Dynamics of ECT Normalization of Low G Protein Function and Immunoreactivity in Mononuclear Leukocytes of Patients With Major Depression
Abstract
OBJECTIVE: Heterotrimeric G proteins were previously implicated in the biochemical mechanisms underlying the pathophysiology and treatment of mood disorders. Low function and immunoreactivity of G proteins were observed in patients with major depression. In the present study the authors evaluated the effects of ECT on the low measures of G proteins in patients with major depression. METHOD: Repeated G protein measurements in mononuclear leukocytes of 10 patients with major depression were made. Each patient was examined while untreated and after successive sessions of ECT; 14 normal subjects were also studied. G protein function was evaluated through β-adrenergic- and muscarinic-agonist-enhanced guanine nucleotide binding capacity, substantiated by quantitative measures of G proteins through immunoblot analyses using polyclonal antibodies against Gsα, Giα, and Gβ proteins. RESULTS: Mononuclear leukocytes of patients with depression showed immunoreactive levels of Gsα and Giα that were significantly lower than those of normal subjects; the depressed patients also had markedly hypofunctional Gs and Gi. The low levels of G protein function and immunoreactivity were alleviated by ECT. Repeated measurements in the same patients after successive ECT sessions showed that the normalization of G protein measures preceded, and thus predicted, clinical improvement. The function and quantity of Gs and Gi proteins in patients given ECT were significantly correlated. CONCLUSIONS: These findings support the implication of G proteins in the pathophysiology and treatment of mood disorders. G protein measurements in patients with depression may potentially serve not only as a biochemical marker for affective state but also for biochemical prediction and evaluation of responses to ECT.
Arole for monoaminergic and muscarinic cholinergic mechanisms in the pathogenesis of mood disorders has been largely suggested by and formulated in the catecholamine hypothesis and in the monoaminergic-cholinergic balance hypothesis of affective disorders (1, 2). Biochemical research in affective disorders has focused on the cascade of events involved in signal transduction: from the level of the primary messenger (the catecholamine or acetylcholine neurotransmitter) to the level of the neurotransmitter receptors and, lately, to information transduction mechanisms beyond receptors, involving guanine-nucleotide-binding G proteins. The family of heterotrimeric G proteins are a crucial point of convergence in the transmission of signals from a variety of hormone and neurotransmitter membrane receptors to a series of downstream cellular events: intracellular second messenger effector enzymes and ionic channels (3, 4). Evidence implicates the involvement of G proteins in the pathophysiology of mood disorders and in the biochemical mechanisms underlying the treatment of these disorders. Hyperfunctional Gs and Gi proteins were detected in mononuclear leukocytes of patients with mania (5, 6), while hypofunctional Gs and Gi proteins were found in mononuclear leukocytes of patients with major depressive disorder (6–9). High immunoreactivity levels and function of Gsα were found in postmortem cerebral cortices of bipolar patients (10–12).
We previously found the function of receptor-coupled Gs and Gi proteins to be altered by lithium (13–19) and other treatments for bipolar disorder (17–19). The results of studies by other groups, using a variety of techniques, have generally agreed with these results and implicate involvement of G proteins in lithium's mechanism of action (20–26). The therapeutic spectrum of ECT, including both depression and mania, is similar to that of lithium and other drugs for bipolar illness. One way of understanding the diversity of ECT's effects on the function of a variety of neurotransmitters and receptors (for review see references 27–29) is to hypothesize that, like lithium, ECT stabilizes disregulated intracellular signaling linked to multiple transmitter systems (27, 29). Indeed, ECT was previously shown to alter various aspects of G protein functioning in animal studies (17–19, 30–32). Searching for the biochemical mechanisms of action of ECT and other treatments used against mood and other mental disorders through animal studies has intrinsic limitations. Usually, mentally disordered patients have disordered neurochemistry that is correlated with their symptomatic state. Thus, the effects of biochemical treatment on normal animals cannot be simply extrapolated to possible biochemical treatment effects in symptomatic patients with known abnormal underlying biochemistry.
