Improving Medical and Psychiatric Outcomes Among Individuals With Bipolar Disorder: A Randomized Controlled Trial
Bipolar disorder is one of the world's ten most disabling conditions ( 1 ) and is associated with significant personal and societal costs ( 2 , 3 , 4 , 5 ). A substantial proportion of these health care costs have been attributed to co-occurring general medical conditions ( 6 , 7 ).
Persons diagnosed as having bipolar disorder also experience more co-occurring general medical conditions—especially cardiovascular disease and conditions related to metabolic syndrome, for example, diabetes—compared with those without bipolar disorder or other chronic mental illnesses ( 8 , 9 , 10 , 11 ). Cardiovascular disease is also the leading cause of morbidity and mortality among individuals with bipolar disorder ( 12 , 13 ). Among individuals with bipolar disorder, some of the most common medical conditions observed (for example, diabetes, hypertension, hyperlipidemia, and obesity) are also the leading risk factors for cardiovascular disease ( 14 ) and subsequent mortality ( 15 , 16 ). The increased use of second-generation antipsychotic medications as mood stabilizers has also increased the risk of diabetes and subsequent cardiovascular disease among these individuals ( 15 ).
Nonetheless, evidence suggests that cardiovascular disease risk factors and other comorbid medical conditions among individuals with bipolar disorder are inadequately treated ( 17 , 18 ). Many individuals with bipolar disorder may consider the mental health specialty setting their "home" site for care because of the focus on their mental illness ( 19 ), and many of them are unaware that they have medical conditions ( 20 ). Poor quality of medical care for persons with bipolar disorder has also been attributed to the organizational and professional separation of mental and physical health care ( 21 ) and to lack of effective self-management strategies that target cardiovascular disease-related risk factors, for example, diet and exercise ( 22 ).
Despite these gaps in health care, there are currently no treatment models specifically tailored to meet the medical and psychiatric needs of persons with bipolar disorder. Previously established treatment models primarily addressed medical care management issues, notably by establishing co-located general medical providers or treatment teams within mental health clinics ( 23 , 24 ). However, these approaches can be costly for smaller sites to implement and sustain over time. Interventions focused on health behavior change have shown some efficacy in clinical outcomes (for example, weight loss) and self-management ( 25 , 26 ), but the relative intensity of these interventions (for example, 24 weeks of educational sessions) may limit their dissemination into routine care settings. Moreover, these interventions have not been adapted to address the unique characteristics of bipolar disorder, notably binge eating, sedentary behavior, and treatment nonadherence associated with alternating manic and depressive symptoms ( 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ).
Alternatively, the chronic care model may provide the framework for improving medical care outcomes because it can be implemented by existing providers and adapted to address the unique behavioral issues of different conditions ( 27 ). The chronic care model focuses on both self-management and care management strategies and has been shown to improve management of chronic illnesses, including diabetes and depression ( 28 , 29 ). The chronic care model promotes self-management education, coordination of care across different providers via a care manager, and guideline implementation ( 27 ). This multifaceted approach is necessary for improving quality and outcomes, because guideline dissemination alone is ineffective in improving quality and outcomes of care ( 30 ). Moreover, behavioral interventions that constitute self-management approaches are most likely sustainable if coupled with ongoing care management ( 28 , 29 ). Care managers, who are usually nurses or social workers, can also assist clients in navigating across multiple providers—that is, medical and psychiatric providers.
The chronic care model has been recently shown to be effective and cost-neutral in improving mental health outcomes for persons with bipolar disorder, notably in the Department of Veterans Affairs Cooperative Studies Program (CSP 430) trial ( 31 , 32 ). However, no models based on the chronic care model have been developed for managing general medical conditions (for example, cardiovascular disease) among persons with bipolar disorder or other chronic mental disorders. Current interventions for bipolar disorder have shown less improvement in outcomes, notably physical health-related quality of life, among those with co-occurring cardiovascular disease-related conditions ( 31 , 33 ).
This article describes the outcomes of a randomized controlled trial of an intervention based on the chronic care model designed to improve medical as well as psychiatric outcomes among individuals with bipolar disorder: the bipolar disorder medical care model (BCM). We hypothesized that among persons with bipolar disorder, compared with those assigned to usual care, those assigned to BCM would have improved physical and mental health-related quality of life, improved overall functioning, and reduced bipolar disorder symptoms.
