Respiratory Psychophysiology of Panic Disorder: Three Respiratory Challenges in 98 Subjects
Abstract
OBJECTIVE: Respiratory abnormalities may play a central role in the pathophysiology of panic disorder. The current study was undertaken to examine the respiratory response in the largest series of subjects to date during three respiratory challenges that used improved methodology. METHOD: Fifty-nine patients with DSM-III-R panic disorder and 39 normal volunteers were challenged with 5% and 7% CO2 inhalation and room air hyperventilation separated by room air breathing with continuous spirometry. RESULTS: Patients with panic disorder were more sensitive to the anxiogenic effects of CO2 than were normal subjects, and CO2 was a more potent stimulus to panic than hyperventilation. Patients increased their respiratory rate more quickly during CO2 inhalation than did comparison subjects, and this increase preceded the panic attacks. Patients who panicked in response to 5% CO2 demonstrated continued rise in end-tidal CO2, while the end-tidal CO2 of the comparison groups stabilized. Low end-tidal CO2 and high variance in minute ventilation at baseline predicted panic attacks during CO2 inhalation. Following CO2 or hyperventilation challenges, respiratory rate dropped sharply, while tidal volume remained elevated longer in patients than in comparison subjects. CONCLUSIONS: The findings confirm the greater behavioral and physiological sensitivity of patients with panic disorder to CO2 inhalation and identify a series of respiratory abnormalities. Panic attacks in panic disorder may be explained by inefficient compensatory mechanisms, primarily of respiratory rate. (Am J Psychiatry 1997; 154:1557–1565)
It is now well established that CO2 inhalation causes more anxiety and panic in patients with panic disorder than in normal volunteers (1–9). CO2-induced panic resembles naturally occurring panic (10). CO2 hypersensitivity is not simply a manifestation of dyspnea induced by CO2 (11) and cannot be explained by baseline anxiety level differences between patients and comparison subjects (12). Panic patients exhibit hypersensitivity to CO2 even when their anxious response is comparable to that of normal comparison subjects (6). Heightened CO2 sensitivity in asymptomatic first-degree relatives of patients with panic disorder suggest that the response may be a trait phenomenon (13). While the enhanced sensitivity of panic disorder patients to inhaled CO2 has been unequivocally established, the nature of the specific respiratory abnormalities in panic disorder remains controversial.
In 1988 we reported the behavioral and physiological effects of a series of respiratory challenges in panic disorder patients (4). We showed, among other findings, that during the first 2.5 minutes of 5% CO2 inhalation, panicking patients increased their minute ventilation more rapidly than nonpanicking patients or normal comparison subjects. This increase took place before the actual panic attack. Following both CO2-induced and room air hyperventilation-induced panic attacks, patients continued to hyperventilate during the recovery phase, unlike the comparison subjects; this may indicate an attempt to reestablish a chronic state of respiratory alkalosis. This finding was one of the first examples of the unusual respiratory physiology of this patient population. Subsequently, a number of studies have also shown that during laboratory-induced panic, panic patients seem to defy the laws of acid-base homeostasis by responding to alkalosis with increased rather than decreased ventilation and a further shift toward alkalosis (14). Many of these findings led to Klein's formulation of a “suffocation false alarm” hypothesis to explain the pathogenesis of panic disorder (15).
The current study was undertaken to attempt to replicate our earlier studies by using 5% and 7% CO2 inhalation and hyperventilation with improved methodology and to thoroughly examine the respiratory response of our largest series of panic disorder patients to date. Specifically, we aimed to determine which one of the two most frequently employed CO2 concentrations should be favored in assessing the respiratory response of patients with panic disorder; to characterize this response by applying novel analytic techniques to respiratory measures identified in our earlier work as discriminating patients and normal comparison subjects; to identify respiratory predictors of panic; and to describe the respiratory pattern accompanying CO2-induced panic. We were particularly interested in the clinical and treatment implications of respiratory abnormalities. We also wanted to address previous methodological shortcomings such as possible order effects of the challenges, absence of self-ratings of panic attacks, possible selection and assessment bias of patients and comparison subjects, and relatively small group size.
The behavioral response to the challenges of a subgroup of 24 patients and 18 normal volunteers has been reported previously (16). Since the behavioral findings in the current extended group are almost identical to those of the earlier report, they will be mentioned only in the interest of clarity. The focus of the current report is respiratory physiology.
METHOD
Subjects
Fifty-nine patients, 21 men and 38 women between the ages of 19 and 58 years (mean=34.0, SD=9.8), with DSM-III-R panic disorder with (N=38) or without agoraphobia were evaluated with standard psychiatric interview. The diagnoses were confirmed by the Structured Clinical Interview for DSM-III-R (SCID) (17). Patients were excluded if they met criteria for current major depressive disorder, obsessive-compulsive disorder, or substance use disorder or if they had a lifetime history of schizophrenia or bipolar disorder. Patients with an anxiety disorder other than panic disorder and those with dysthymia were included if the disorder was secondary to panic disorder.
The comparison group consisted of 20 men and 19 women between the ages of 20 and 60 years (mean=32.1, SD=9.9). They were assessed by either the Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (18) or the SCID. They were free of any lifetime history of anxiety disorders, major affective disorders, schizophrenia, and current substance use disorders. All subjects underwent medical evaluations and were in good health.