Therefore, in the present study we sought to evaluate the effects of repeated electroconvulsive treatments on the low quantitative and functional measures of Gs and Gi proteins in mononuclear leukocytes of patients with major depression, addressing the following questions: 1) Do the dynamics of normalization of Gs and Gi protein function during ECT parallel the dynamics of normalization of Gsα and Giα immunoreactivity? and 2) Does normalization of the biochemical measures of Gs and Gi proteins follow, and thus reflect, clinical improvement or precede, and thus predict, clinical improvement?
METHOD
Patients
All patients were diagnosed according to DSM-IV criteria by at least two senior psychiatrists (G.S., G.R.). The inclusion criteria were normal results of physical examination, ECG, and laboratory tests for renal, hepatic, hematologic, and thyroid function. After complete description of the study to the subjects, written informed consent was obtained for repeated blood examinations. The study was approved by the institutional review board.
The 10 untreated hospitalized patients with major depression had a mean score on the Hamilton Depression Rating Scale of 26.4 (SD=5.7); there were three women and seven men, and their average age was 53.7 years (range=26–72). The healthy volunteer group consisted of 14 subjects, 10 men and four women with an average age of 50.8 years (range=25–74), from the staff of the Beer Sheva Mental Health Center and the staff's families.
ECT was administered twice a week at 3–4-day intervals by means of a MECTA-D apparatus with bitemporal electrode placement. No antidepressant medication was given to the patients during the course of ECT treatment. Anesthesia was induced by sodium pentothal (3.0 mg/kg), and the patients received succinylcholine (1.5 mg/kg), followed by oxygenation. Seizure duration was monitored centrally by the MECTA single-channel EEG monitor. A constant-current bidirectional brief-pulse square-wave stimulus was applied (pulse width, 1.5 msec; frequency, 70 Hz; duration, 1.25–2.00 seconds). In all cases blood was drawn between 8:00 and 10:00 a.m. In the course of ECT, blood was drawn at least 48 hours after the previous ECT session. The Beck Depression Inventory and the Hamilton Depression Rating Scale were administered before blood sampling.
Isolation of Mononuclear Leukocytes
Mononuclear leukocytes were isolated from 60 ml of heparinized fresh blood by using Ficoll-Paque gradient. Cells were homogenized in 25 mM Tris hydrochloride, pH 7.4, and 1 mM dithiotreitol. The homogenate was passed through two layers of cheesecloth to remove debris, and membranes were collected by further centrifugation at 18,000 g for 10 minutes. The membranes were then either freshly used for the functional binding measures or suspended in homogenization buffer containing 1 mM EGTA and 30% sucrose weight per volume and frozen at –70° until assayed by the quantitative measures. Aliquots were taken for protein concentration determination by Bradford's assay.
Guanine Nucleotide Binding Assay
Binding reactions were carried out for 15 minutes at room temperature in a final volume of 200 µl. The reaction buffer consisted of 25 mM Tris hydrochloride, pH 7.4, 1 mM ATP, 1 mM Mg2+, 1 mM EGTA, and 1 mM dithiotreitol, with varying concentrations of [3H]5′-guanylylimidodiphosphate ([3H]Gpp(NH)p) (0.05–5.00 µM). Reactions were started by adding 50 µg of membrane protein and were terminated with 5 ml of ice-cold buffer containing 10 mM Tris hydrochloride, pH 7.4, and 100 mM NaCl, followed by rapid filtration through GF/C glass filters. The filters were subsequently washed twice with 3 ml of cold buffer and taken for scintillation counting. Agonist effects on [3H]Gpp(NH)p binding were assessed by adding isoproterenol (25 µM) or carbamylcholine (50 µM) to the reaction mixture. These are the minimum concentrations resulting in the maximum effect of the agonists. Nonspecific binding was determined by using 100 µM GTPγS and usually represented less than 30% of total binding.
