March 17, 1999. From the Child Psychiatry Branch, NIMH; Yale University, New Haven, Conn.; the University of Maryland, Baltimore; and Columbia University, New York. Address reprint requests to Dr. Nicolson, Bldg. 10, Rm. 3N202, 10 Center Dr. MSC 1600, Bethesda, MD 20892-1600; [email protected] (e-mail)
 |
1. Kendler KS, Diehl SR: The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull 1993; 19:261–285Crossref, Medline, Google Scholar
2. Tsuang MT, Faraone SV: The case for heterogeneity in the etiology of schizophrenia. Schizophr Res 1995; 17:161–175Crossref, Medline, Google Scholar
3. Karayiorgou M, Gogos JA: A turning point in schizophrenia genetics. Neuron 1997; 19:967–979Crossref, Medline, Google Scholar
4. DeLisi LE, Friedrich U, Wahlstrom J, Boccio-Smith A, Forsman A, Eklund K, Crow TJ: Schizophrenia and sex chromosome anomalies. Schizophr Bull 1994; 20:495–505Crossref, Medline, Google Scholar
5. Bassett AS: Chromosomal aberrations and schizophrenia: autosomes. Br J Psychiatry 1992; 161:323–334Crossref, Medline, Google Scholar
6. Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Eisen H, Childs B, Kazazian HH, Kucherlapati R, Antonarakis SE, Pulver AE, Housman DE: Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA 1995; 92:7612–7616Google Scholar
7. Burn J, Goodship J: Congenital heart disease, in Emery and Rimoin’s Principles and Practice of Medical Genetics, 3rd ed. Edited by Rimoin DL, Connor JM, Pyeritz RE. New York, Churchill Livingstone, 1996, pp 767–828Google Scholar
8. Papolos DF, Faedda GL, Veit S, Goldberg R, Morrow B, Kucherlapati R, Shprintzen RJ: Bipolar spectrum disorders in patients diagnosed with velo-cardio-facial syndrome: does a hemizygous deletion of chromosome 22q11 result in bipolar affective disorder? Am J Psychiatry 1996; 153:1541–1547Google Scholar
9. Childs B, Scriver CR: Age at onset and causes of disease. Perspect Biol Med 1986; 29:437–460Crossref, Medline, Google Scholar
10. Jacobsen LK, Rapoport JL: Research update: childhood-onset schizophrenia: implications of clinical and neurobiological research. J Child Psychol Psychiatry 1998; 39:101–113Crossref, Medline, Google Scholar
11. Gordon CT, Frazier JA, McKenna K, Giedd J, Zametkin A, Zahn T, Hommer D, Hong W, Kaysen D, Albus KE, Rapoport JL: Childhood-onset schizophrenia: an NIMH study in progress. Schizophr Bull 1994; 20:697–712Crossref, Medline, Google Scholar
12. McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL: Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry 1994; 33:636–644Crossref, Medline, Google Scholar
13. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
14. Yan W, Jacobsen LK, Krasnewich DM, Guan XY, Lenane MC, Paul SP, Dalwadi HN, Zhang H, Long RT, Kumra S, Martin BM, Scambler PJ, Trent JM, Sidransky E, Ginns EI, Rapoport JL: Chromosome 22q11.2 interstitial deletions among childhood-onset schizophrenics and “multidimensionally impaired.” Am J Med Genet 1998; 81:41–43Crossref, Medline, Google Scholar
15. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 157–169Google Scholar
16. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
17. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
18. Wechsler D: The Wechsler Intelligence Scale for Children, 3rd ed. Cleveland, Psychological Corp, 1991Google Scholar
19. Jacobsen LK, Hong WL, Hommer DW, Hamburger SD, Castellanos FX, Frazier JA, Giedd JN, Gordon CT, Karp BI, McKenna K, Rapoport JL: Smooth pursuit eye movements in childhood-onset schizophrenia: comparison with attention-deficit hyperactivity disorder and normal controls. Biol Psychiatry 1996; 40:1144–1154Google Scholar
20. Thaker G, Kirkpatrick B, Buchanan RW, Ellsberry R, Lahti A, Tamminga C: Oculomotor abnormalities and their clinical correlates in schizophrenia. Psychopharmacol Bull 1989; 25:491–497Medline, Google Scholar
21. Lawrie SM, Abukmeil SS: Brain abnormality in schizophrenia: a systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 1998; 172:110–120Crossref, Medline, Google Scholar
22. Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL: Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry 1996; 53:617–624Crossref, Medline, Google Scholar
23. Lewis SW, Owen MJ, Murray RM: Obstetric complications and schizophrenia: methodology and mechanisms, in Schizophrenia: Scientific Progress. Edited by Schulz SC, Tamminga CA. New York, Oxford University Press, 1989, pp 56–68Google Scholar
24. Hollis C: Child and adolescent (juvenile onset) schizophrenia: a case control study of premorbid developmental impairments. Br J Psychiatry 1995; 166:489–495Crossref, Medline, Google Scholar
25. Fyer AJ, Mannuzza SM, Klein DF, Endicott J: Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA). New York, New York State Psychiatric Institute, 1985Google Scholar
26. Pfohl B, Blum N, Zimmerman M: Structured Interview for DSM-IV Personality: SIDP-IV. Iowa City, University of Iowa, Department of Psychiatry, 1995Google Scholar
27. Reich W, Leacock N, Shanfeld K: Diagnostic Interview for Children and Adolescents (DICA-R). St Louis, Washington University, 1995Google Scholar
28. Kumra S, Wiggs E, Krasnewich D, Meck J, Smith AC, Bedwell J, Fernandez T, Jacobsen LK, Lenane M, Rapoport JL: Brief report: association of sex chromosome anomalies with childhood-onset psychotic disorders. J Am Acad Child Adolesc Psychiatry 1998; 37:292–296Crossref, Medline, Google Scholar
29. Gordon CT, Krasnewich D, White B, Lenane M, Rapoport JL: Brief report: translocation involving chromosomes 1 and 7 in a boy with childhood-onset schizophrenia. J Autism Dev Disord 1994; 24:537–545Crossref, Medline, Google Scholar
30. Nicolson R, Rapoport JL: Childhood-onset schizophrenia: what can it teach us? in Childhood Onset of “Adult” Psychopathology: Clinical and Research Advances. Edited by Rapoport JL. Washington, DC, American Psychiatric Press (in press)Google Scholar
31. Kendler KS: The impact of diagnostic hierarchies on prevalence estimates for psychiatric disorders. Compr Psychiatry 1988; 29:218–227Crossref, Medline, Google Scholar
32. Nielsen J, Wohlert M: Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet 1991; 87:81–83Crossref, Medline, Google Scholar
33. Bassett AS, Chow E, Scutt L, Hodgkinson K, Weksberg R: Psychiatric phenotype of a genetic subtype of schizophrenia (abstract). Schizophr Res 1999; 36:87Crossref, Google Scholar
34. Weis S, Weber G, Neuhold A, Rett A: Down syndrome: MR quantification of brain structures and comparison with normal control subjects. AJNR Am J Neuroradiol 1991; 12:1207–1211Google Scholar
35. Murphy DG, DeCarli C, Daly E, Haxby JV, Allen G, White BJ, McIntosh AR, Powell CM, Horwitz B, Rapoport SI: X-chromosome effects on female brain: a magnetic resonance imaging study of Turner’s syndrome. Lancet 1993; 342:1197–1200Google Scholar
36. Jernigan TL, Bellugi U, Sowell E, Doherty S, Hesselink JR: Cerebral morphologic distinctions between Williams and Down syndromes. Arch Neurol 1993; 50:186–191Crossref, Medline, Google Scholar
37. Bingham PM, Zimmerman RA, McDonald-McGinn D, Driscoll D, Emanuel BS, Zackai E: Enlarged sylvian fissures in infants with interstitial deletion of chromosome 22q11. Am J Med Genet 1997; 74:538–543Crossref, Medline, Google Scholar
38. Murray RM, Lewis SW: Is schizophrenia a neurodevelopmental disorder? BMJ 1987; 295:681–682Google Scholar
39. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
40. Risch N: Linkage strategies for genetically complex traits, I: multilocus models. Am J Hum Genet 1990; 46:222–228Medline, Google Scholar
41. Jones P, Murray RM: The genetics of schizophrenia is the genetics of neurodevelopment. Br J Psychiatry 1991; 158:615–623Crossref, Medline, Google Scholar
42. Beitchman JH: Childhood schizophrenia: a review and comparison with adult-onset schizophrenia. Psychiatr Clin North Am 1985; 8:793–814Crossref, Medline, Google Scholar
43. Gustavson KH, Holmgren G, Blomquist HK: Chromosomal aberrations in mildly mentally retarded children in a northern Swedish county. Ups J Med Sci Suppl 1987; 44:165–168Medline, Google Scholar



