Project Harmony: A Meta-Analysis With Individual Patient Data on Behavioral and Pharmacologic Trials for Comorbid Posttraumatic Stress and Alcohol or Other Drug Use Disorders
Abstract
Objective:
Treatment efficacy for co-occurring posttraumatic stress disorder (PTSD) and substance use disorders is well established, yet direct evidence for comparative effectiveness across treatments is lacking. The present study compared the effectiveness of several behavioral and pharmacological therapies for adults with co-occurring PTSD and alcohol or other drug use disorders.
Methods:
A systematic search of PsycINFO, MEDLINE, and ClinicalTrials.gov was conducted through December 2020 for trials targeting PTSD, alcohol or other drug use disorders, or both disorders (36 studies, N=4,046). Primary outcomes were severity scores for PTSD, alcohol use, and drug use, estimated via moderated nonlinear factor analysis. Propensity score weight–adjusted multilevel models were used. Model-predicted effect sizes were estimated for each treatment, and comparative effect sizes for each active arm against treatment as usual, at end of treatment and at 12-month follow-up.
Results:
Compared with treatment as usual, combining trauma-focused therapy and pharmacotherapy for substance use disorders showed the largest comparative effect sizes for PTSD severity (d=−0.92, 95% CI=−1.57, −0.30) and alcohol use severity (d=−1.10, 95% CI=−1.54, −0.68) at end of treatment. Other treatments with large comparative effect sizes included pharmacotherapies for alcohol or other drug use disorders, trauma-focused integrated therapies, and trauma-focused nonintegrated therapies. Reductions in outcomes for PTSD symptoms and alcohol use were observed for nearly all treatments.
Conclusions:
The findings provide support for treating comorbid PTSD and substance use disorders using a variety of approaches, with alcohol-targeted pharmacotherapies and trauma-focused behavioral therapies as a combination of treatments that lead to early and sustained improvements in PTSD and alcohol use severity. Further treatment development is indicated for combining behavioral and pharmacological treatments for synergized impact and understanding the mechanisms of action and conditions under which each treatment type is optimized.
Comorbid posttraumatic stress disorder (PTSD) and substance use disorder is globally prevalent and costly. PTSD affects between 1.3% and 8.8% of the global population, depending on locale (1). Rates of substance use disorder are similarly high, with approximately 269 million people using drugs per year worldwide—a number that has increased by ∼30% over 10 years—and some 35 million people suffering from a drug use disorder (2). Nearly three decades of published research has documented the devastating impact of comorbid PTSD and substance use disorder: longer hospital stays, lower treatment adherence, poorer treatment outcomes than either disorder alone, worse functioning, and higher suicide risk (3), all of which are linked to increased mortality (4–6).
The efficacy of various pharmacotherapies and behavioral treatments in addressing the complex comorbidity of PTSD and substance use disorder has increasingly been established, but these treatments vary in their approaches. The behavioral treatments in particular vary in the extent to which they focus on a single disorder (e.g., relapse prevention for substance use disorder [7] and prolonged exposure for PTSD [8]) or integrate PTSD and substance use treatment foci (e.g., Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure [COPE] [9]). Within PTSD-focused and integrated PTSD and substance use treatments, interventions vary with respect to whether they focus on trauma-related memories and content (trauma-focused treatments; e.g., COPE) or do not (non-trauma-focused treatment; e.g., Seeking Safety therapy). While behavioral treatments have been shown to be effective for comorbid PTSD and substance use disorders, pharmacotherapies have typically targeted either alcohol use or PTSD, although there are some medications (such as sertraline) that may target both. Indeed, evidence from basic science research points to several neurobiological abnormalities that are common to both sets of disorders, including disruptions in dopamine, norepinephrine, and serotonin systems (10, 11).
The fact that most of these treatment approaches have rarely been compared with each other (see reference 12 for an exception) has made it difficult to determine best practices in treating comorbid PTSD and alcohol or other drug use disorders. Furthermore, data syntheses, such as systematic reviews and meta-analyses, are marked by small sample sizes, high attrition, and lack of demographic diversity (6, 13–20; D.A. Hien et al., 2021, unpublished). Hence, researchers and policy makers have been unable to make recommendations for practitioners about comparative effectiveness across treatment modalities or their combinations.
To provide definitive guidance on the most effective treatment strategies for comorbid PTSD and substance use disorder, we integrated raw individual patient data from 36 representative randomized controlled trials using a “virtual clinical trial” (13) model combining integrative data analysis, propensity score weighting, and meta-analysis of individual patient data frameworks to accomplish what no single, multisite, or traditional meta-analysis could accomplish given the real-world clinical challenges of studying this common, yet underserved population. In our focal analysis of data for the Project Harmony Virtual Clinical Trials study, we conducted a comparative effectiveness analysis of each of eight active treatment classifications and one pharmacotherapy treatment classification, compared with behavioral treatment as usual on outcomes for PTSD and alcohol or other drug use at end of treatment and at an estimated 12-month follow-up.
Methods
The methods are described in detail in our study protocol (13).
Study Design, Study Selection, and Participants
The analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-ANALYSIS (PRISMA) Individual Patient Data Statement (21). Criteria for study inclusion were 1) randomized clinical trial of a psychological and/or pharmacological intervention targeting either PTSD symptoms, alcohol or other drug use disorder symptoms, or both; 2) pre- and posttreatment collection of PTSD and substance use outcomes; and 3) an adult sample (age 18 and above) with a current diagnosis of full or subthreshold PTSD according to DSM-IV or DSM-5 criteria (subthreshold PTSD was defined as meeting PTSD criteria A, B, E, F, and either C or D [22]) and a current substance use disorder diagnosis (alcohol or other drug abuse or dependence in DSM-IV, and substance use disorder in DSM-5). Upon confirmation of study eligibility, data were requested from authors of eligible studies (i.e., the participating principal investigators). The present study was reviewed and approved by the institutional review boards of RTI International and Rutgers University. Permission to use data from 49 studies was requested, and we acquired data from 36 trials (N=4,046; see Table 1, the PRISMA chart in Figure 1, and the online supplement).