Methods
Study design
We conducted a prospective, randomized, single-site, single-blind intervention pilot study at a large Department of Veterans Affairs (VA) mental health facility to determine whether BCM care, compared with usual care, improved overall physical and mental health-related outcomes for persons with bipolar disorder. This randomized controlled trial was reviewed and approved by the VA Pittsburgh local institutional review board.
Participant selection and randomization
This was an effectiveness study in which we tested a model that would have potentially wide appeal to a broad segment of individuals with bipolar disorder in usual care settings, and hence, broad inclusion criteria were used. Adults were eligible if they were from the mental health clinic and had a clinician diagnosis of bipolar disorder (I, II, or not otherwise specified); had a current diagnosis of a cardiovascular disease-related risk factor, for example, hypertension, hyperlipidemia, obesity or a body mass index (BMI) over 25, or diabetes mellitus (diagnosis was determined by medical record review and was based on a previously established chart abstraction tool [34]); and had an assigned primary care provider at the facility.
Persons were excluded if they had unresolved substance intoxication or were experiencing withdrawal symptoms from substance abuse at the time of enrollment, were already enrolled in a mental health program with a mobile outreach component in which clinical caregivers delivered services in the community (for example, assertive community treatment), or were unwilling or unable to provide informed consent or comply with study requirements at the time of enrollment (for example, unwilling or unable because of serious illness or substantial functional limitations).
Eligible individuals were then randomly selected by the data analyst and were contacted by a survey coordinator. After complete description of the study was given to selected patients, those agreeing to participate provided written informed consent. Another data analyst not affiliated with this study randomly assigned enrolled participants to receive BCM or usual care.
Assessments and outcomes
Because our intervention was designed to improve physical health as well as mental health through a combination of self-management education and care management, our primary outcomes included physical and mental health-related quality of life. We chose physical and mental health-related quality of life to be our primary outcome, because the National Institutes of Health and World Health Organization have indicated that quality of life is a key indicator of health ( 35 , 36 ), and self-reported poor health-related quality of life in one study was associated with a two- to threefold increased risk in cardiovascular disease-related mortality ( 37 ). Secondary outcomes included global functioning and bipolar disorder symptoms. Data on demographic characteristics, access to care, and behavioral factors were also collected from the assessment. Assessments were self-completed and administered at baseline and at three and six months by a survey coordinator who was blinded to randomized group assignment.
Health-related quality of life was assessed with the 12-item Short-Form Health Survey (SF-12), which is a self-reported measure of general health functioning ( 38 ). The SF-12 generates two summary scores reflecting physical and mental health-related quality of life. Possible scores on each scale range from 0 to 100, with higher scores indicating better health. The SF-12 was found to be highly correlated with the SF-36 on summary scores for mental health-related quality of life (r=.91) and physical health-related quality of life (r=.92).
Global functioning was measured with the World Health Organization Disability Assessment Scale (WHO-DAS) ( 39 ), a 12-item assessment of the degree of functional impairment experienced within the past month. The assessment is based on a Likert scale regarding self-care (for example, bathing and dressing), mobility (for example, standing and walking), cognition (for example, remembering), social functioning (for example, conversing), and role functioning. A total score representing degree of impairment was generated by summing scores for each item. Possible scores on the WHO-DAS range from 0 to 48, with higher scores indicating more impairment.
Bipolar disorder symptoms were assessed with the Internal State Scale (ISS), a 15-item self-completed instrument that generates subscales reflecting depressive and manic symptoms and well-being ( 40 ). The ISS has a high test-retest reliability, and the subscales are highly correlated with clinician ratings of current episode (for example, manic and depressive). The person rates each symptom on an 11-point scale (scores range from 0, not at all, to 10, very much so).