Immunoblot Analysis
On the day of assay, the membranes were thawed, 10-µg aliquots of membranes were taken for protein separation by gel electrophoresis with sodium dodecyl sulfate and 10% polyacrylamide, and the resulting proteins were transferred to nitrocellulose paper by use of an electroblotting apparatus. The blots were washed in Tris-buffered saline containing 3% polysorbate 20 and were blocked by incubation with 5% bovine serum albumin for 1 hour in Tris-buffered saline containing 0.1% polysorbate 20. After two washes in this solution, the blots were incubated overnight with each of the antisera (NEN-DuPont, Boston) directed specifically against Gsα (dilution 1:2,500), Gi1,2α (dilution 1:5,000), and Gβ (dilution 1:1,000) and were subsequently incubated with goat antirabbit IgG labeled with horseradish peroxidase. Immunoreactivity was detected with the Enhanced Chemiluminescence Western Blot Detection System (Amersham, Buckinghamshire, England) followed by exposure to X-ray film.
A preliminary assay of mononuclear leukocyte membranes from the healthy volunteers was carried out for determination of the range of linearity of the assay with respect to protein concentration. Linearity was found between 2.5 and 15.0 µg of membrane protein. Quantitation of the immunoblots was performed by densitometric scanning by means of an image analysis system. An aliquot of pooled standard membranes from rat brain cortex was run on one lane of every gel, and the immunolabeling was calculated in relation to this standard. This normalization procedure was carried out to minimize the between-blot variability. Although anti-Gsα detects both the 52- and 45-kDa Gsα species, only the 45-kDa species is consistently clearly detected in leukocytes, while rat cortex membranes show predominantly the 52-kDa species. The other subunits assayed in leukocytes were found to migrate at the expected molecular weights, similarly to those labeled in the rat cortex membranes.
Statistical Analysis
Correlations of the functional and quantitative measures of G proteins in the mononuclear leukocytes of the depressed patients before ECT to those evaluated during the course of ECT were conducted by using the Spearman rank correlation. Differences in the agonist-induced increases in Gpp(NH)p binding capacity and the immunoreactivities of the various G protein subunits between the depressed patients before the initiation of ECT and the healthy volunteer group and between the depressed patients after completion of treatment and the healthy volunteers were determined through Bonferroni-corrected t tests for multiple comparisons of three groups by dividing alpha by 3. The results before and after ECT within the patient group were compared through paired t tests.
RESULTS
The G protein function in mononuclear leukocyte membranes was measured through increases in [3H]~Gpp(NH)p binding capacity induced by the β-adrenergic agonist isoproterenol or the muscarinic agonist carbamylcholine. Both agonists induced significant increases in Gpp(NH)p binding capacity without substantially affecting the affinity of binding. Isoproterenol-enhanced Gpp(NH)p binding capacity was specifically blockable by the β-adrenergic antagonist propranolol and by pretreatment with cholera toxin, with no effect of pertussis toxin, indicating a specific effect through Gs protein. Carbamylcholine effects on guanine nucleotide binding were specifically blockable by the M2 selective antagonist ADFX-116 and were exerted in a specific pertussis-toxin-sensitive manner, suggesting coupling with Gi protein (33).
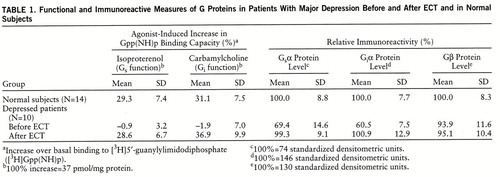
As shown by Bonferroni t tests, in comparison with the age- and sex-matched healthy subjects, the patients with depression, while untreated, had statistically significant lower function of mononuclear leukocyte Gs (t=13.58, df=22, p<0.001) and Gi (t=11.05, df=22, p<0.001) proteins and significantly lower mononuclear leukocyte immunoreactivity of the Gsα (t=5.91, df=22, p<0.001) and Giα (t=12.50, df=22, p<0.001) subunit proteins. The immunoreactive levels of the Gβ subunit protein in the mononuclear leukocytes of the depressed patients and the healthy subjects were similar (table 1).