| Study and Reference | Population | Intervention(s) | Number of Sessions | Number Receiving Intervention(s) | Control Condition | Number in Control Group | Outcome Measure for PTSD | Outcome Measure for Substance Use | Treatment Completion Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Back et al. (49) | Veteran | N-Acetylcysteine | 8 | 13 | Placebo medication | 14 | CAPS, PCL | VAS | 70 |
| Back et al. (50) | Veteran | COPE | 12 | 54 | RP | 27 | CAPS, PCL | TLFB | 61 |
| Boden et al. (51) | Veteran | SS | 24 | 49 | TAU | 49 | CAPS, IES | ASI, CRI | 93 |
| Hien et al. (52) | Civilian | SS, RP | 24 | 45, 47 | Community care | 34 | CAPS | SUI | 76 |
| Hien et al. (11) | Civilian | SS + sertraline | 12 | 32 | SS | 37 | CAPS | ASI, SUI | 58 |
| Hien et al. (53) | Civilian | SS | 12 | 176 | Women’s health | 177 | CAPS | TLFB | 56 |
| Ruglass et al. (54) | Civilian | COPE, RP | 12 | 37, 42 | Community care | 28 | CAPS, MPSS | ASI, SUI, SCID | 50 |
| McDevitt-Murphy et al. (55) | Veteran | MI | 2 | 35 | Feedback only | 33 | CAPS | TLFB, AUDIT, DrInC | NR |
| McGovern et al. (29) | Civilian | I-CBT | 8–12 | 32 | Individual addiction counseling | 21 | CAPS, PCL | TLFB, ASI | 49 |
| McGovern et al. (56) | Civilian | I-CBT | 8–12 | 94 | TAU | 95 | CAPS, PCL | TLFB, ASI | NR |
| Mills et al. (57) | Civilian | COPE | 13 | 55 | TAU | 48 | CAPS | TLFB, CIDI, OTI | 13 |
| Myers et al. (58) | Civilian | SS | 25 | 22 | Twelve-step | 18 | CAPS | TLFB | 45 |
| Norman et al. (12) | Veteran | COPE | Unknown | 63 | SS | 56 | CAPS | TLFB | 100 |
| Haller et al. (59) | Veteran | Modified CPT | 12 | 63 | Integrated CBT | 63 | PCL | TLFB | NR |
| Petrakis et al. (60) | Civilian | Prazosin | 13 | 50 | Placebo medication | 46 | CAPS | TLFB | 78 |
| Petrakis et al. (39) | Veteran | Zonisamide | Unknown | 18 | CPT | 6 | SCID, CAPS, PCL | TLFB, OCDS | 70 |
| Saladin (unpublished) | Civilian | Propranolol | 1 | 21 | Placebo medication | 23 | CAPS, MINI | TLFB | 68 |
| Sannibale et al. (61) | Civilian | I-CBT | 12 | 33 | CBT for alcohol support | 29 | CAPS | TLFB | 73 |
| Schacht et al. (62) | Civilian | PE + contingency management | 14 | 28 | PE | 30 | TLEQ, MPSS-R | UDS, ASI | 19 |
| Schäfer et al. (63) | Civilian | SS | 16 | 111 | RP | 115 | PDS, CTQ, SAEs | SCID, ASI-Lite | 37 |
| Sonne (unpublished) | Civilian | Paroxetine | 12 | 13 | Placebo medication | 12 | CAPS, DTS, TOP-8 | TLFB | 44 |
| Zlotnick et al. (31) | Prison | SS | 12 | 10 | Residential TAU | 10 | CAPS | ASI | 83 |
| Zlotnick et al. (64) | Prison | SS | 20 | 27 | Residential TAU | 22 | CAPS | ASI, TLFB | 92 |
| Vujanovic et al. (32) | Civilian | TIPSS (I-CBT) | 12 | 21 | CBT for substance use disorder | 32 | CAPS, PCL | UDS, TLFB | |
| Foa et al. (38) | Veteran and civilian | PE + naltrexone, PE, naltrexone | 24 | 40, 40, 42 | TAU | 43 | PSSI | TLFB, PACS | 64 |
| Brief et al. (65) | Veteran | VetChange | 8 | 404 | Waiting list | 196 | PCL | QDS, AUDIT | 36 |
| van Dam et al. (66) | Civilian | CBT + SWT | 15 | 19 | CBT for substance use disorder | 15 | PDS, SCID | TLFB, InDUC | 53 |
| Rosenthal (67) | Veteran | PE + VR | 10 | 19 | PE | 19 | CAPS | TLFB | 53 |
| Batki et al. (68) | Veteran | Topiramate | 12 | 14 | Placebo medication | 16 | CAPS, PCL | TLFB, OCDS | 93 |
| Brady et al. (69) | Civilian | Sertraline | 12 | 49 | Placebo medication | 45 | CAPS, SCID | TLFB, OCDS, ASI | NR |
| Frisman et al. (70) | Civilian | TARGET | 8–9 | 141 | Trauma-sensitive TAU | 72 | CAPS | GAIN | 27 |
| Petrakis et al. (71) | Veteran and civilian | Desipramine + naltrexone or paroxetine + naltrexone | 12 | 22, 22 | Desipramine + placebo or paroxetine + placebo | 24, 20 | CAPS | TLFB | 70 |
| Perez-Dandieu and Tapia (30) | Civilian | EMDR | 8 | 6 | TAU | 6 | PCL | ASI | NR |
| Stappenbeck et al. (72) | Veteran and civilian | Cognitive restructuring or experiential acceptance | 4 | 31, 29 | TAU | 20 | SCID, PSSI, PCL | TLFB | NR |
| Simpson et al. (73) | Veteran | Prazosin | 6 | 15 | Placebo medication | 15 | PSSI, PCL | TLFB, PACS | NR |
| Kehle-Forbes et al. (74) | Veteran | Integrated MET + PE | 16 | 88 | Phased MET + PE | 95 | SCID, PSS, PCL | TLFB | NR |
TABLE 1. Characteristics of the 36 studies on treatment of comorbid PTSD and substance use disorders that were included in the analysis (N=4,046)a
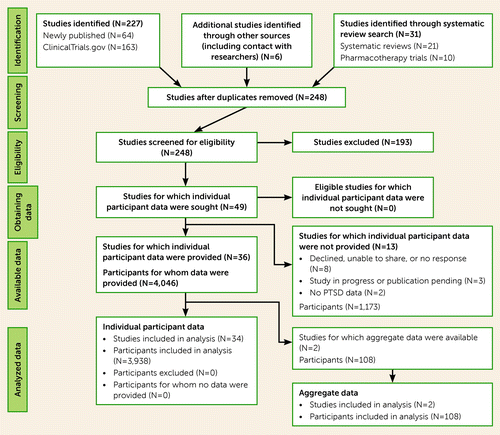
FIGURE 1. PRISMA study selection flow diagram for a meta-analysis of comorbid PTSD and substance use disordersa
a For inclusion criteria, see the Methods section in the text.