Additional data on demographic characteristics and intermediate outcomes (that is, potential mediators) were collected with a self-completed survey. Potential intermediate outcomes are considered exploratory and included self-efficacy in managing chronic medical and psychiatric illness and access to general medical care. The Lorig Self-Management Efficacy in Chronic Disease 6-Item Scale ( 41 ) was used to rate individuals' self-efficacy in managing chronic medical illness (interrater reliability=.91). This scale includes six questions regarding chronic disease management, including symptom control, role function, emotional functioning, and communicating with physicians. The individual rates his or her perceived confidence in managing each domain (1, not at all confident, to 10, totally confident). A total possible score, ranging from 1 to 60, is generated by adding up each item. We assessed access to care with the Cunningham access survey, which includes a specific question regarding access to general medical providers ("I am able to get medical care whenever I need it"), with responses ranging from strongly agree, agree, uncertain, disagree, or strongly disagree ( 42 ). Problems accessing medical care were defined as responding "strongly disagree" or "disagree" to this statement.
We also ascertained current medication use, number of outpatient medical visits, and diagnoses of comorbid medical conditions. For comorbid medical conditions we chose the eight most common conditions seen in persons with bipolar disorder that contribute to poor functioning ( 34 )—diabetes (type 2), obesity or BMI over 25, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, coronary artery disease, chronic hepatitis, and osteoarthritis. Conditions were assessed by using a previously established assessment by Fenn and colleagues ( 34 ).
Intervention
The BCM core elements included self-management education, care management, and guideline implementation. Each component is described below.
Self-management. The BCM self-management program was adapted from the Life Goals Program, a group-based psychoeducational program for bipolar disorder used in the CSP 430 ( 31 , 43 , 44 ). The original Life Goals Program consisted of six one-hour sessions that combined psychoeducation regarding bipolar disorder with active discussions on symptom management and behavior change ( 44 ). For this intervention, we retained the Life Goals Program but combined sessions into three two-hour sessions and added content pertaining to cardiovascular disease-related risk factors related to mania and depression. We also added active discussions regarding the importance of managing chronic diseases, such as diabetes, hypertension, and hyperlipidemia ( Table 1 ). A fourth session was also added to provide guidance on practical strategies for improving diet and exercise habits (for example, making healthier choices at fast-food restaurants and finding opportunities to increase physical activity in everyday life) and engaging general medical providers (for example, having a list of questions to ask, having a list of current medications, and going through assertiveness training). Sessions were held on a weekly basis, and participants were given workbooks with additional information covered in the sessions. Although self-management sessions were delivered in group format, participants were allowed to make up sessions over the phone as long as they attended at least one of the sessions in person.
 |
Care management. The care management component was implemented at the completion of the self-management program and was based on approaches used in previous chronic care models for bipolar disorder ( 31 , 32 ). A nurse care manager served as a liaison between participants and providers regarding ongoing issues with medical and psychiatric care, referring urgent matters to providers and following up with providers and participants regarding medical visits. Through monthly phone calls, the care manager also reviewed with participants the lessons learned in the self-management sessions and followed up when appointments were missed. The care manager also documented the participants' clinical and recovery status over time.
Guideline implementation. Two one-hour in-house sessions for continuing medical education (CME) credit were held for mental health and general medical providers. These sessions addressed the unique risk factors for cardiovascular disease among individuals with bipolar disorder and recommendations for managing these risk factors. Guidelines for managing these risk factors were based on guidelines from the American Diabetes Association and American Heart Association ( 45 , 46 ). Pocket cards were also handed out as part of the educational sessions that summarized these recommendations for monitoring cardiovascular disease-related risk factors, monitoring psychotropic drug toxicity, and promoting good diet and exercise habits.
Fidelity
Fidelity to the intervention was promoted by using an effectiveness-oriented approach. That is, instead of tightly controlled treatment team meetings that are infeasible in routine care settings, we used the following techniques developed for CSP 430 ( 31 ) to maintain and monitor fidelity to BCM without burdening staff. First, the care manager was trained by study staff over a three-day period in August 2005. Second, we implemented fidelity measures based on data from care manager logs and chart review, including the number of self-management sessions completed and number of calls. Also assessed was the proportion of persons completing the four self-management sessions and the minimum six monthly phone contacts.
Usual care
Providers of participants randomly assigned to the usual care group received guidelines as well (for example, in-house sessions for CME credit), but they provided their choice of routine care. Participants in the usual care arm did not receive the self-management or care management.