The low Gs and Gi protein function (measured through isoproterenol- and carbamylcholine-enhanced Gpp(NH)p binding capacity) and immunoreactive measures in the mononuclear leukocytes of the untreated patients with depression were found to be normalized by ECT in a statistically significant manner, according to paired t tests (Gs function: t=11.20, df=9, p<0.001; Gi function: t=12.23, df=9, p<0.001; Gsα immunoreactivity: t=4.43, df=9, p<0.002; Giα immunoreactivity: t=4.86, df=9, p<0.001). The G protein measures after ECT were found to be not significantly different from those characterizing the group of healthy subjects, according to Bonferroni t tests (Gs function: t=0.24, df=22, n.s.; Gi function: t=1.56, df=22, n.s.; Gsα immunoreactivity: t=0.18, df=22, n.s.; Giα immunoreactivity: t=0.20, df=22, n.s.) (table 1).
Repeated measurements of Gs and Gi protein function and of the immunoreactivity of the Gsα and Giα subunit proteins were conducted during the course of ECT in parallel with repeated clinical evaluations of depressive symptoms. Figure 1 is a representative example of immunoblots obtained for the quantitation of Gsα and Giα proteins from mononuclear leukocytes of a patient with major depression undergoing ECT. The figure depicts normalization of the low Gsα and Giα immunoreactivity in the course of ECT. The detailed dynamics of normalization of the measures of G protein function (figure 2, top) and immunoreactivity (figure 2, bottom) in the mononuclear leukocytes of the depressed patients during the course of ECT in relation to the dynamics of clinical improvement reveal that the biological normalization preceded clinical improvement by at least a week.
There were significant positive correlations between the biochemical functional measures of G proteins, namely isoproterenol- and carbamylcholine-enhanced Gpp(NH)p binding capacity, and the respective immunoreactive measures of Gsα and Giα in the mononuclear leukocytes of the depressed patients undergoing ECT (Gsα: rs=0.76, N=38, p<0.001; Giα: rs=0.74, N=39, p<0.001). It can therefore be concluded that the dynamics of normalization of Gs and Gi protein function during ECT parallel the dynamics of normalization of Gsα and Giα immunoreactivity.
DISCUSSION
Our results show that the dynamics of normalization by ECT of the low function of Gs and Gi proteins parallels the dynamics of normalization of the low immunoreactivity of Gsα and Giα subunit proteins. The correlation between the function of Gs and Gi and their immunoreactive measures in mononuclear leukocytes of depressed patients at various stages of ECT supports a direct alteration at the G protein level. It can be concluded that the normalization of the low Gs and Gi function by ECT is related to a normalization of the low quantities of these proteins as shown by Gsα and Giα immunoreactivity.
The mechanisms underlying the alterations in G protein levels and function in mononuclear leukocytes of depressed patients and their normalization by ECT are still unknown. Increasing evidence indicates the existence of neurally mediated immunomodulatory mechanisms (34), involving the hypothalamic-pituitary axis and the sympathetic and parasympathetic innervation of primary and secondary lymphoid organs (35). Thus, alterations in mononuclear leukocyte G proteins may reflect 1) primary systemic alterations in G proteins similar to possible biochemical alterations in CNS signal transduction induced by the depressive episode and/or by ECT; 2) secondary influences of circulatory primary messengers, altered by the depressive state and/or by ECT; 3) secondary influences of altered sympathetic and parasympathetic innervation of lymphoid organs induced by the depressive state and/or by ECT. Most of the circulating lymphocytes are T cells whose lifespan is long: months or years (36). The lifespan of B cells is generally shorter: days or weeks (36). Both types of cells may have shorter transit times in the blood and tend to migrate to lymphoid organs. The majority of the long-living cells belong to a recirculating pool (36). Since repeated measurements of mononuclear leukocyte G proteins during a time period of around 3 weeks were conducted in the present study, it should be noted that the population of peripheral mononuclear leukocytes might be turning over or turning around through lymphoid organs throughout the course of ECT. Thus, the effects of depression and of ECT are probably exerted on the G proteins of cells in bone marrow and other lymphoid organs in addition to those in mononuclear leukocytes circulating in blood.