Subjects were required to have been drug free for at least 2 weeks before baseline assessment. However, the use of benzodiazepines up to the equivalent of 5 mg of diazepam/day was permitted until 48 hours before testing. Only one patient took benzodiazepines within 2 weeks of testing. All subjects signed informed consent forms that explained that they would breathe air mixed with CO2 and also would be asked to hyperventilate room air, that the procedures were not dangerous, and that anxiety or panic could occur during the study. The study was approved by the institutional review board.
Procedure
Subjects were tested on two mornings separated by not more than 10 days. The first morning involved CO2 response testing that used the Read rebreathing method (19), a traditional pulmonary assessment of ventilatory sensitivity. These results have been reported elsewhere (20). On the second morning, following an overnight fast, subjects underwent the “canopy” procedure reported here.
First, subjects were randomly assigned to watch one of two videotapes that gave different sets of instructions regarding the effects of CO2 inhalation. The data showing that the instructions did not meaningfully influence the responses to respiratory challenges (including no interactions) have been reported separately (21). Next, the subject was asked to lie down, and his or her head was placed in a clear plastic canopy described in detail elsewhere (16). The canopy was sealed, but the subject could see and hear and be seen and heard at all times. The subject knew how to open the canopy quickly by flipping a latch (no subject actually did this). Subjects could also signal to have the procedure terminated at any time. The canopy was vented by a source of external air at 40 liters per minute. End-tidal CO2 concentration was measured by capnograph. End-tidal CO2 correlates well with arterial CO2 concentration (22). Respiratory rate and tidal volume were recorded in a spirometer.
The experiment consisted of seven periods: 1) room air breathing for 20 minutes, 2) according to random assignment either 5% CO2 in room air for 20 minutes or room air hyperventilation for 15 minutes (for the hyperventilation, a metronome was placed on top of the canopy with a flashing light, and the subject was told to take a deep breath every time the light flashed [30 breaths per minute]), 3) room air breathing for 15 minutes, 4) the intervention that was not assigned in period 2, 5) room air breathing for 15 minutes, 6) 7% CO2 in room air for 20 minutes, and 7) room air breathing for 15 minutes. Subjects were not informed about the timing and type of each period.
Ten raters were trained to identify panic attacks by watching videotapes of lactate infusions. The occurrence of a panic attack was determined in two ways. The rater, blind to diagnosis, used DSM-III-R criteria to assess the presence of a panic attack (four or more DSM-III-R symptoms plus a clear and sudden increase in anxiety). A few months after the study began a second determination of panic was added. Here the subject was asked to indicate whether he or she had experienced a panic attack by pointing to answers on a card. The rater was not permitted to observe the subject's rating. Because the subject's rating of panic was included after the study began, fewer subject assessments, compared to rater assessments, were available. In addition to the varying extent to which subjects participated in the experiment, this is the other reason why the number varies in certain analyses.
Four rating scales were administered at the end of each test period to determine anxiety and distress: the 27-item Acute Panic Inventory (23), 10-point Likert-scored Anxiety and Apprehension Scales, and the Borg Scale of Exertion (24), which measures effort required to breathe. Whenever a subject could not tolerate the intervention (indicated by raising his or her hand), room air breathing resumed, and the scales were administered.
Data Analysis
Panic rate. Rates of panic among the three interventions, between patients and normal volunteers, and also separately for raters' and subjects' ratings and by gender were compared with chi-square (or Fisher's exact) tests.
Physiology. The respiratory physiology data consisted of continuous (every other breath) recordings of end-tidal CO2, respiratory rate, and tidal volume. Before statistical analysis aberrant values were edited according to preset criteria (i.e., end-tidal CO2 less than 10 torr during room air, less than 33 torr during 5% CO2, less than 48 torr during 7% CO2; respiratory rate greater than 80; tidal volume less than 100 ml). Data points with three standard deviations outside the mean were also eliminated. These corrections resulted in the elimination of less than 3% of the data. Vital capacity was covaried in comparisons involving tidal volume.
In order to correct for multiple dependent variables, whenever appropriate, multivariate analyses were performed first with subject groups, respiratory measures (end-tidal CO2, tidal volume, respiratory rate), and gender included as factors. Significant differences were followed up by separate comparisons. However, given our a priori directional hypotheses (25), the reported prominent gender differences in respiratory response (26), and our unequal sex distribution, all analyses were repeated in men and women separately whenever group sizes were meaningful (greater than five), regardless of the results of the multivariate analyses. These “exploratory” analyses are listed separately within each section.
Five-minute mean values (and standard deviations) were calculated for each variable throughout the study. In the first set of analyses the last 5-minute mean of each period was used as a single value. Following up on significant multivariate tests, first we compared patients with normal subjects within each period for each variable by using analysis of variance (ANOVA). These analyses assessed group differences at baseline and during the interventions regardless of panic rates. The three baselines (room air breathing) were also compared with ANOVAs for each variable in order to determine if the interventions caused any differences in “recovery.”
Second, we divided the patients into those panicking in response to 5% CO2 and those who did not (also into those who did and did not panic in response to 7% CO2, to hyperventilation, and to both 5% and 7% CO2), separately according to the raters' and the subjects' ratings, and performed three group ANOVAs for each period and each variable.