Coding of treatment classifications.
Treatment conditions, based on groupings of each within-study treatment arm, were defined and coded according to whether the treatment was 1) trauma focused, 2) an integrated PTSD and alcohol or other drug treatment, 3) a pharmacotherapy that targeted PTSD (e.g., sertraline, paroxetine), 4) a pharmacotherapy that targeted alcohol or other drug use (e.g., methadone, naltrexone), 5) solely a behavioral treatment for alcohol or other drug use disorder, or 6) a nonmanualized, community-based treatment (i.e., treatment as usual). These groupings were not mutually exclusive.
Individual-level covariates.
The following variables were examined as covariates: gender, age, race/ethnicity, education level, population type (civilian, veteran, incarcerated), treatment dosage (proportion of available sessions attended or study medication doses taken), baseline diagnosis of current major depressive disorder, and concomitant non-study psychotropic medication use at baseline. Because treatments were grouped across studies, the original within-study randomization structure no longer holds, leading to possible relations between covariates and the “new” treatment classifications, which required mitigation using propensity score weighting (13, 23).
Primary Outcomes of Interest
Latent PTSD severity.
A total of 42 PTSD indicators (21 symptoms from a clinical interview and 21 self-report symptoms) were harmonized across the studies that had item-level PTSD data and were used to formulate the indicators of a 42-item latent PTSD construct estimated under the moderated nonlinear factor analysis (MNLFA) framework (24, 25) (considerable detail on MNLFA scale score estimation and item parameters is provided in the online supplement). The 42 symptoms include a mix of the 16 PTSD symptoms that are common to both the DSM-IV and DSM-5 diagnostic criteria, the one symptom that is unique to DSM-IV (sense of foreshortened future), and the four symptoms that are unique to DSM-5.
Latent substance use severity.
Latent substance use severity scores were also estimated under MNLFA. Binary indicators of any past-30-day use of the following substances were used to support a six-indicator latent substance use variable: cocaine, heroin, opioids (excluding heroin), sedatives, other stimulants (excluding cocaine), and hallucinogens.
Latent alcohol use.
A latent alcohol use variable was estimated under MNLFA using two indicators: number of days of alcohol use in the past 30 days and any alcohol use to intoxication in the past 30 days.
Results
Sample Characteristics
The mean age in the sample was 39.0 years (SD=11.2); 53% of participants were male, 65% were White, 25% were African American, and 7% were Hispanic. Additional descriptive statistics (overall and treatment class–specific) are provided in the online supplement. To ensure raw data integrity, descriptive statistics for each study were checked for comparability against published descriptive data from the original trials. An analysis comparing descriptive data from the trials that were included here with the published descriptive data for the trials whose raw data we were unable to acquire revealed no statistically significant differences (all p values >0.17), suggesting that the data we acquired generalize to the universe of eligible randomized controlled trials.
Mitigation of Covariate Imbalance via Propensity Score Weighting
Propensity scores were estimated using a multinomial logit model with all covariates and study-level fixed effects using SAS Proc GLIMMIX. Covariate balance across treatment classes was achieved for all covariates after propensity score weighting, with all postweighting balance checks below a d value of |0.10| (see the online supplement). Thus, the inverse probability treatment weightings were of sufficient quality for use in primary outcomes analysis.
Outcomes Models
The primary analysis model was an inverse probability treatment weighting, three-level linear mixed model (using Proc MIXED in SAS) to account for 1) clustering of repeated observations within participants and 2) participants clustered within studies (i.e., one-stage meta-analysis of individual patient data). A study-level random effect was specified for the intercept, and participant-level random effects for the intercept and slopes were also included. Model results for all fixed-effect parameter estimates and standard errors, including two-way interaction effects with treatment phase and posttreatment phase time variables (i.e., single intervention treatment effects) and three-way interaction effects (i.e., treatment effects of combinations of interventions), were combined across the 20 multiply imputed, synthetic data sets using Proc MIANALYZE in SAS. Model estimates were then converted into model-based Cohen’s d effect sizes by using the methods of conversion outlined in reference 26.
PTSD Severity Outcomes
End-of-treatment data.
For the treatment-as-usual primary comparator condition, reductions in PTSD symptom severity by end of treatment corresponded to a d value of −0.61 (95% CI=−0.72, −0.52). Comparative effect sizes against treatment as usual (see Table 2 and the online supplement) that were both statistically significant and reached at least Cohen’s convention for small comparative effect sizes (d>|0.20|) were, in ascending order, placebo medication (d=−0.32, 95% CI=−0.53, −0.12), pharmacotherapy for alcohol or other drug use (d=−0.41, 95% CI=−0.77, −0.08), integrated trauma-focused therapies (d=−0.47, 95% CI=−0.94, −0.01), and behavioral therapies for alcohol or other drug use (d=−0.60, 95% CI=−0.80, −0.38). The largest comparative effect size overall was for the combination of trauma-focused therapies and pharmacotherapy for alcohol or other drug use (d=−0.92, 95% CI=−1.57, −0.30). Comparative effect sizes that exceeded a d value of |0.20| but were not statistically significant were observed for nonintegrated trauma-focused therapies (d=−0.24, 95% CI=−0.50, 0.01) and pharmacotherapies for PTSD (d=−0.41, 95% CI=−0.79, 0.24). The study-level intraclass correlation in this model was significant (τ2=0.116, z=3.54, p<0.001), suggesting significant heterogeneity in latent PTSD severity across studies. (Comparisons between pharmacotherapies and combination behavioral and pharmacotherapy interventions against placebo medication were also conducted; see Table S12 in the online supplement.)