The differences in G protein measures between the depressed patients and the healthy subjects were not merely due to motor hyper- or hypoactivity, as normal subjects after intensive physical exercise (37) show G protein measures no different from the values for our healthy volunteer subjects. Also, G protein measures in the mononuclear leukocytes of elderly volunteer subjects, who in general are less physically active, did not differ from the measures in active, young subjects (38).
We are aware that the involvement of G proteins in the pathophysiology of depression and/or ECT, as implicated by the data presented here, should be considered cautiously, since extrapolations from findings obtained in blood cells peripheral to the CNS are not straightforward. We have discussed this issue in detail in a previous paper (8).
Our previous findings of high Gs and Gi protein function and Gsα and Giα immunoreactivity levels in mononuclear leukocytes of manic patients (5, 6) and of low Gs and Gi protein function and low Gsα and Giα immunoreactivity levels in unipolar and bipolar depressed patients (6–8) suggest that Gs and Gi protein quantitative and functional measures in mononuclear leukocytes are biochemical indicators of the affective state, rather than trait markers of bipolar mood disorder. Moreover, the function and quantity of both Gs and Gi were found to be significantly correlated with the severity of depressive symptoms (7, 8). The functional measurements of β-adrenergic-receptor-coupled Gs protein conducted in the present and previous studies are in accord with other functional findings of low β-adrenergic-coupled adenylate cyclase activity in patients with depression (39–43). In accord with the findings suggesting that the abnormalities in G protein measures in mononuclear leukocytes of mood-disordered patients should be considered state rather than trait markers, it should be noted that no structural or regulatory abnormalities in the gene for the G protein stimulatory α subunit were found in patients with bipolar disorder (44). Previous results have shown that the abnormalities in G protein functional measures are normalized by specific treatments: lithium-treated eu~thymic bipolar patients (5, 37) and antidepressant-treated mood-disordered patients (37) showed G protein function similar to that of control subjects, and those findings support the notion that alterations in G protein function in mononuclear leukocytes of affective disorder patients reflect their mood state. In this regard, the present findings showing low function and immunoreactivity of Gs and Gi proteins in unipolar depressed patients, and normalization of the function and immunoreactive levels of Gs and Gi proteins in the course of ECT, support the notion that mononuclear leukocyte G protein measures in mood disorders are state rather than trait markers.
The normalization by ECT of the biochemical measures of Gs and Gi function and of Gsα and Giα immunoreactivity levels did not follow, and thus reflect, the clinical improvement of the depressed patients. Rather, they preceded clinical improvement by at least a week. Gs and Gi protein functional and quantitative measurements in mononuclear leukocytes of patients with depression may thus potentially serve not only as a biochemical marker for the affective state of these patients but also as a biochemical means of predicting and evaluating response to ECT. It would be interesting to explore in the future whether psychotherapeutic intervention in depression can induce normalization of low G protein measures and, if so, whether the normalization of the biochemical measures precedes, as in the present study, or follows clinical improvement.
 |
Received July 7, 1997; revision received Nov. 24, 1997; accepted Dec. 22, 1997. From the Departments of Clinical Pharmacology and Psychiatry, Faculty of Health Sciences, Ben-Gurion University of the Negev. Address reprint requests to Dr. Avissar, Department of Clinical Pharmacology, Faculty of Health Sciences, Ben-Gurion University of the Negev, P.O. Box 653, 84105 Beer Sheva, Israel; [email protected] (e-mail). Supported in part by research grants from the Chief Scientist Office of the Israel Ministry of Health and by the Yadgarov Family Foundation to Dr. Schreiber and Dr. Avissar.