During CO2 inhalation and hyperventilation it takes approximately 10 minutes to reach steady state respiratory conditions in the canopy. Therefore, the last 5-minute means of these interventions were used as single values. This method of analysis is adequate for subjects who were able to complete the challenges. Subjects who panicked during any of the interventions and stopped the procedure early were exposed to the challenge for a shorter time period than nonpanicking subjects. For these subjects we considered the 5-minute mean immediately before the panic attack. Subjects whose panic attack occurred sooner than 5 minutes into the period (eight for 5% CO2, four for hyperventilation, and 19 for 7% CO2) were not included in this analysis.
Since the overall respiratory response to CO2 is hyperbolic, we also performed a time analysis to examine the first few minutes of linear respiratory response to CO2. This ANOVA included all subjects. One-minute means of respiratory rate, tidal volume, minute ventilation, and end-tidal CO2 were calculated at the following six time points: 2 minutes before CO2 and 1, 2, 3, 4, and 5 minutes of CO2 inhalation. Many patients who panicked had their panic attack by minute 5, so further comparison was meaningless because of diminishing group size. CO2 sensitivity (delta minute ventilation divided by delta end-tidal CO2) was calculated on a minute-by-minute basis by using the difference between the values at each time point and baseline. ANOVAs were used to compare the same time periods across the three groups (panickers, nonpanickers, and comparison subjects). We also performed this comparison by using respiratory rate (delta respiratory rate divided by delta end-tidal CO2) and tidal volume (delta tidal volume divided by delta end-tidal CO2) and self- and raters' ratings separately.
In order to compare the pattern of response in the three subject groups exposed to CO2 for the full 15 minutes, we also plotted tidal volume, respiratory rate, minute ventilation, and end-tidal CO2 curves by using 1-minute means throughout the CO2 challenges for comparison subjects, nonpanicking patients, and self-rated panickers and performed ANOVAs followed up by pairwise comparisons when appropriate.
The recovery analyses compared the pattern of change in tidal volume, respiratory rate, minute ventilation, and end-tidal CO2 from the end of the interventions through the subsequent 10 minutes of room air breathing by using ANOVAs on 1-minute means. The recovery analyses were repeated by using self- and raters' ratings separately.
In order to identify predictors of panic response, logistic regression analyses were performed with the forward stepping procedure by using eight preselected baseline measures (respiratory rate, tidal volume, minute ventilation, end-tidal CO2, and their standard deviations) with self-rated panic as the outcome measure and sex and age as control measures (probability in and out criteria were set at 0.20 and 0.25, respectively).
All p values reported are two-tailed, with significance levels of 0.05. When indicated by unequal variances, the separate variance estimate statistic was used.
RESULTS
Panic Rates
Table 1 shows the panic rates during the interventions according to the rater and according to the subject. Significantly more patients panicked in response to 7% than 5% CO2, and the panic rates in response to CO2 were significantly higher than those in response to hyperventilation, determined by using either ratings. The order of the interventions did not affect the rates of panic. During 5% CO2 inhalation, of the 51 patients for whom both ratings were available, 31 panic patients rated themselves as panicking, but only 14 were noted by the clinician. There was only one case in which the clinician-rated panic attack was not endorsed by the subject. During 7% CO2 inhalation, of the 47 patients for whom both ratings were available, 34 panic patients rated themselves as panicking, and 26 were so rated by clinicians. Four clinician-rated panic attacks were not endorsed by the patient. Agreement between blind and self-ratings of panic did not reach statistically significant differences. The only sex difference was a significantly higher self-rating of panic in female patients (N=26 of 31) than in male patients (N=8 of 16) during 7% CO2 inhalation (χ2=6.05, df=1, p<0.01).
The behavioral rating scales demonstrated more anxiety, apprehension, and breathlessness in patients than in comparison subjects. Compared to our preliminary report (16), these differences became more accentuated.
Respiratory Physiology
Baseline. The physiology data (means and standard deviations) are summarized in table 2. The physiological differences were most prominent between panicking and nonpanicking patients when the self-ratings to 5% CO2 inhalation were used. Unless stated otherwise, this rating is used in all subsequent analyses and tables.
At first baseline the multivariate test revealed significant sex effects only (F=3.84, df=3,79, p<0.01). Follow-up exploratory comparisons showed that baseline end-tidal CO2 for patients was significantly lower than that for the comparison group (F=7.64, df=1,91, p<0.007). Both panicking and nonpanicking patients had significantly lower end-tidal CO2 values than normal subjects (F=3.12, df=2,81, p<0.05) but did not differ from each other. Respiratory rate, tidal volume, and minute ventilation were all higher in patients than in comparison subjects, but the differences did not reach significance. The overall baseline comparison of standard deviations again revealed significant sex effects (F=4.53, df=1,90, p<0.04). The subsequent comparisons within gender showed significantly higher individual standard deviations in tidal volume (t=2.25, df=51, p<0.03, separate variance estimate) and minute ventilation (t=3.19, df=46, p<0.003) in female patients than in female comparison subjects.
We compared the three baselines (periods 1, 3, and 5) in order to assess possible differential order effects of CO2 or hyperventilation in periods 2 and 4. The overall multivariate test showed no significant order effects.