Analyses
We assessed the effect of BCM care, compared with usual care, on changes in outcomes between baseline and three months and six months by using mixed-effects regression modeling, adding in the main effects of BCM, time, and the interaction between BCM and time. Two-tailed statistical tests were used for t statistics generated from the repeated-measures analyses, and p values of <.05 were considered statistically significant. Scores for SF-12, WHO-DAS, symptoms, and self-management efficacy were normally distributed and were not transformed. Participants with missing follow-up data were dropped from the analyses. Given that our secondary outcomes were exploratory, we report effect sizes (Cohen's d) along with the main results ( 47 ). For remaining intermediate outcomes (access to care and utilization), we examined participants' charts to compare the BCM and usual care groups for the percentage of participants reporting problems accessing medical care at six months and the percentage reporting no medical visits.
Results
Between March and June 2006, 79 of 299 patients from the clinic were identified as being eligible for the BCM study. Of the 79 patients, 61 were randomly selected to be contacted for participation. Of the 61, all completed the three-month assessment, and three were lost to follow-up at six months, leaving 58 participants who completed the baseline survey and the three- and six-month surveys (27 in the BCM group and 31 from the usual care group completed all three surveys).
Participant characteristics are presented in Table 2 . The mean age of the overall sample was 55.3±8.4 years (range 30–73 years). Overall, five (9%) were female, 52 (90%) were white, and six (10%) were African American. The demographic characteristics of our participants were similar to those of all veterans in care who were diagnosed as having bipolar disorder (that is, mean age of 52 years, 13% women, and 9% African American) ( 48 ). The most common cardiovascular disease-related diagnosis was obesity or a BMI over 25 (N=52, or 90%), followed by hypertension (N=47, or 81%), hyperlipidemia (N=44, or 76%), and diabetes (N=19, or 33%). Almost half (N=28, or 48%) had three or more of the major comorbid conditions out of the eight queried for. There were no significant differences in other factors, including diagnoses or medication use between the BCM and usual care groups except for lamotrigine use ( Table 2 ).
 |
As shown in Table 3 , significant differences in changes in SF-12 scores for physical health-related quality of life were observed from baseline to six months between the two groups (change in scores: .8±6.7 for the BCM group versus –.6±6.6 for the usual care group) ( β =2.50; t=2.01, df=173, p=.04). That is, a significant improvement in physical health-related quality-of-life scores was observed in the BCM group, and a significant decline in physical health-related quality of life was seen in the usual care group. Although the BCM group had a positive change in scores over the study period for SF-12 mental health-related quality of life (indicating improved mental health), this finding was not statistically significant (change in scores: 1.0±7.7 for the BCM group versus –.9±6.6 for the usual care group). No significant differences between the two groups were found over the study period in changes in scores for overall functioning or bipolar disorder symptoms ( Table 3 ).
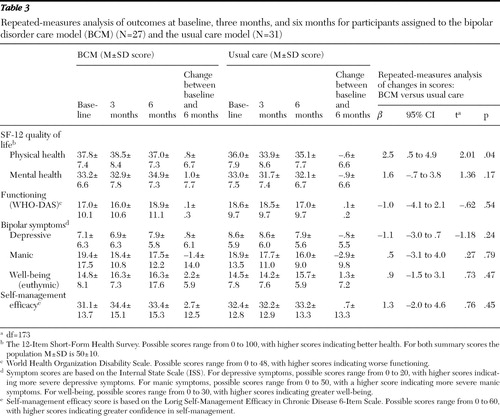 |
The effect size for observed changes in physical health-related quality-of-life scores was moderate (Cohen's d=.32). The effect size for observed changes in mental health-related quality-of-life scores was slightly smaller (Cohen's d=.20). Smaller effects were observed for changes in global functioning (Cohen's d=.18) and bipolar symptom change scores for depression (Cohen's d=.17), mania (Cohen's d=.10), and well-being (Cohen's d=.10).