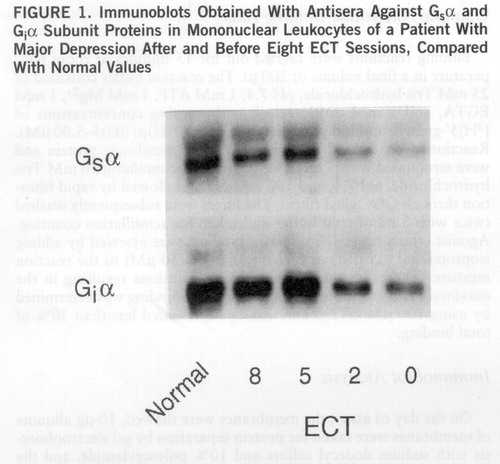
FIGURE 1. Immunoblots Obtained With Antisera Against Gsα and Giα Subunit Proteins in Mononuclear Leukocytes of a Patient With Major Depression After and Before Eight ECT Sessions, Compared With Normal Values

FIGURE 2. Rate of Normalization by ECT of Low G Protein Function and Immunoreactivity in Mononuclear Leukocytes of 10 Patients With Major Depression, Compared With Rate of Clinical Improvementa
aECT sessions were administered every 3 or 4 days over 3–4 weeks, for a total of eight sessions.
1 Schatzberg AF, Schildkraut JJ: Recent studies on norepinephrine systems in mood disorders, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 911–920Google Scholar
2 Janowsky DS, Overstreet DH: The role of acetylcholine mechanisms in mood disorders. Ibid, pp 945–956Google Scholar
3 Gilman AG: G proteins: transducers of receptor-generated signals. Annu Rev Biochem 1987; 56:615–649Crossref, Medline, Google Scholar
4 Hepler JR, Gilman AG: G proteins. Trends Biochem Sci 1992; 17:383–387Crossref, Medline, Google Scholar
5 Schreiber G, Avissar S, Danon A, Belmaker RH: Hyperfunctional G proteins in mononuclear leukocytes of patients with mania. Biol Psychiatry 1991; 29:273–280Crossref, Medline, Google Scholar
6 Avissar S, Nechamkin Y, Barki-Harrington L, Roitman G, Schreiber G: Differential G protein measures in mononuclear leukocytes of patients with bipolar mood disorder are state dependent. J Affect Disord 1997; 43:85–93Crossref, Medline, Google Scholar
7 Avissar S, Barki-Harrington L, Nechamkin Y, Roitman G, Schreiber G: Reduced β-adrenergic receptor-coupled Gs protein function and Gsα immunoreactivity in mononuclear leukocytes of patients with depression. Biol Psychiatry 1996; 39:755–760Crossref, Medline, Google Scholar
8 Avissar S, Nechamkin Y, Roitman G, Schreiber G: Reduced G protein functions and immunoreactive levels in mononuclear leukocytes of patients with depression. Am J Psychiatry 1997; 154:211–217Link, Google Scholar
9 Karege F, Bovier P, Stepanian R, Gaillard J-M: Change in platelet GTP-binding protein in drug-free depressed patients. Human Psychopharmacology 1996; 11:115–121Crossref, Google Scholar
10 Friedman E, Wang HY: Receptor-mediated activation of G proteins is increased in postmortem brains of bipolar affective disorder subjects. J Neurochem 1996; 67:1145–1152Crossref, Medline, Google Scholar
11 Young LT, Li PP, Kish SJ, Siu KP, Warsh JJ: Post mortem cerebral cortex Gsα subunit levels are elevated in bipolar affective disorder. Brain Res 1991; 553:323–326Crossref, Medline, Google Scholar
12 Young LT, Li PP, Kish SJ, Siu KP, Kamble A, Hornykiewics O, Warsh JJ: Cerebral cortex Gs protein levels and forskolin-stimulated cyclic AMP formation are increased in bipolar affective disorder. J Neurochem 1993; 61:890–898Crossref, Medline, Google Scholar
13 Avissar S, Schreiber G, Danon A, Belmaker RH: Lithium inhibits adrenergic and cholinergic increases in GTP binding in rat cortex. Nature 1988; 331:440–442Crossref, Medline, Google Scholar
14 Avissar S, Schreiber G: Muscarinic receptor subclassification and G-proteins: significance for lithium action in affective disorders and for the treatment of the extrapyramidal side effects of neuroleptics. Biol Psychiatry 1989; 26:113–130Crossref, Medline, Google Scholar