5% CO2 inhalation. Both nonpanickers (mean=16.65 minutes, SD=5.33) (t=2.05, df=50, p<0.05) and comparison subjects (mean=19.06 minutes, SD=2.10) (t=4.83, df=36,13, p<0.001, separate variance estimate) continued to breathe 5% CO2 significantly longer than panickers (mean=12.95 minutes, SD=6.89) (F=10.36, df=2,86, p<0.001). The multivariate test with three groups, three respiratory measures, and sex, which used pre- and intervention values, showed overall sex effect (F=3.19, df=3,69, p<0.03), time effect (F=101.1, df=3,69, p<0.0001), and sex-by-time interaction (F=3.64, df=3,69, p<0.02). The follow-up ANOVAs showed group effect for end-tidal CO2 (F=4.14, df=2,69, p<0.02). Both panicking and nonpanicking patients had lower end-tidal CO2 than comparison subjects. The group-by-sex interaction for end-tidal CO2 (F=8.98, df=1,81, p<0.004) was due to significantly lower values in both panicking and nonpanicking patients than in comparison subjects; female patients had the lowest values followed by male comparison subjects, male patients, and female comparison subjects.
Through use of the last 5-minute means the multivariate test showed a significant sex effect (F=2.85, df=3,75, p<0.04), and the follow-up exploratory ANOVAs found that the differences were in respiratory rate (patients: mean=20.37, SD=5.09; comparison subjects: mean=18.11, SD=3.92) (F=4.54, df=1,83, p<0.04) and end-tidal CO2 (patients: mean=49.41, SD=3.78; comparison subjects: mean=51.87, SD=3.03) (F=10.66, df=1,81, p<0.002). The group-by-time near-significant interaction for respiratory rate (F=2.65, df=2,43, p<0.08, with Greenhouse-Geisser correction) indicated a larger increase in panicking female patients than in the other two female groups.
The time analysis elaborated on this finding. The multivariate comparison showed significant sex effect (F=3.63, df=3,61, p<0.02), time effect (F=46.16, df=15,49, p<0.001), and significant sex-by-time (F=3.94, df=15,49, p<0.001) and group-by-time (F=1.65, df=30,96, p<0.04) interactions. The follow-up comparison showed a near-significant group difference and significant group-by-time and group-by-time-by-sex interactions in respiratory rate (figure 1). Patients who panicked in response to 5% CO2 increased their respiratory rate much sooner than comparison subjects or nonpanickers. The interactions were due to a more variable course for panickers than for nonpanickers and comparison subjects and a more accentuated increase in women than in men.
Using 1-minute means we performed the multivariate test comparing the three groups (comparison subjects, self-rated panickers, nonpanicking patients) exposed to CO2 for the full 15 minutes. The significant overall sex effect (F=6.18, df=3,44, p<0.001) and group-by-sex interaction (F=2.93, df=6,86, p<0.01) were due to a steeper, smoother hyperbolic tidal volume rise in comparison subjects than nonpanicking patients or self-rated panickers (F=1.80, df=7,180, p<0.09, with Greenhouse-Geisser correction). Respiratory rate showed an almost opposite pattern; here the significant group effects in women (F=3.85, df=2,26, p<0.03) was the result of a steep and chaotic increase in patients, with relatively smaller changes in comparison subjects. The group-by-sex interaction for end-tidal CO2 (figure 2) was due to significant group differences in women (F=6.26, df=2,27, p<0.006); comparison and nonpanicking women developed steady levels by minute 5, while the end-tidal CO2 in self-rated panickers continued to rise. The within-subject correlation coefficients between minute ventilation and end-tidal CO2 confirmed this pattern; the mean correlation in self-rated panickers (r=0.75) was significantly higher than that in either comparison subjects (r=0.59) (t=2.13, df=43, p<0.04;) or nonpanicking patients (r=0.41) (t=2.70, df=28, p<0.02).
Hyperventilation. Panicking patients hyperventilated in room air for a mean of 11.48 minutes (SD=4.62), nonpanickers for a mean of 12.35 minutes (SD=3.44), and the comparison subjects for a mean of 12.99 minutes (SD=2.98) (n.s.). Panicking patients reached a mean end-tidal CO2 of 31.27 torr (SD=6.23), while nonpanicking patients reached a mean of 30.28 torr (SD=6.13) and comparison subjects reached a mean of 31.87 torr (SD=6.13). All subjects were encouraged to maintain a respiratory rate of 30 breaths per minute. The actual respiratory rates, shown in table 2, were in fact very close to 30 breaths per minute for all groups. The multivariate comparison of pre- and intervention means showed sex effect (F=6.12, df=3,65, p<0.001), time effect (F=94.71, df=3,65, p<0.001), and group-by-sex (F=2.27, df=6,128, p<0.04) and sex-by-time (F=3.84, df=3,65, p<0.01) interactions. Follow-up ANOVAs showed no group differences for any of the measures.
Through use of the last 5-minute means the multivariate analysis showed sex effect only (F=3.49, df=3,68, p<0.02). The follow-up exploratory comparisons showed no significant differences between patients and comparison subjects with respect to tidal volume and respiratory rate. For end-tidal CO2 significant group-by-sex interaction (F=4.49, df=2,78, p<0.01) showed that panicking (mean=26.62 torr, SD=3.51) and nonpanicking (mean=27.35 torr, SD=5.82) female patients had significantly lower end-tidal CO2 than comparison women (mean=32.88 torr, SD=7.63). The same analysis in men would have been meaningless, since only two men panicked during this intervention.