The mean change in the Lorig self-management efficacy score was higher for the BCM group than for the usual care group, although the finding was not significant ( Table 3 ). Moderate effects were observed for changes in self-management efficacy (Cohen's d=.26). For other intermediate outcomes, seven (23%) in the usual care group reported problems accessing medical care at the six-month follow-up, and only four (13%) in the BCM group reported such problems at the six-month follow-up, although this finding was not statistically significant. Only a small percentage of participants had no medical visits between baseline and the six-month follow-up: one (4%) participant in the BCM group and three (10%) in the usual care group had no outpatient medical visits, although the difference between the groups was not significant.
The mean number of completed self-management sessions (group or phone) was 3.7±.7 (range of two to four), and 23 (85%) of the participants in the BCM group completed all four self-management sessions. The mean number of care management calls completed over the six-month intervention period for the BCM group was 10.6±4.8 (range of three to 18), and 22 (73%) from the BCM group completed the minimum of six care management contacts.
Discussion
To our knowledge, this is the first randomized controlled trial of an intervention designed to improve both medical and psychiatric outcomes for persons with bipolar disorder. BCM is a manual-based model designed to be implemented by existing health care providers in a routine care setting. There have been few treatment models specifically focused on improving medical outcomes for persons with chronic mental illness, and none have been tailored to address the unique concerns of individuals with bipolar disorder. BCM addresses this gap by combining self-management education tailored to risk factor management of cardiovascular disease within the context of bipolar disorder symptoms, care management to enhance linkages with general medical providers, and dissemination of practice guidelines focused on the medical risk factors in bipolar disorder.
We found significant differences in changes in scores for physical health-related quality of life over the study period between the BCM and usual care groups, even within a relatively short time period (six months). The significant treatment effect on physical health-related quality of life appears to be driven by both an improvement in score in the BCM group and a decline in score for the usual care group. Perhaps BCM slowed the decline in physical health-related quality of life among participants randomly assigned to receive BCM care, which could be a result of BCM's focus on general medical provider engagement.
On the basis of observed effect sizes, we found that BCM might have led to a positive change in mental health-related quality of life among persons enrolled in BCM, compared with usual care; however, perhaps because of the small sample, this finding was not statistically significant. This lack of significant effect on mental health-related quality of life was in contrast to previous interventions based on the chronic care model for bipolar disorder. Notably, in the CSP 430 improved scores in mental health-related quality of life were observed over a three-year period among persons randomly assigned to the CSP 430 intervention group, compared with usual care (31,32). Lack of a significant change in mental health-related quality of life in our study may have been partly due to our selection criteria—that is, all enrollees had at least one cardiovascular disease-related condition, compared with only one-third of the CSP 430 sample ( 31 ). Because of these comorbid medical conditions, perhaps in the short term, participants in our study were more sensitive to declines in physical health-related quality of life than mental health-related quality of life.
Nonetheless, the effect size we observed in physical health-related quality of life using the SF-12 has been found in different study samples to be associated with changes in cardiovascular disease-related measures among medically ill individuals ( 49 ). In addition our observed effect size (.20) in mental health-related quality of life was similar to effect sizes observed in the CSP 430 trial ( 31 ). Modest improvements in clinical outcomes are often observed in effectiveness-oriented trials, and even small differences in clinical outcomes can be associated with significant changes in hospital admission rates among persons with mood disorders ( 50 ). Additional effectiveness intervention trials also demonstrated that even modest changes (5% to 17%) in quality-of-life measures can have clinically meaningful benefits ( 51 ).
We found little effect of BCM care on our secondary outcomes, notably depressive or manic symptoms. This may have been attributed to our relatively short follow-up period (six months), compared with the CSP 430 trial (three years), because manic and depressive episodes may not occur as frequently. Furthermore, we found little change in our exploratory outcomes, notably in outpatient medical utilization and problems in accessing care. Although this may suggest that BCM had little impact on these factors, the limited sample size precluded us from fully exploring these potential mediators of treatment effect on outcome
Treatment fidelity findings suggest that BCM was acceptable to participants and was overall a feasible intervention. BCM fidelity was on par with fidelity to the chronic care model for the CSP 430 trial (for example, 80% completed self-management sessions in the CSP) and was greater than fidelity for other bipolar disorder psychosocial interventions reported elsewhere (for example, 50% to 60%) ( 52 ). BCM was designed to minimize participant burden, relying on a limited number of self-management sessions and ongoing telephone care management that could be implemented by existing providers. Thus BCM has the potential to be more easily adopted in settings with relatively few resources and to be appropriate for persons with bipolar disorder who are not functioning well enough to make extensive commitments to long-term psychosocial treatment.