| PTSD | Alcohol Use | Drug Use | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| End of Treatment | 12 Months Posttreatment | End of Treatment | 12 Months Posttreatment | End of Treatment | 12 Months Posttreatment | |||||||
| Treatment | d | 95% CI | d | 95% CI | d | 95% CI | d | 95% CI | d | 95% CI | d | 95% CI |
| Trauma-focused therapy and pharmacotherapy for alcohol or other drug use | −0.92b | −1.57, −0.30 | −2.00b | −3.38, −0.68 | −1.10b | −1.54, −0.68 | −1.24b | −2.03, −0.40 | n.s. | n.s. | −0.27c | −1.07, 0.53 |
| Trauma-focused nonintegrated behavioral therapy | −0.24c | −0.50, 0.01 | −0.46b | −0.93, −0.04 | −0.45b | −0.64, −0.26 | −0.24c | −0.51, 0.03 | n.s. | n.s. | n.s. | n.s. |
| Trauma-focused integrated behavioral therapy | −0.47b | −0.94, −0.01 | n.s. | n.s. | −0.42b | −0.74, −0.10 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Pharmacotherapy for alcohol or other drug use | −0.41b | −0.77, −0.08 | −1.37b | −2.15, −0.63 | −0.83b | −1.07, −0.60 | −0.84b | −1.30, −0.41 | n.s. | n.s. | −0.31c | −0.79, 0.11 |
| Placebo medication | −0.32b | −0.53, −0.12 | −0.57b | −1.08, −0.01 | −0.47b | −0.64, −0.31 | −0.29c | −0.65, −0.09 | n.s. | n.s. | n.s. | n.s. |
| Behavioral intervention for alcohol or other drug use | −0.60b | −0.80, −0.38 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Non-trauma-focused integrated behavioral therapy | n.s. | n.s. | −0.22c | −0.54, 0.09 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| PTSD pharmacotherapy | −0.41c | −0.79, 0.24 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.82d | −1.20, 2.99 |
| Treatment as usuale | −0.61 | −0.72, −0.52 | −1.16 | −1.40, −0.92 | −0.37 | −0.44, −0.30 | −0.36 | −0.50, −0.22 | −0.53 | −0.61, −0.47 | −0.63 | −0.78, −0.47 |
TABLE 2. Comparative effect sizes for eight treatment classifications (with treatment as usual as the comparator) on PTSD and severity of alcohol or drug use outcomes at end of treatment and 12 months posttreatmenta
12-month follow-up.
For the treatment-as-usual primary comparator condition, reductions in PTSD symptom severity at 12-month follow-up corresponded to a d value of −1.16 (95% CI=−1.40, −0.92). The following treatments or treatment combinations were statistically superior to treatment as usual, with meaningful comparative effect sizes, in ascending order: nonintegrated trauma-focused therapies (d=−0.46, 95% CI=−0.93, −0.04), placebo medication (d=−0.57, 95% CI=−1.08, −0.01), pharmacotherapy for alcohol or other drug use (d=−1.37, 95% CI=−2.15, −0.63), and combined trauma-focused therapy and pharmacotherapy for alcohol or other drug use (d=−2.00, 95% CI=−3.38, −0.68). Integrated therapies showed meaningful effect size differences compared with treatment as usual but were nonsignificant (d=−0.22, 95% CI=−0.54, 0.09).
Alcohol Severity Outcomes
End-of-treatment data.
For the treatment-as-usual primary comparator condition, reductions in alcohol severity at end of treatment corresponded to a d value of −0.37 (95% CI=−0.44, −0.30). Comparative effect sizes against treatment as usual that were statistically significant and reached Cohen’s convention for a small comparative effect size (d>|0.20|) were (in ascending order) trauma-focused integrated behavioral treatments (d=−0.42, 95% CI=−0.74, −0.10), trauma-focused nonintegrated behavioral treatments (d=−0.45, 95% CI=−0.64, −0.26), placebo medication (d=−0.47, 95% CI=−0.64, −0.31), and pharmacotherapy for alcohol or other drug use (d=−0.83, 95% CI=−1.07, −0.60). Again, the largest comparative effect size overall was for the combination of trauma-focused therapies and pharmacotherapy for alcohol or other drug use (d=−1.10, 95% CI=−1.54, −0.68). The study-level intraclass correlation in this model was significant (τ2=0.067, z=4.02, p<0.001), suggesting significant heterogeneity in latent alcohol use severity across studies.
12-month follow-up data.
For the treatment-as-usual primary comparator condition, reductions in alcohol use severity by 12-month follow-up corresponded to a d value of −0.36 (95% CI=−0.50, −0.22). The following treatments or treatment combinations were statistically superior to treatment as usual, with meaningful comparative effect sizes: pharmacotherapy for alcohol or other drug use alone (d=−0.84, 95% CI=−1.30, −0.41) and the combination of trauma-focused therapy and pharmacotherapy for alcohol or other drug use (d=−1.24, 95% CI=−2.03, −0.40). Placebo medication (d=−0.29, 95% CI=−0.65, −0.09) and nonintegrated trauma-focused therapies (d=−0.24, 95% CI=−0.51, 0.03) showed meaningful effect size differences compared with treatment as usual but were nonsignificant.
Drug Use Severity Outcomes
End-of-treatment data.
For the treatment-as-usual primary comparator condition, reductions in drug use severity by end of treatment corresponded to d value of −0.53 (95% CI=−0.61, −0.47). There were no comparative effect sizes that were statistically significant or larger than Cohen’s convention for a small comparative effect size (d>|0.20|). The study-level intraclass correlation in this model was significant (τ2=0.246, z=3.99, p<0.001), suggesting significant heterogeneity in latent drug use severity across studies.
12-month follow-up data.
For the treatment-as-usual primary comparator condition, reductions in drug use severity by 12-month follow-up corresponded to a d value of −0.63 (95% CI=−0.78, −0.47). None of the treatment combinations was statistically superior to treatment as usual, but pharmacotherapy for drug use (d=−0.31, 95% CI=−0.79, 0.11) and the combination of trauma-focused therapy and pharmacotherapy for drug use (d=−0.27, 95% CI=−1.07, 0.53) had small comparative effect sizes, respectively, compared with treatment as usual. Pharmacotherapy for PTSD (d=0.82, 95% CI=−1.20, 2.99) had a comparative effect size that suggested worse drug use outcomes compared with treatment as usual.
Risk of bias analysis.