7% CO2 inhalation. The mean times of 7% CO2 exposure for each group were as follows: panickers, mean=8.74 minutes, SD=7.01; nonpanickers, mean=10.87 minutes, SD=6.06; normal comparison subjects, mean=15.75 minutes, SD=5.43 (F=9.88, df=2,75, p<0.001). Comparison subjects lasted significantly longer than either panickers (t=4.38, df=61, p<0.001) or nonpanickers (t=2.60, df=40, p<0.02). The multivariate comparison of pre- and intervention values showed sex effect (F=8.41, df=3,48, p<0.001) and group-by-sex (F=2.80, df=6,94, p<0.02) and sex-by-time (F=9.53, df=3,48, p<0.001) interactions. The follow-up ANOVAs showed significant sex effect (F=20.1, df=1,52, p<0.001) and sex-by-time interaction (F=22.82, df=1,52, p<0.001) for tidal volume, with panicking male patients having the lowest tidal volume response followed by nonpanickers and then by comparison subjects. This difference resulted in significantly higher tidal volume during the last 5 minutes of CO2 exposure in comparison subjects (mean=1,956 ml, SD=729) than in patients (mean=1,613 ml, SD=642) (F=4.23, df=1,69, p<0.04). The significant group-by-sex-by-time interaction for respiratory rate (F=3.25, df=2,52, p<0.05) was due to panicking female patients having a significantly larger respiratory rate increase than comparison subjects (group-by-time interaction in women: F=4.65, df=2,30, p<0.02). Panicking female patients started with a lower respiratory rate but ended up with a higher rate than the other two groups of women.
CO2 Sensitivity
No overall conventional CO2 sensitivity (delta minute ventilation divided by delta end-tidal CO2) differences emerged with use of either the 5% or the 7% values against baseline. However, sensitivity attributable to respiratory rate showed significant overall sex effect (F=4.67, df=1,71, p<0.03). Female patients (mean=0.20, SD=0.19) had significantly higher response than female comparison subjects (mean=0.10, SD=0.11) during 5% CO2 inhalation (t=–2.25, df=41.44, p<0.03, separate variance estimate). Similar but more accentuated respiratory rate response differences were found during 7% CO2. Here, the significant overall sex effect (F=7.42, df=1,55, p<0.01), group effect (F=3.39, df=2,55, p<0.04), and group-by-sex interaction (F=3.31, df=2,55, p<0.05) were due to significantly higher respiratory rate response in female patients (mean=0.30, SD=0.29) than in female comparison subjects (mean=0.10, SD=0.13) (F=6.03, df=2,31, p<0.01), resulting in a significant overall patient/comparison subject difference in respiratory rate response (patients: mean=0.23, SD=0.25; comparison subjects: mean=0.13, SD=0.13). While it was not significant, panicking patients had the highest response (0.26) followed by nonpanicking patients (0.17) and comparison subjects (0.13).
During 5% CO2 female comparison subjects had higher tidal volume response (mean=40.97, SD=21.78) than female patients (mean=28.63, SD=12.66) (F=2.87, df=2,31, p<0.07), which produced an overall patient/comparison subject difference in tidal volume response (F=2.63, df=2,71, p<0.08).
“Recovery” Analyses
The recovery period showed similar patterns after all three interventions, with the most pronounced differences emerging after 5% CO2 was switched to room air. Here, the multivariate analysis showed sex effect (F=3.51, df=3,52, p<0.02), time effect (F=53.61, df=30,25, p<0.001), and a near-significant group effect (F=2.07, df=6,102, p<0.06). The follow-up ANOVA for tidal volume demonstrated that patients maintained their increased tidal volume longer than comparison subjects (group-by-time interaction: F=2.03, df=10,285, p<0.03). While patients lowered their respiratory rate sharply, they showed a delay in lowering their minute ventilation during the recovery phase, resulting in a more pronounced drop in end-tidal CO2 than in comparison subjects (figure 3).
Predictors of Panic
The logistic regression analysis identified low end-tidal CO2 (Wald F=4.16, df=1, p<0.05) for the entire group and low end-tidal CO2 (F=6.20, df=1, p<0.02) and high within-subject standard deviation of minute ventilation (F=4.98, df=1, p<0.01) in women only as baseline predictors of panic.
DISCUSSION
In our largest group to date, we have replicated and substantially extended many earlier findings concerning the respiratory physiology of panic disorder and failed to replicate a few others. Clearly, panic disorder patients exhibit both behaviorally and physiologically abnormal responses to respiratory challenges. Patients reported significantly more panic attacks and anxiety during the challenges than normal volunteers. The behavioral responses as assessed by the rating scales were comparable with our earlier report in a subgroup (16) with more accentuated patient/comparison subject differences. The inhalation of either concentration of CO2 was significantly more panicogenic than hyperventilation. Hyperventilation at 30 breaths a minute, in spite of significant drops in end-tidal CO2 (greater in patients than in comparison subjects) to conventionally accepted levels of hypocapnia, seemed once again a less convincing panicogenic challenge than CO2. It is possible that a more drastic hyperventilation-induced reduction in end-tidal CO2 would have resulted in more panic attacks as some studies suggest (27, 28). However, on the basis of our experience, the demands of a more vigorous hyperventilation test only result in increasing noncompliance, especially in panic disorder patients.
While not significant, agreement between blind raters and subjects about the occurrence of a CO2-induced panic attack was higher during 7% than 5% CO2 inhalation. The panic attack induced by 7% CO2 is more dramatic and therefore easier to identify by a blind rater. This finding is consistent with that of Roth et al. (29), who argue that blind rating of 5% CO2-induced panic is unreliable. On the basis of these findings from our expanded group, we continue to recommend that when the goal is to differentiate patients with panic disorder from other groups on the basis of panic attack rate, 7% CO2 is the superior concentration.