There are limitations to this study that warrant consideration, notably the small sample, brief follow-up period, and reliance on self-reported outcomes. These limitations suggest the need for caution in interpreting both the positive finding in physical health-related quality of life and the nonsignificant finding for mental health-related quality of life, which may reflect inadequate power and a relatively brief study duration. The lack of a strong effect of the BCM on depressive or manic symptoms may be partly attributable to the relatively short follow-up period (six months). In addition, self-reported symptom assessments, as opposed to standardized psychiatric rating scales, may not be sensitive enough to detect changes in mood symptoms that can be detected by clinician-based assessments, and the self-reporting of symptoms might have been influenced by the fact that participants were not blinded to treatment assignment.
Moreover, we did not assess more objective outcomes, including physiologic measures related to cardiovascular disease risk. A more definitive study of BCM is currently under way that will determine whether BCM, compared with usual care, leads to reduced cardiovascular disease risk factors—for example, lower blood pressure and cholesterol and weight loss. However, we chose to use self-reported measures in order to minimize respondent burden and to have this effectiveness study reflect as closely as possible the means by which it would likely be implemented in routine practice—that is, most community-based practices do not have the personnel or time to conduct formal assessments of participants. We were also unable to collect additional information on treatment model fidelity, such as the extent to which self-management or care management sessions covered specified material. To minimize provider burden, we chose minimally invasive fidelity measures used in similar bipolar disorder intervention trials ( 31 ). Furthermore, determining whether BCM's impact on outcomes was mediated by model fidelity was beyond the scope of the study presented here.
Finally, the intervention was limited to a single VA site, and hence, study findings may not generalize to populations outside of the VA setting or to other VAs. The sociodemographic characteristics of the VA population in general differ from individuals served in non-VA settings. Veterans are on average older and more indigent than those served by non-VA mental health treatment programs ( 37 ). Furthermore, the VA system is more highly integrated than many non-VA systems (for example, in the VA system medical and psychiatric services are often provided in the same location and use a common electronic medical record system).
Conclusions
Overall, compared with usual care, BCM may have positively affected health outcomes among persons with bipolar disorder, notably by slowing the decline in physical health-related quality of life. Although this pilot study is limited by a small sample, brief follow-up period, and reliance on self-reported outcomes, our preliminary results suggest that BCM is a potentially promising and feasible treatment model for individuals with bipolar disorder. Further studies are needed to determine whether BCM leads to long-term positive changes in physical and mental health-related quality of life, as well as reduced cardiovascular disease risk (for example, through weight loss and lower blood pressure and cholesterol). Ultimately, BCM may be potentially attractive as an integrated care model for sites that cannot afford co-locating providers from different specialties. The model's emphasis on self-management and coordination of care is also likely to represent an important enhancement to many systems of care for individuals with complex comorbid medical and psychiatric conditions.
Acknowledgments and disclosures
This research was supported by the Department of Veterans Affairs and the Veterans Health Administration and by an investigator-initiated trial grant from Eli Lilly and Company.
The authors report no competing interests.