Studies were assessed for risk of bias based on the Cochrane risk-of-bias assessment tool, version 2 (27, 28), with two independent raters per study. A reanalysis of all outcome models was conducted, with six of the 36 studies removed from the analysis; three were judged as having a high risk of bias (29–31), one was judged as having an unclear risk of bias because the report on primary outcomes has yet to be published (32), and two were unpublished (M. Saladin, unpublished; S. Sonne, unpublished). A comparison of comparative effect sizes from the full data set versus the data set with studies at high risk of bias excluded showed no differences in effect sizes greater than |0.06| except for one effect: the PTSD medication comparative effect size on PTSD symptom severity at 12-month follow-up was −1.01 when restricted to the studies at low to moderate risk of bias (it was −0.04 with the full data set).
Discussion
To date, systematic reviews and conventional meta-analyses in the area of treating comorbid PTSD and substance use disorders have left unanswered questions regarding the comparative effectiveness of behavioral and pharmacological treatments because, among other limitations (6, 33; D.A. Hien et al., unpublished), these methodologies have not allowed for direct head-to-head comparisons. The present study used a “virtual clinical trial” framework (13) to compare multiple sets of behavioral and pharmacological treatments for comorbid PTSD and alcohol or other drug use disorders using a single-stage individual-patient meta-analysis framework. The findings revealed that two broad groups of treatments—pharmacotherapy for alcohol use, and trauma-focused behavioral interventions—were significantly and consistently more likely to lead to improvements by end of treatment, which were sustained at 12-month follow-up for PTSD and alcohol use symptom severity. Notably, the strongest comparative effects appeared to favor the combination of trauma-focused treatments and alcohol-targeted pharmacotherapy when compared with behavioral treatment as usual and with placebo medication (see the online supplement). Indeed, this was the only treatment class that was superior to both treatment as usual and placebo medication.
Our findings regarding trauma-focused behavioral PTSD treatments provide support for some of the more tentative conclusions regarding efficacy previously drawn from systematic reviews and traditional meta-analyses (6, 13–17, 19, 20, 33). The present study extends our knowledge substantially by demonstrating direct and significant impacts of treatment interventions on alcohol use severity for the first time, and underscores the efficacy of pharmacotherapy targeting alcohol use disorders that are comorbid with PTSD. In contrast, none of the treatment groups were superior to treatment as usual for drug use severity at either time point, although there were two pharmacotherapy effect sizes (for alcohol pharmacotherapy and for trauma-focused and alcohol pharmacotherapy combined) for drug use severity at 12 months posttreatment that were clinically meaningful. We have posited that for drug use outcomes, the mechanisms for improvement may differ from those for alcohol use, and involve more downstream impacts where changes in PTSD symptoms mediate changes in drug use (e.g., references 34–36). Interestingly, pharmacotherapies for PTSD appeared to lead to clinical worsening across both drug use and alcohol use outcomes by 12-month follow-up; further study is needed to determine conditions for efficacy (such as moderator and mediator analyses).
Many single-site randomized controlled trials and conventional meta-analyses of treatments addressing comorbid PTSD and substance use disorders have shown robust clinical impacts on PTSD outcomes (6, 14) but have not shown consistent superiority on alcohol or drug use. The direct impacts of treatments for comorbid PTSD and substance use disorders on alcohol and other drug outcomes have been modest, if any, providing practitioners with far less clarity on the best research-informed practices to address alcohol and drug use disorders. In contrast, the present study is the first analysis to show consistent and direct effects of interventions for comorbid PTSD and alcohol or other drug use disorders—and specifically PTSD interventions with pharmacotherapies for substance use disorders—on alcohol severity outcomes above and beyond treatment as usual.
While in line with anecdotal clinical practice and knowledge (37), support for a particular intervention combination—trauma-focused behavioral treatment paired with an alcohol-targeting medication—may advance the field significantly (37, 38), although it should be noted that this treatment class was based on only two trials (cognitive processing therapy and zonisamide [39], and prolonged exposure and naltrexone [38]) and thus should be replicated for confirmation. To date, the number of pharmacotherapy trials for comorbid PTSD and substance use disorders remains small (20, 40) and formal meta-analytic study is lacking, so pharmacotherapy trial results have been unable to provide a strong signal in any particular direction. In contrast, the methods in the present study integrated 36 trials to 1) enlarge sample size, 2) address measurement variation, 3) increase population diversity, and 4) boost statistical power, and the results have illuminated previously unknown synergies between trauma-focused behavioral treatment and alcohol-targeted pharmacotherapy, suggesting that their combination may amplify their respective effects. Notably, even when offered alone or as a placebo, pharmacotherapies for alcohol use disorder (N-acetylcysteine, naltrexone, paroxetine, prazosin, sertraline, topiramate, and zonisamide) exerted medium to large effects on PTSD and alcohol use outcomes. However, the underrepresentation of studies combining pharmacotherapy with behavioral treatments (i.e., trauma-focused integrated treatments and PTSD-targeted pharmacotherapies combined with a behavioral platform) in the present study underscores the need for investments in novel combined behavioral and pharmacotherapy trials.
Integrated non-trauma-focused approaches—the most widely disseminated treatments for comorbid PTSD and substance use disorders provided in community-based settings, due to feasibility and acceptability—had small to medium effects on PTSD outcomes. They showed lower impacts on drug use outcomes but, notably, were similar to treatment as usual and behavioral approaches in outcomes for alcohol and other drug use across the end-of-treatment and 12-month time frames. Integrated non-trauma-focused treatments typically provide psychoeducation on the relationship between PTSD and alcohol or drug use. In contrast to trauma-focused treatments, these behavioral approaches largely avoid explicit trauma processing and focus on addressing trauma’s current impact (e.g., identifying triggers for relapse, setting boundaries in relationships, distinguishing between safe and unsafe situations, and managing intense emotional responses). Although we see that these models do have benefits (and do not lead to symptom worsening across the board), the effect sizes are much smaller relative to treatment as usual and do not reach statistical significance, in contrast to the trauma-focused integrated and trauma-focused nonintegrated models overall.
Eight of the nine treatments evaluated for comorbid PTSD and alcohol or other drug use disorders, including the treatment-as-usual and placebo comparators, had positive effects on PTSD, alcohol, and drug use severity outcomes at end of treatment. This was observed regardless of type, category, or target of approach (effect sizes ranging from small to large), except for PTSD pharmacotherapy at 12 months posttreatment on drug use outcomes. Also, regardless of whether or not the substance use disorder or PTSD is directly targeted, our analysis indicates that many approaches have some benefits—benefits that may extend beyond the targeted outcomes of the individual type of approach. For example, naltrexone, which is typically used to reduce alcohol cravings and other symptoms of alcohol dependence, also has effects on reducing PTSD symptom severity. Likewise, prolonged exposure, typically used to address PTSD symptoms, also has effects on both alcohol and drug use outcomes.