By contrast, self-ratings of panic during 5% CO2 inhalation, possibly representing “limited” panic attacks, seemed to differentiate the panic group on most respiratory measures from nonpanickers and comparison subjects better than any other panic rating. This difference may simply reflect large group sizes; self-rating during 5% CO2 inhalation yields the highest panic group, subjects tolerate the lower concentration better and stay in the canopy longer, and therefore significantly more data are collected during 5% than 7% CO2 inhalation. Therefore, when the goal is to differentiate panic patients from other groups on the basis of physiological responses, 5% CO2 seems preferable. This conclusion is also supported by receiver operating characteristic analysis showing that a relatively modest increment of anxiety may discriminate best between panic patients and comparison subjects (30).
As in several previous studies, we again find that the CO2 level in panic patients, whether measured by capnography (end-tidal CO2) or directly from arterial blood (PaCO2), is lower at baseline than in normal comparison subjects. Relative hypocapnia appears to predict the occurrence of panic both to CO2 inhalation and to lactate infusion (31). We interpret this to mean that some degree of respiratory stimulation precedes the induction of panic in the laboratory. It is unclear, however, whether this decrease in CO2 level is the result of anticipation or represents an ongoing pattern of increased ventilation.
We also confirm earlier findings of irregular breathing at rest in panic disorder patients, although this time limited to the female patient group. Increased within subject standard deviation of minute ventilation and tidal volume indicate a chaotic breathing pattern and may predict vulnerability to panic. This is also consistent with data showing increased irregular breathing in panic disorder patients undergoing CO2 challenges (32), while asleep in the laboratory (33) or at home and wearing an ambulatory respiratory monitor (34).
Perhaps the most interesting finding from this study is the respiratory profile of change during CO2 inhalation. Normal comparison subjects and nonpanicking patients responded to CO2 mainly by increasing tidal volume but panicking patients demonstrated an exaggerated respiratory rate and blunted tidal volume response. This difference resulted in significantly higher respiratory sensitivity attributable to respiratory rate in response to CO2 in patients than in comparison subjects. This differs somewhat from our 1988 finding of increased minute ventilation response but is consistent with more recent findings from our group (20) and others (5). The discrepancy may be due to the heterogeneity of panic groups, the homogeneity of comparison populations, and differences in the equipment and methodologies used. Nevertheless, a consistent picture of increased respiratory rate response during CO2-induced panic is apparent.
The differential reactivity in response to CO2 inhalation was paralleled during the “recovery” phase. After switching to room air, most patients responded with respiratory rate while their tidal volume remained more elevated than in comparison subjects. We believe that the pattern of respiratory rate response to CO2 coupled with sustained tidal volume during recovery can be explained as a physiological response to CO2 intolerance. While panicking in room air the patient attempts to decrease CO2 concentration by taking deep breaths (35). Taking deep breaths during CO2 inhalation, however, would only serve to increase the amount of CO2 inspired, hence the shallow rapid breathing. Once room air breathing resumes, it becomes physiologically sensible again for the CO2 intolerant patient to maintain an increased tidal volume to rapidly expel CO2. However, this response to CO2 appears inefficient; the chaotic increase in respiratory rate does not work, the end-tidal CO2 levels of patients who subsequently panic continue to rise. The response seems analogous to a nonswimmer tossed into deep water who begins frantically flaying arms and legs, only increasing the chances of drowning.
Teaching patients the technique of slow abdominal breathing is standard for behavioral therapies of panic disorder (36) and may be effective by increasing control over breathing and decreasing chaotic respiratory rate responses. We have also found recently that antidepressant medications decrease the respiratory rate component of CO2 response in patients with panic disorder (Gorman et al., unpublished data). Antipanic treatments may work by directly influencing respiratory control.
The evidence implicating respiratory abnormalities in the pathogenesis of panic is rapidly accumulating. While the data presented here support a theory advocating that panic represents a primary respiratory abnormality (37), there remain a number of critical areas in need of exploration before such assertion can be fully validated. These areas include the need to establish that CO2-induced panic is specific to patients with panic disorder; whether it varies with the age or sex of the patients and to what extent they are cognitively, as opposed to biologically, mediated; whether respiratory abnormalities are trait or state related and finally; whether there is a direct central nervous system correlate of any of the behavioral findings observed during respiratory challenges.
 |
 |
Received Oct. 15, 1996; revisions received April 14 and May 21, 1997; accepted May 29, 1997. From the Biological Studies Unit, New York State Psychiatric Institute, College of Physicians and Surgeons, Columbia University; New York University Medical Center; and the Phobia, Anxiety and Stress Disorders Clinic, Hillside Hospital, New York. Address reprint requests to Dr. Papp, 722 West 168th St., New York, NY 10032. Supported in part by NIMH grants MH-41778 and MH-30906, Scientist Development Award for Clinicians MH-00858 (Dr. Papp), Research Scientist Award MH-00416 (Dr. Gorman), and Scientist Development Award for Clinicians MH-01039 (Dr. Coplan).