1. Murray CJ, Lopez AD: Evidence-based health policy—Global Burden of Disease Study. Science 274:740–743, 1996Google Scholar
2. Bauer M, Unützer J, Pincus HA, et al: Bipolar disorder. Mental Health Services Research 4:225–229, 2002Google Scholar
3. Bauer MS, Kirk G, Gavin C, et al: Correlates of functional and economic outcome in bipolar disorder: a prospective study. Journal of Affective Disorders 65:231–241, 2001Google Scholar
4. Kleinman L, Lowin A, Flood E, et al: Costs of bipolar disorder. Pharmacoeconomics 21:601–622, 2003Google Scholar
5. Bryant-Comstock L, Stender M, Devercelli G: Health care utilization and costs among privately insured patients with bipolar I disorder. Bipolar Disorders 4:398–405, 2002Google Scholar
6. Peele PB, Xu Y, Kupfer DJ: Insurance expenditures on bipolar disorder: clinical and parity implications. American Journal of Psychiatry 160:1286–1290, 2003Google Scholar
7. Simon GE, Unützer J: Health care utilization and costs among patients treated for bipolar disorder in an insured population. Psychiatric Services 50:1303–1308, 1999Google Scholar
8. Kilbourne AM, Brar J, Drayer RA, et al: Cardiovascular disease and metabolic risk factors in patients with schizophrenia, schizoaffective disorder, and bipolar disorder. Psychosomatics 48:412–417, 2007Google Scholar
9. Kilbourne AM, Cornelius J, Pincus HA, et al: Burden of general medical comorbidities among individuals with bipolar disorder. Bipolar Disorders 6:368–373, 2004Google Scholar
10. Cassidy F, Ahearn E, Carroll BJ: Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. American Journal of Psychiatry 156:1417–1420, 1999Google Scholar
11. Kilbourne AM, Cornelius JR, Han X, et al: General-medical conditions in older patients with serious mental illness. American Journal of Geriatric Psychiatry 13:250–254, 2005Google Scholar
12. Hennekens C: Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. Journal of Clinical Psychiatry 68(suppl 4):4–7, 2007Google Scholar
13. Angst F, Stassen HH, Clayton PJ, et al: Mortality of patients with mood disorders: follow-up over 34–38 years. Journal of Affective Disorders 68:167–181, 2002Google Scholar
14. Khot UN, Khot MB, Bajzer CT, et al: Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 290:898–904, 2003Google Scholar
15. Newcomer JW: Metabolic disturbances associated with antipsychotic use. Journal of Clinical Psychiatry 27:3–4, 2001Google Scholar
16. Kupfer DJ: The increasing medical burden in bipolar disorder. JAMA 293:2528–2530, 2005Google Scholar
17. Kilbourne AM, Post EP, Bauer MS, et al: Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. Journal of Affective Disorders 102:145–151, 2007Google Scholar
18. Druss BG, Bradford WD, Rosenheck RA, et al: Quality of medical care and excess mortality in older patients with mental disorders. Archives of General Psychiatry 58:565–572, 2001Google Scholar
19. Druss BG, Rosenheck RA: Locus of mental health treatment in an integrated service system. Psychiatric Services 51:890–892, 2000Google Scholar
20. Kilbourne AM, McCarthy J, Welsh D, et al: Recognition of co-occurring medical conditions among patients with serious mental illness. Journal of Nervous and Mental Disease 194:598–602, 2006Google Scholar
21. Horvitz-Lennon M, Kilbourne AM, Pincus HA: From silos to bridges: meeting the general health care needs of adults with severe mental illnesses. Health Affairs 25:659–669, 2006Google Scholar
22. Kilbourne AM, Rofey D, McCarthy J, et al: Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disorders 9:443–452, 2007Google Scholar
23. Lehman AF, Goldman HH, Dixon LB, et al: Evidence-Based Mental Health Treatments and Services: Examples to Inform Public Policy. New York, Milbank Memorial Fund, 2004. Available at www.milbank.org/reports/2004lehman/2004lehman.htmlGoogle Scholar
24. Druss BG, Rohrbaugh RM, Levinson CM, et al: Integrated medical care for patients with serious psychiatric illness: a randomized trial. Archives of General Psychiatry 58:861–868, 2001Google Scholar
25. McKibbin CL, Patterson TL, Norman G, et al: A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophrenia Research 86:36–44, 2006Google Scholar
26. Weber M, Wyne K: A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophrenia Research 83:95–101, 2006Google Scholar
27. Wagner EH, Austin BT, Von Korff M: Organizing care for patients with chronic illness. Milbank Quarterly 74:511–544, 1996Google Scholar
28. Badamgarav E, Weingarten SR, Henning JM, et al: Effectiveness of disease management programs in depression: a systematic review. American Journal of Psychiatry 160:2080–2090, 2003Google Scholar