This analysis focused primarily on the question regarding overall comparative effectiveness across treatment platforms for comorbid PTSD and substance use disorders. Although additional analyses that are specific to optimizing matches between treatment platforms and patient characteristics (i.e., moderation) and the extent to which alcohol and other drug effects across treatment platforms are transmitted through PTSD (i.e., mediation) are planned (13), these issues are beyond the scope of this report. Therefore, we underscore that the findings presented are limited to the overall sample; we caution practitioners away from drawing conclusions about comparative effectiveness for subsamples (such as women only or veterans only). Our comparative effectiveness analysis focused on treatment effects in comparison with behavioral treatment as usual and did not aim to address direct head-to-head comparisons between the eight active classifications; however, the information needed to calculate specific comparative effect sizes between any two treatments not involving treatment as usual as the comparator is available in the online supplement. Instead, we highlight the importance of having many different treatment options for individuals with comorbid PTSD and substance use disorder. This may be one reason that the findings for significant pharmacotherapy effects were spotlighted, given that the typical pharmacotherapy trial compares active pharmacotherapies with placebo and not treatment as usual.
Our 36 studies included trials of 10 different medications (or combinations of medications) and multiple trauma-focused therapies, with at least three trials of the COPE intervention and eight trials of interventions based on Seeking Safety therapy. This represents many active treatment classifications, extracted from the 36 studies for which we were able to obtain data. We present the review findings based on categorization that we reference from the literature (6) and a recent publication from our team (41) that focuses on how interventions impacted outcomes (PTSD only, alcohol or other drug use disorder only, or comorbid PTSD and alcohol or other drug use disorder), and have reorganized the intervention types along several different vectors of classification, including target of the intervention (integrated vs. nonintegrated), disorder(s) of focus (PTSD only, alcohol or other drug use disorder only, or comorbid PTSD and alcohol or other drug use disorder), treatment modality (behavioral or pharmacologic), and whether the behavioral intervention is trauma focused or non–trauma focused.
All individual patient data meta-analyses are limited by what trials are available, and in the case of comorbid PTSD and alcohol and other drug use disorders, the studies are quite diverse, and therefore our findings still need replication, and further analyses focusing on individual differences and other comparators are needed to determine treatment-matching goals (13). Yet, these findings can serve as guideposts for which promising combinations of treatments and understudied treatments warrant further examination in future trials.
Limitations
While our combination of integrative data analysis and “one-stage” meta-analysis of individual patient data constitutes a more robust and precise method for estimating cross-study variation in measurement and treatment outcomes compared with other approaches (e.g., “two-stage” meta-analysis of individual patient data, conventional meta-analysis) (42), this study has some limitations. First, as with all treatment outcomes for randomized controlled trials, the findings of this study can only be generalized to individuals who volunteer to participate in randomized clinical studies. Future analysis of the Project Harmony data set could explore estimation of treatment effects that could generalize to the larger population of treatment seekers (43). Second, the results from the study suggested statistically significant study-level heterogeneity for all three outcomes. The more immediate cause of study-level heterogeneity in outcomes is the differences in inclusion criteria across studies (e.g., full PTSD vs. subthreshold PTSD), which both directly and indirectly impact variation in the study-level mean values of baseline severity measures of PTSD and substance use disorders at baseline. As structured, the covariates in the propensity score weighting model and the treatment class variables vary within the trial, and are thus between-individual predictors, which do not reduce unexplained variability at the study level. Inclusion of study-aggregated and (study-centered) individual-level moderators will reduce unexplained variability at the study level in continuing analysis of the Project Harmony data set (13).
Although the included trials aimed to be diverse with regard to inclusion, quasi-experimental designs and single-group studies were excluded; however, selection biases that may be created by “mixing and matching” treatment classes across randomized controlled trials in meta-analyses of individual patient data (13, 44, 45) were mitigated by propensity score weighting specifically for those types of designs (46, 47). Further, our risk-of-bias analyses, in which studies at high risk of bias were excluded, were robust to risk of bias across all comparative effect sizes except one, suggesting that our findings are robust to variation in trial design quality. Finally, our study generalizability analysis suggested that the studies we were able to include were representative of the larger universe of randomized controlled trials that we identified for potential inclusion in the study.
Conclusions
The comparative effectiveness results from this meta-analysis of individual patient data extend and offer greater precision than other forms of evidence synthesis as to which behavioral and pharmacological treatments, or their combination, are most effective in addressing PTSD and substance use disorder in individuals with both disorders, when holding a number of critical individual characteristics constant. The findings show that there are several effective options and that trauma-focused therapies combined with pharmacotherapy for alcohol use disorder significantly and consistently led to early and sustained improvements on both PTSD and alcohol use outcomes.
The indication of benefits of trauma-focused and combined alcohol pharmacotherapy may be an important beacon for service providers and users. This is particularly salient given the earlier adoption by the substance use treatment community of non-trauma-focused treatments (48), largely due to clinician and/or client fears that trauma processing may worsen PTSD or substance use. The present study findings demonstrate not only that individuals with comorbid PTSD and alcohol use disorders derive direct benefits in their drinking outcomes from trauma processing therapies, but also that they are able to tolerate the trauma-focused approaches, with PTSD effect sizes similar to those observed in individuals without substance use disorders (15). The findings show that this was the case whether or not treatments are offered in an integrated fashion (i.e., the behavioral treatment includes some content that addresses the relationship between PTSD and substance use across the majority of treatment sessions); study participants did not relapse in the process. On a policy level, our findings support the contention that there are “no wrong doors” (17) regarding the delivery of substance use intervention services that integrate PTSD treatment alongside them, as opposed to isolating approaches to substance use and PTSD in distinct services. However, as the findings provide clearer support for trauma-focused interventions, alcohol-targeted pharmacotherapy, and their combination, it does appear that “some doors are better than others.”