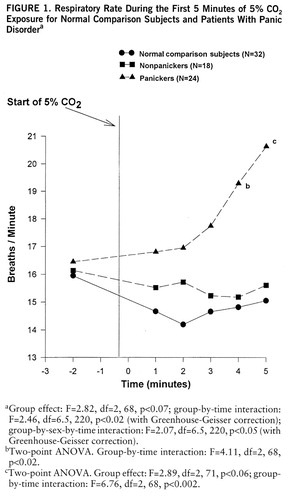
FIGURE 1. Respiratory Rate During the First 5 Minutes of 5O2 Exposure for Normal Comparison Subjects and Patients With Panic Disordera
aGroup effect: F=2.82, df=2,68, p<0.07; group-by-time interaction: F=2.46, df=6.5,220, p<0.02 (with Greenhouse-Geisser correction); group-by-sex-by-time interaction: F=2.07, df=6.5,220, p<0.05 (with Greenhouse-Geisser correction).
bTwo-point ANOVA. Group-by-time interaction: F=4.11, df=2,68, p<0.02.
cTwo-point ANOVA. Group effect: F=2.89, df=2,71, p<0.06; group-by-time interaction: F=6.76, df=2,68, p<0.002.
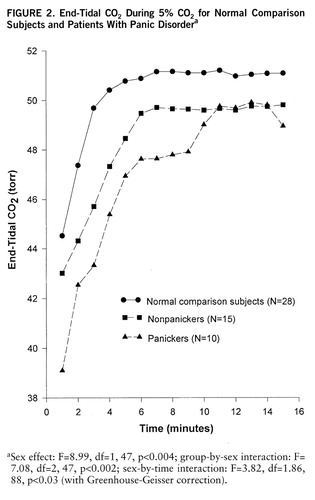
FIGURE 2. F IGURE 2End-Tidal CO2 During 5O2 f or N ormal Comparison Su bjects and Patients With Panic Disordera
aSex effect: F=8.99, df=1,47, p<0.004; group-by-sex interaction: F=7.08, df=2,47, p<0.002; sex-by-time interaction: F=3.82, df=1.86,88, p<0.03 (with Greenhouse-Geisser correction).
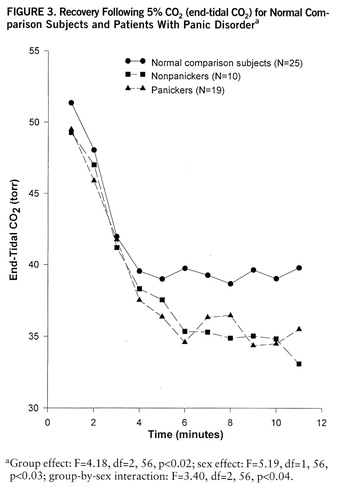
FIGURE 3. Recovery Following 5O2 (end-tidal CO2) for Normal Com p arison Subjects and Patients With Panic Disordera
aGroup effect: F=4.18, df=2,56, p<0.02; sex effect: F=5.19, df=1,56, p<0.03; group-by-sex interaction: F=3.40, df=2,56, p<0.04.
1. Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, Klein DF: Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry 1984; 141:857–861Link, Google Scholar
2. Woods SW, Charney DS, Lake J, Henninger GR: Carbon dioxide sensitivity in panic anxiety. Arch Gen Psychiatry 1986; 43:900–909Crossref, Medline, Google Scholar
3. Griez E, Lousberg H, van den Hout MA, Zandbergen J: CO2 vulnerability in panic disorder. Psychiatry Res 1987; 20:87–95Crossref, Medline, Google Scholar
4. Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, Kinney J, Klein DF: Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry 1988; 45:31–39Crossref, Medline, Google Scholar
5. Pain MCF, Biddle N, Tiller JWG: Panic disorder, the ventilatory response to carbon dioxide and respiratory variables. Psychosom Med 1988; 50:541–548Crossref, Medline, Google Scholar
6. Papp LA, Goetz R, Cole R, Klein DF, Jordan F, Liebowitz MR, Fyer AJ, Hollander E, Gorman JM: Hypersensitivity to carbon dioxide in panic disorder. Am J Psychiatry 1989; 146:779–781Link, Google Scholar
7. Sanderson WC, Rapee RM, Barlow DH: The influence of an illusion of control on panic attacks induced via inhalation of 5.5% carbon dioxide-enriched air. Arch Gen Psychiatry 1989; 46:157–162Crossref, Medline, Google Scholar
8. Fishman SM, Carr DB, Beckett A, Rosenbaum JF: Hypercapnic ventilatory response in patients with panic disorder before and after alprazolam treatment and in pre- and postmenstrual women. J Psychiatr Res 1994; 28:165–170Crossref, Medline, Google Scholar
9. Perna G, Gabriele A, Caldirola D, Bellodi L: Hypersensitivity to inhalation of carbon dioxide and panic attacks. Psychiatry Res 1995; 57:267–273Crossref, Medline, Google Scholar
10. Sanderson WC, Wetzler S: Five percent carbon dioxide challenge: valid analogue and marker of panic disorder? Biol Psychiatry 1990; 27:689–701Google Scholar
11. Papp LA, Klein DF, Martinez J, Schneier F, Cole R, Liebowitz MR, Hollander E, Fyer AJ, Jordan F, Gorman JM: The diagnostic and substance specificity of carbon-dioxide-induced panic. Am J Psychiatry 1993; 150:250–257Link, Google Scholar
12. Griez E, de Loof C, Pols H, Zandbergen J, Lousberg H: Specific sensitivity of patients with panic attacks to carbon dioxide inhalation. Psychiatry Res 1990; 31:193–199Crossref, Medline, Google Scholar
13. Perna G, Cocchi S, Bertani A, Arancio C, Bellodi L: Sensitivity to 35% CO2 in healthy first-degree relatives of patients with panic disorder: Am J Psychiatry 1995; 152:623–625Google Scholar
14. Papp LA, Coplan J, Gorman JM: Neurobiology of anxiety, in American Psychiatric Press Review of Psychiatry, vol 11. Edited by Tasman A, Riba MB. Washington, DC, American Psychiatric Press, 1992, pp 347–367Google Scholar
15. Klein DF: False suffocation alarms, spontaneous attacks, and related conditions. Arch Gen Psychiatry 1993; 50:306–317Crossref, Medline, Google Scholar