15 Avissar S, Murphy DL, Schreiber G: Magnesium reverses lithium inhibition of beta adrenergic and muscarinic receptor coupling to G proteins. Biochem Pharmacol 1991; 41:171–175Crossref, Medline, Google Scholar
16 Schreiber G, Avissar S, Aulakh CS, Murphy DL: Lithium-selective alteration of brain vs cardiac Gs protein function. Neuropharmacology 1990; 29:1067–1071Crossref, Medline, Google Scholar
17 Avissar S, Schreiber G, Aulakh CS, Murphy DL: Carbamazepine and electroconvulsive shock attenuate beta-adrenoceptor and muscarinic cholinoceptor coupling to G proteins in rat cortex. Eur J Pharmacol (Mol Pharmacol) 1990; 100:99–103Crossref, Google Scholar
18 Avissar S, Schreiber G: The involvement of G proteins in the pathogenesis and treatment of affective disorders. Biol Psychiatry 1992; 31:435–459Crossref, Medline, Google Scholar
19 Avissar S, Schreiber G: Interaction of antibipolar and antidepressant treatments with receptor-coupled G proteins. Pharmaco~psychiatry 1992; 25:44–50Crossref, Medline, Google Scholar
20 Meyniel G, Doly M, Gaillard G: [Inhibition of transducin by lithium: electrophysiological demonstration using the isolated retina]. C R Acad Sci (III) 1989; 309:289–294 (French)Medline, Google Scholar
21 Mork A, Geisler A: Effects of GTP on hormone-stimulated aden~ylate cyclase activity in cerebral cortex, striatum and hippocampus from rats treated chronically with lithium. Biol Psychiatry 1989; 26:279–288Crossref, Medline, Google Scholar
22 Newman ME, Shapira B, Lerer B: Effects of lithium and desipramine on second messenger responses in rat hippocampus: relation to G protein effects. Neuropharmacology 1991; 30:1297–1310Crossref, Medline, Google Scholar
23 Manji HK, Bitran J, Masana M, Chen G, Hsiao JK, Risby ED, Rudorfer MW, Potter WZ: Signal transduction modulation by lithium: cell culture, cerebral microdialysis and human studies. Psychopharmacol Bull 1991; 27:199–208Medline, Google Scholar
24 Song L, Jope RS: Chronic lithium treatment impairs phosphatidylinositol hydrolysis in membranes from rat brain regions. J Neurochem 1992; 58:2200–2206Crossref, Medline, Google Scholar
25 Li PP, Tam YK, Young LT, Warsh JJ: Lithium decreases Gs, Gi-1, Gi-2 α subunit mRNA levels in rat cortex. Eur J Pharmacol (Mol Pharmacol) 1991; 206:165–166Crossref, Medline, Google Scholar
26 Lesch KP, Aulakh CS, Tolliver TJ, Hill JL, Wolozin BL, Murphy DL: Differential effects of long-term lithium and carbamazepine administration on Gsα and Giα protein in rat brain. Eur J Pharmacol (Mol Pharmacol) 1991; 207:355–359Crossref, Medline, Google Scholar
27 Lerer B: Neurochemical and other neurobiological consequences of ECT: implications for the pathogenesis and treatment of affective disorders, in Psychopharmacology: The Third Generation of Progress. Edited by Meltzer HY. New York, Raven Press, 1987, pp 577–588Google Scholar
28 Sackeim HA, Devanand DP, Nobler MS: Electroconvulsive therapy, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 1123–1141Google Scholar
29 Dubovsky SL: Electroconvulsive therapy, in Comprehensive Textbook of Psychiatry, 6th ed, vol 2. Edited by Kaplan HI, Sadock BJ. Baltimore, Williams & Wilkins, 1995, pp 2129–2140Google Scholar