1. : Epidemiology of posttraumatic stress disorder: prevalence, correlates, and consequences. Curr Opin Psychiatry 2015; 28:307–311Crossref, Medline, Google Scholar
2. : World Drug Report 2021: Executive Summary: Policy Implications. Vienna, United Nations, Office on Drugs and Crime, Division for Policy Analysis and Public Affairs, June 2021. https://www.unodc.org/res/wdr2021/field/WDR21_Booklet_1.pdf Google Scholar
3. : The burden of co-occurring alcohol use disorder and PTSD in US military veterans: comorbidities, functioning, and suicidality. Psychol Addict Behav 2018; 32:224–229Crossref, Medline, Google Scholar
4. : Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID-19. Am J Psychiatry 2020; 177:1031–1037 Link, Google Scholar
5. : Co-occurring mental and substance abuse disorders: a review on the potential predictors and clinical outcomes. Psychiatry Res 2011; 186:159–164Crossref, Medline, Google Scholar
6. : Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: a systematic review and meta-analysis. Clin Psychol Rev 2015; 38:25–38Crossref, Medline, Google Scholar
7. : Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol 1996; 4:46–54 Crossref, Google Scholar
8. : The efficacy of 90-minute versus 60-minute sessions of prolonged exposure for posttraumatic stress disorder: design of a randomized controlled trial in active duty military personnel. Psychol Trauma 2019; 11:307–313Crossref, Medline, Google Scholar
9. : Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE): Therapist Guide. Oxford, UK, Oxford University Press, 2014 Google Scholar
10. : PTSD: from neurobiology to pharmacological treatments. Eur J Psychotraumatol 2016; 7:31858Crossref, Medline, Google Scholar
11. : Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology 2012; 62:542–551Crossref, Medline, Google Scholar
12. : Efficacy of integrated exposure therapy vs integrated coping skills therapy for comorbid posttraumatic stress disorder and alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 2019; 76:791–799Crossref, Medline, Google Scholar
13. : Evaluating treatments for posttraumatic stress disorder, alcohol and other drug use disorders using meta-analysis of individual patient data: design and methodology of a virtual clinical trial. Contemp Clin Trials 2021; 107:106479Crossref, Medline, Google Scholar
14. : Efficacy and acceptability of interventions for co-occurring PTSD and SUD: a meta-analysis. J Anxiety Disord 2021; 84:102490Crossref, Medline, Google Scholar
15. : Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update (Comparative Effectiveness Review, No 207). Rockville, Md, Agency for Healthcare Research and Quality, May 2018. https://www.ncbi.nlm.nih.gov/books/NBK525132/ Crossref, Google Scholar
16. : Effectiveness of Seeking Safety for co-occurring posttraumatic stress disorder and substance use. J Couns Dev 2016; 94:51–61 Crossref, Google Scholar
17. : No wrong doors: findings from a critical review of behavioral randomized clinical trials for individuals with co-occurring alcohol/drug problems and posttraumatic stress disorder. Alcohol Clin Exp Res 2017; 41:681–702Crossref, Medline, Google Scholar
18. : Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: a systematic review. Clin Psychol Rev 2012; 32:202–214Crossref, Medline, Google Scholar
19. : Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev 2006; 26:162–178Crossref, Medline, Google Scholar
20. : Posttraumatic stress disorder and alcohol use disorder: a critical review of pharmacologic treatments. Alcohol Clin Exp Res 2017; 41:226–237Crossref, Medline, Google Scholar
21. : Preferred Reporting Items for Systematic Review and Meta-Analyses of Individual Participant Data: the PRISMA-IPD statement. JAMA 2015; 313:1657–1665Crossref, Medline, Google Scholar
22. : Subthreshold PTSD in primary care: prevalence, psychiatric disorders, healthcare use, and functional status. J Nerv Ment Dis 2005; 193:658–664Crossref, Medline, Google Scholar
23. : Addressing methodologic challenges and minimizing threats to validity in synthesizing findings from individual level data across longitudinal randomized trials. Prev Sci 2018; 19(suppl 1):60–73Crossref, Medline, Google Scholar
24. : A more general model for testing measurement invariance and differential item functioning. Psychol Methods 2017; 22:507–526Crossref, Medline, Google Scholar
25. : Psychometric approaches for developing commensurate measures across independent studies: traditional and new models. Psychol Methods 2009; 14:101–125Crossref, Medline, Google Scholar
26. : Meta-analysis with standardized effect sizes from multilevel and latent growth models. J Consult Clin Psychol 2017; 85:262–266Crossref, Medline, Google Scholar
27. : Assessing risk of bias in a randomized trial, in Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ, John Wiley & Sons, 2019, pp 205–228 Crossref, Google Scholar
28. : Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J Clin Epidemiol 2018; 97:26–34Crossref, Medline, Google Scholar
29. : A randomized controlled trial comparing integrated cognitive behavioral therapy versus individual addiction counseling for co-occurring substance use and posttraumatic stress disorders. J Dual Diagn 2011; 7:207–227Crossref, Medline, Google Scholar
30. : Treating trauma in addiction with EMDR: a pilot study. J Psychoactive Drugs 2014; 46:303–309Crossref, Medline, Google Scholar
31. : A cognitive-behavioral treatment for incarcerated women with substance abuse disorder and posttraumatic stress disorder: findings from a pilot study. J Subst Abuse Treat 2003; 25:99–105Crossref, Medline, Google Scholar
32. : Development of a novel, integrated cognitive-behavioral therapy for co-occurring posttraumatic stress and substance use disorders: a pilot randomized clinical trial. Contemp Clin Trials 2018; 65:123–129Crossref, Medline, Google Scholar
33. : Maximizing effectiveness trials in PTSD and SUD through secondary analysis: benefits and limitations using the National Institute on Drug Abuse Clinical Trials Network “Women and Trauma” study as a case example. J Subst Abuse Treat 2015; 56:23–33Crossref, Medline, Google Scholar
34. : Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA’s Clinical Trials Network. Am J Psychiatry 2010; 167:95–101Link, Google Scholar
35. : The impact of treatment condition and the lagged effects of PTSD symptom severity and alcohol use on changes in alcohol craving. Behav Res Ther 2016; 79:7–14Crossref, Medline, Google Scholar
36. : Indirect effects of 12-session Seeking Safety on substance use outcomes: overall and attendance class-specific effects. Am J Addict 2014; 23:218–225Crossref, Medline, Google Scholar