29. Weingarten SR, Henning JM, Badamgarav E, et al: Interventions used in disease management programmes for patients with chronic illness—which ones work? Meta-analysis of published reports. British Medical Journal 325:925, 2002Google Scholar
30. Bodenheimer T, Wagner EH, Grumbach K: Improving primary care for patients with chronic illness. JAMA 288:1775–1779, 2002Google Scholar
31. Bauer MS, McBride L, Williford WO, et al: Collaborative care for bipolar disorder: part II. impact on clinical outcome, function, and costs. Psychiatric Services 57:937–945, 2006Google Scholar
32. Simon GE, Ludman EJ, Bauer MS, et al: Long-term effectiveness and cost of a systematic care program for bipolar disorder. Archives of General Psychiatry 63:500–508, 2006Google Scholar
33. Kilbourne AM, Biswas K, Sajatovic M, et al: Is the chronic care model effective for complex patients? Analyzing moderators of treatment effect. Journal of Mental Health Policy and Economics 10:S70, 2007Google Scholar
34. Fenn HH, Bauer MS, Alschuler L, et al: Medical comorbidity and health-related quality of life in bipolar disorder across the adult age span. Journal of Affective Disorders 86:47–60, 2005Google Scholar
35. Hays RD, Hahn H, Marshall G: Use of the SF-36 and other health-related quality of life measures to assess persons with disabilities. Archives of Physical Medicine and Rehabilitation 83:S4–S9, 2002Google Scholar
36. Feeny DH, Torrance GW: Incorporating utility-based quality-of-life assessment measures in clinical trials: two examples. Medical Care 27:S190–S204, 1989Google Scholar
37. Bosworth HB, Siegler IC, Brummett BH, et al: The association between self-rated health and mortality in a well-characterized sample of coronary artery disease patients. Medical Care 37:1226–1236, 1999Google Scholar
38. Ware J Jr, Kosinski M, Keller SD: A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 34:220–233, 1996Google Scholar
39. Rehm J, Ustun T, Saxena S, et al: On the development and psychometric testing of the WHO screening instrument to assess disablement in the general population. International Journal of Methods in Psychiatric Research 8:110–122, 1999Google Scholar
40. Bauer MS, Vojta C, Kinosian B, et al: The Internal State Scale: replication of its discriminating abilities in a multisite, public sector sample. Bipolar Disorders 2:340–346, 2000Google Scholar
41. Lorig KR, Sobel DS, Ritter PL, et al: Effect of a self-management program for patients with chronic disease. Effective Clinical Practice 4:256–262, 2001Google Scholar
42. Cunningham WE, Hays RD, Williams KW, et al: Access to medical care and health-related quality of life for low-income persons with symptomatic human immunodeficiency virus. Medical Care 33:739–754, 1995Google Scholar
43. Kilbourne AM, Nossek A, Drill L, et al: Service delivery in older patients with bipolar disorder: a review and development of a medical care model. Bipolar Disorders, 2008Google Scholar
44. Bauer MS, McBride L: Structured Group Psychotherapy for Bipolar Disorder: The Life Goals Program, 2nd ed. New York, Springer, 2003Google Scholar
45. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al: Consensus development conference on antipsychotic drugs and obesity and diabetes. Journal of Clinical Psychiatry 65:267–272, 2004Google Scholar
46. Pearson TA, Blair SN, Daniels SR, et al: AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases: American Heart Association Science Advisory and Coordinating Committee. Circulation 106:388–389, 2002Google Scholar
47. Cohen J: A power primer. Psychological Bulletin 112:155–159, 1992Google Scholar
48. Blow F, McCarthy JM, Valenstein M, et al: Care in the VHA for Veterans With Psychosis: FY2005. First Annual Report on Veterans With Psychoses. Ann Arbor, Mich, Department of Veterans Affairs National Serious Mental Illness Treatment Research and Evaluation Center, Nov 2005Google Scholar
49. Wyrwich KW, Nienaber NA, Tierney WM, et al: Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Medical Care 37:469–478, 1999Google Scholar
50. Kessing LV, Andersen PK: Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? Journal of Neurology, Neurosurgery, and Psychiatry 75:1662–1666, 2004Google Scholar
51. Nierenberg AA, Ostacher MJ, Calabrese JR, et al: Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. American Journal of Psychiatry 163:210–216, 2006Google Scholar
52. Miklowitz DJ, Otto MW, Frank E, et al: Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Archives of General Psychiatry 64:419–426, 2007Google Scholar