16. Gorman JM, Papp LA, Coplan JD, Martinez JM, Lennon S, Goetz RR, Ross D, Klein DF: Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am J Psychiatry 1994; 151:547–553Link, Google Scholar
17. Spitzer RL, Williams JBW, Gibbon M, First MB: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1988Google Scholar
18. Fyer AJ, Mannuzza SM, Klein DF, Endicott J: Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA). New York, New York State Psychiatric Institute, 1985Google Scholar
19. Read DJC: A clinical method for assessing the ventilatory response to carbon dioxide. Aust Ann Med 1967; 16:20–27Medline, Google Scholar
20. Papp LA, Martinez JM, Coplan JD, Gorman JM: Rebreathing tests in panic disorder. Biol Psychiatry 1995; 38:240–245Crossref, Medline, Google Scholar
21. Papp LA, Welkowitz L, Martinez JM, Klein DF, Browne S, Gorman JM: Instructional set does not alter outcome of respiratory challenges in panic disorder. Biol Psychiatry 1995; 38:826–830Crossref, Medline, Google Scholar
22. Waldau T, Oberg B, Larsen VH: Reliability of CO2 measurements from the airway by a pharyngeal catheter in unintubated, spontaneously breathing subjects. Acta Anaesthesiol Scand 1995; 39:637–642Crossref, Medline, Google Scholar
23. Dillon D, Gorman JM, Liebowitz MR, Fyer AJ, Klein DF: The measurement of lactate-induced panic and anxiety. Psychiatry Res 1987; 20:97–105Crossref, Medline, Google Scholar
24. Borg GAV: Psychosocial bases of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381Medline, Google Scholar
25. Klein DF, Ross DC: Reanalysis of the National Institute of Mental Health Treatment of Depression Collaborative Research Program General Effectiveness Report. Neuropsychopharmacology 1993; 8:241–251Crossref, Medline, Google Scholar
26. Papp LA, Martinez J, Klein DF, Liebowitz MR, Fyer AJ, Hollander E, Gorman JM: Arterial blood gas changes during lactate induced panic. Psychiatry Res 1989; 28:171–180Crossref, Medline, Google Scholar
27. Hornsveld H, Garssen B, van Spiegel P: Voluntary hyperventilation: the influence of duration and depth on the development of symptoms. Biol Psychol 1995; 40:299–312Crossref, Medline, Google Scholar
28. Maddock RJ, Carter CS: Hyperventilation-induced panic attacks in panic disorder with agoraphobia. Biol Psychiatry 1991; 29:843–854Crossref, Medline, Google Scholar
29. Roth WT, Margraf J, Ehlers A, Barr TC, Maddock RJ, Davies S, Agras WS: Stress test reactivity in panic disorder. Arch Gen Psychiatry 1992; 49:301–310Crossref, Medline, Google Scholar
30. Battaglia M, Perna G: The 35% CO2 challenge in panic disorder: optimization by receiver operating characteristic (ROC) analysis. J Psychiatr Res 1995; 29:111–119Crossref, Medline, Google Scholar
31. Liebowitz MR, Gorman JM, Fyer AJ, Dillon D, Appleby L, Levy G, Anderson S, Levitt M, Palij M, Davies SO, Klein DF: Lactate provocations of panic attacks, II: biochemical and physiologic findings. Arch Gen Psychiatry 1984; 41:764–770Crossref, Medline, Google Scholar
32. Bystritsky A, Shapiro D: Continuous physiological changes and subjective reports in panic patients: a preliminary methodological report. Biol Psychiatry 1992; 32:766–777Crossref, Medline, Google Scholar
33. Stein MB, Millar TW, Larsen DK, Kryger MH: Irregular breathing during sleep in patients with panic disorder. Am J Psychiatry 1995; 152:1168–1173Google Scholar
34. Martinez JM, Papp LA, Coplan JD, Anderson DE, Mueller CM, Klein DF, Gorman JM: Ambulatory monitoring of respiration in anxiety. Anxiety 1996; 2:296–302Medline, Google Scholar
35. Goetz RR, Gully R, Dillon DJ, Kahn J, Liebowitz MR, Fyer AJ, Klein DF, Gorman JM: Panic attacks during laboratory placebo procedures: physiology and symptomatology. Arch Gen Psychiatry 1993; 50:280–285Crossref, Medline, Google Scholar
36. Barlow DH: Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. New York, Guilford Press, 1988Google Scholar
37. Papp LA, Klein DF, Gorman JM: Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry 1993; 150:1149–1157Google Scholar