37. : A guide to guidelines for the treatment of posttraumatic stress disorder in adults: an update. Psychotherapy 2019; 56:359–373Crossref, Medline, Google Scholar
38. : Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA 2013; 310:488–495Crossref, Medline, Google Scholar
39. : Zonisamide as an adjunctive treatment to cognitive processing therapy for veterans with posttraumatic stress disorder and comorbid alcohol use disorder: a pilot study. Am J Addict 2020; 29:515–524Crossref, Medline, Google Scholar
40. : Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology 2012; 62:542–551Crossref, Medline, Google Scholar
41. : What’s in a name? A data-driven method to identify optimal psychotherapy classifications to advance treatment research on co-occurring PTSD and substance use disorders. Eur J Psychotraumatol 2022; 13:2001191Crossref, Medline, Google Scholar
42. : Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med 2017; 36:855–875Crossref, Medline, Google Scholar
43. : Implementing statistical methods for generalizing randomized trial findings to a target population. Addict Behav 2019; 94:124–132Crossref, Medline, Google Scholar
44. : Testing moderation in network meta-analysis with individual participant data. Stat Med 2016; 35:2485–2502Crossref, Medline, Google Scholar
45. : Design and methodology for an integrative data analysis of coping power: direct and indirect effects on adolescent suicidality. Contemp Clin Trials 2022; 115:106705Crossref, Medline, Google Scholar
46. : The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55 Crossref, Google Scholar
47. : Propensity scores for multiple treatments: a tutorial for the mnps function in the twang package. October 20 2021. http://cran.r-project.org/web/packages/twang/vignettes/mnps.pdf Google Scholar
48. : PTSD/substance use disorder comorbidity: treatment options and public health needs. Curr Treat Options Psychiatry 2020; 7:544–558Crossref, Medline, Google Scholar
49. : A double-blind, randomized, controlled pilot trial of N-acetylcysteine in veterans with posttraumatic stress disorder and substance use disorders. J Clin Psychiatry 2016; 77:e1439–e1446Crossref, Medline, Google Scholar
50. : Concurrent treatment of substance use disorders and PTSD using prolonged exposure: a randomized clinical trial in military veterans. Addict Behav 2019; 90:369–377Crossref, Medline, Google Scholar
51. : Seeking Safety treatment for male veterans with a substance use disorder and post-traumatic stress disorder symptomatology. Addiction 2012; 107:578–586Crossref, Medline, Google Scholar
52. : Promising treatments for women with comorbid PTSD and substance use disorders. Am J Psychiatry 2004; 161:1426–1432Link, Google Scholar
53. : Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. J Consult Clin Psychol 2009; 77:607–619Crossref, Medline, Google Scholar
54. : Concurrent treatment with prolonged exposure for co-occurring full or subthreshold posttraumatic stress disorder and substance use disorders: a randomized clinical trial. Psychother Psychosom 2017; 86:150–161Crossref, Medline, Google Scholar
55. : Randomized controlled trial of two brief alcohol interventions for OEF/OIF veterans. J Consult Clin Psychol 2014; 82:562–568Crossref, Medline, Google Scholar
56. : A randomized controlled trial of treatments for co-occurring substance use disorders and post-traumatic stress disorder. Addiction 2015; 110:1194–1204Crossref, Medline, Google Scholar
57. : Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: a randomized controlled trial. JAMA 2012; 308:690–699Crossref, Medline, Google Scholar
58. : Treatment engagement: female survivors of intimate partner violence in treatment for PTSD and alcohol use disorder. J Dual Diagn 2015; 11:238–247Crossref, Medline, Google Scholar
59. : Integrated cognitive behavioral therapy versus cognitive processing therapy for adults with depression, substance use disorder, and trauma. J Subst Abuse Treat 2016; 62:38–48Crossref, Medline, Google Scholar
60. : Prazosin for veterans with posttraumatic stress disorder and comorbid alcohol dependence: a clinical trial. Alcohol Clin Exp Res 2016; 40:178–186Crossref, Medline, Google Scholar
61. : Randomized controlled trial of cognitive behaviour therapy for comorbid post-traumatic stress disorder and alcohol use disorders. Addiction 2013; 108:1397–1410Crossref, Medline, Google Scholar
62. : Incentivizing attendance to prolonged exposure for PTSD with opioid use disorder patients: a randomized controlled trial. J Consult Clin Psychol 2017; 85:689–701Crossref, Medline, Google Scholar
63. : A multisite randomized controlled trial of Seeking Safety vs relapse prevention training for women with co-occurring posttraumatic stress disorder and substance use disorders. Eur J Psychotraumatol 2019; 10:1577092Crossref, Medline, Google Scholar
64. : Randomized controlled pilot study of cognitive-behavioral therapy in a sample of incarcerated women with substance use disorder and PTSD. Behav Ther 2009; 40:325–336Crossref, Medline, Google Scholar
65. : Web intervention for OEF/OIF veterans with problem drinking and PTSD symptoms: a randomized clinical trial. J Consult Clin Psychol 2013; 81:890–900Crossref, Medline, Google Scholar
66. : Trauma-focused treatment for posttraumatic stress disorder combined with CBT for severe substance use disorder: a randomized controlled trial. BMC Psychiatry 2013; 13:172Crossref, Medline, Google Scholar
67. : Virtual reality and cellular phones as a complementary intervention for veterans with PTSD and substance use disorders. Durham, NC, Duke University Medical Center, 2013. https://apps.dtic.mil/sti/pdfs/AD1025212.pdf Google Scholar
68. : Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial. Alcohol Clin Exp Res 2014; 38:2169–2177Crossref, Medline, Google Scholar
69. : Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res 2005; 29:395–401Crossref, Medline, Google Scholar
70. : Outcomes of trauma treatment using the TARGET model. J Groups Addict Recover 2008; 3:285–303Crossref, Google Scholar
71. : Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacol 2012; 37:996–1004Crossref, Medline, Google Scholar
72. : A controlled examination of two coping skills for daily alcohol use and PTSD symptom severity among dually diagnosed individuals. Behav Res Ther 2015; 66:8–17Crossref, Medline, Google Scholar
73. : A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res 2015; 39:808–817Crossref, Medline, Google Scholar
74. : A randomized controlled trial evaluating integrated versus phased application of evidence-based psychotherapies for military veterans with comorbid PTSD and substance use disorders. Drug Alcohol Depend 2019; 205:107647Crossref, Medline, Google Scholar