30 McGowen S, Eastwood SL, Mead A, Burnet PWJ, Smith C, Flanigan TP, Harrison PJ: Hippocampal and cortical G protein (Gsα, Goα and Gi2α) mRNA expression after electroconvulsive shock or lithium carbonate treatment. Eur J Pharmacol 1996; 306:249–255Crossref, Medline, Google Scholar
31 Ozawa H, Rasenick MM: Chronic electroconvulsive treatment augments coupling of the GTP-binding protein Gs to the catalytic moiety of adenylyl cyclase in a manner similar to that seen with chronic antidepressant drugs. J Neurochem 1991; 56:330–338Crossref, Medline, Google Scholar
32 Newman ME, Solomon H, Lerer B: Electroconvulsive shock and cyclic AMP signal transduction: effects distal to the receptor. J Neurochem 1986; 46:1667–1669Crossref, Medline, Google Scholar
33 Avissar S, Schreiber G: Measurement of early events in signal transduction beyond receptors involving G proteins function in mononuclear leukocytes. J Neuroimmunol 1996; 70:81–86Crossref, Medline, Google Scholar
34 Roszman TL, Brooks WH: Neural modulation of immune function. J Neuroimmunol 1985; 10:59–69Crossref, Medline, Google Scholar
35 Livnat S, Felten SY, Carlson SL, Bellinger DL, Felten DL: Involvement of peripheral and central catecholamine systems in neural immune interactions. J Neuroimmunol 1985; 10:5–30Crossref, Medline, Google Scholar
36 Paraskevas F, Foerster J: The lymphocytes, in Wintrobe's Clinical Hematology, 9th ed, vol 1. Edited by Lee GR, Bithell TC, Foerster J, Athens JW, Lukens JN. London, Lea & Febiger, 1993, pp 354–430Google Scholar
37 Avissar S, Schreiber G: Antidepressant and antibipolar treatments effects on receptor-coupled G proteins measures in lymphocytes of patients with mood disorders (abstract). Neuropsychopharmacology 1994; 10(suppl):170SGoogle Scholar
38 Barki-Harrington L, Nechamkin Y, Schreiber G, Avissar S: Functional and quantitative measures of receptor-coupled G proteins in human mononuclear leukocytes: no change with age. Exp Gerontol 1996; 31:351–363Crossref, Medline, Google Scholar
39 Pandey GN, Dysken MW, Garver DL, Davis JM: Beta-adrenergic receptor function in affective illness. Am J Psychiatry 1979; 136:675–678Link, Google Scholar
40 Extein I, Tallman J, Smith CC, Goodwin FK: Changes in lymphocyte beta-adrenergic receptors in depression and mania. Psychiatry Res 1979; 1:191–197Crossref, Medline, Google Scholar
41 Mann JJ, Brown RP, Halper JP, Sweeney JA, Kocsis JH, Stokes PE, Bilezikian JP: Reduced sensitivity of lymphocyte beta-adrenergic receptors in patients with endogenous depression and psychomotor agitation. N Engl J Med 1985; 313:715–720Crossref, Medline, Google Scholar
42 Halper JP, Brown RP, Sweeney JA, Kocsis JH, Peters A, Mann JJ: Blunted β-adrenoceptor responsivity of peripheral blood mononuclear cells in endogenous depression: isoproterenol dose-response studies. Arch Gen Psychiatry 1988; 45:241–244Crossref, Medline, Google Scholar
43 Ebstein RP, Lerer B, Shapira B, Shemesh Z, Moscovich DG, Kindler S: Cyclic AMP second-messenger signal amplification in depression. Br J Psychiatry 1988; 152:665–669Crossref, Medline, Google Scholar
44 Ram A, Guedj F, Cravchik A, Weinstein L, Cao Q, Badner JA, Goldin LR, Grisaru N, Manji HK, Belmaker RH, Gershon ES, Gejman PV: No abnormality in the gene for the G protein stimulatory alpha subunit in patients with bipolar disorder. Arch Gen Psychiatry 1997; 54:44–48Crossref, Medline, Google Scholar



