Disrupted Dorsal Mid-Insula Activation During Interoception Across Psychiatric Disorders
Abstract
Objective:
Maintenance of bodily homeostasis relies on interoceptive mechanisms in the brain to predict and regulate bodily state. While altered neural activation during interoception in specific psychiatric disorders has been reported in many studies, it is unclear whether a common neural locus underpins transdiagnostic interoceptive differences.
Methods:
The authors conducted a meta-analysis of neuroimaging studies comparing patients with psychiatric disorders with healthy control subjects to identify brain regions exhibiting convergent disrupted activation during interoception. Bibliographic, neuroimaging, and preprint databases through May 2020 were searched. A total of 306 foci from 33 studies were extracted, which included 610 control subjects and 626 patients with schizophrenia, bipolar or unipolar depression, posttraumatic stress disorder, anxiety, eating disorders, or substance use disorders. Data were pooled using a random-effects model implemented by the activation likelihood estimation algorithm. The preregistered primary outcome was the neuroanatomical location of the convergence of peak voxel coordinates.
Results:
Convergent disrupted activation specific to the left dorsal mid-insula was found (Z=4.47, peak coordinates: −36, −2, 14; volume: 928 mm3). Studies directly contributing to the cluster included patients with bipolar disorder, anxiety, major depression, anorexia, and schizophrenia, assessed with task probes including pain, hunger, and interoceptive attention. A series of conjunction analyses against extant meta-analytic data sets revealed that this mid-insula cluster was anatomically distinct from brain regions involved in affective processing and from regions altered by psychological or pharmacological interventions for affective disorders.
Conclusions:
These results reveal transdiagnostic, domain-general differences in interoceptive processing in the left dorsal mid-insula. Disrupted mid-insular activation may represent a neural marker of psychopathology and a putative target for novel interventions.
Arguably, the most vital function of the nervous system for survival is to detect and regulate the body’s internal state to maintain key physiological variables within viable operating ranges. Interoception provides our sense of the physiological condition of the internal milieu (1) and requires the brain to integrate temporally and spatially complex information carried by afferent projections from diverse bodily systems. Mapping the body’s internal state often occurs entirely outside of conscious awareness until an urgent bodily need arises—a lack of oxygen, for instance, or a pressing need for food or water (2). Altered interoceptive processes are reported in many neuropsychiatric conditions, including addiction (3, 4), anxiety and depression (5, 6), eating disorders (7–11), panic disorder (12–15), and somatoform disorders (16–18). Theoretical models have proposed that disrupted cortical processing drives such alterations in interoceptive processing, conferring vulnerability to a range of mental health symptoms (2, 19–22).
Homeostatic regulation requires the brain to perform two functions: monitoring (what is my current bodily state with respect to viable physiological operating parameters?) and prediction (how will putative actions change this bodily state?) (20). One class of influential theories posits that prospective control (allostasis) is used to avoid potential departures from homeostatic operating ranges (2, 19, 20, 23, 24). To support prospective regulation of bodily state, a neural circuit involving the anterior insula cortex, anterior cingulate cortex, and orbitofrontal cortex is thought to receive bodily state information from the mid- and posterior insula and send predictions to the hypothalamus, brainstem, and spinal cord nuclei (19, 20, 23, 25, 26). This circuitry allows arbitration between external environmental information and the state of the internal milieu (physiology, motivational state, etc.) (20, 23, 25–27).
In light of the increasing recognition that dimensions of psychopathology cut across traditional nosological boundaries (28, 29), disruptions in interoception may originate from a transdiagnostic perturbation within this neural circuitry. Identifying the anatomical location of this disruption would shed light on the origins of behavioral transdiagnostic disruptions in interoception (30). For example, convergent disrupted signaling in the primary interoceptive cortex—usually attributed to the posterior insula—would indicate a common alteration in bodily state representation across disorders (31). In contrast, altered activity in the anterior insula may indicate a transdiagnostic disruption in abstract representation of affective state (31), perhaps the aspect of interoceptive processing most strongly implicated in psychopathology (32–34). Alternatively, disruptions at the top level of the hierarchy—in anterior prefrontal regions—would support recent theories suggesting aberrant interoceptive meta-cognition (that is, confidence in one’s own ability to regulate homeostasis) (19).
To date, the vast majority of individual neuroimaging studies measuring neural correlates of interoception in psychiatric conditions are limited in their ability to identify such transdiagnostic mechanisms because of study and sample specificity. Neuroimaging meta-analysis is a powerful technique to aggregate data across studies to identify whether there exist any common loci of disruption that manifest across psychiatric disorders (35, 36). This approach has particular significance for interoception, given the rationale that diverse interoceptive signals, assayed with a variety of tasks, are nevertheless integrated in common neural regions (31). Establishing whether the diverse measures and samples in individual neuroimaging studies converge on a neuroanatomical locus could eventually facilitate development of novel transdiagnostic interventions translated from basic neuroscience.
We therefore examined differential neural activation across a collated transdiagnostic sample of patients and control subjects during a variety of interoceptive probes, synthesized from multiple neuroimaging studies using neuroimaging meta-analysis. This allowed us to uncover whether there exists a common interoceptive locus of disruption across psychiatric disorders and across interoceptive domains, and where this locus is situated within established interoceptive brain circuitry.
We additionally explored two secondary aims. First, does this locus of disruption overlap with the brain’s affect circuitry, a wide-reaching network thought to be critical to psychopathology? Previous work may suggest some overlap: emotional experience is profoundly influenced by bodily state (32), and interoceptive accuracy (assessed by heartbeat detection) is associated with better affect regulation (33) and verbalization (37). We assessed this by quantitatively contrasting our findings with a large existing database of neuroimaging studies of emotion processing (38). Second, does this locus of disruption overlap with the neural targets of existing evidence-based psychiatric interventions? We assessed this by contrasting our findings with two previous meta-analyses of the neural effects of psychological (39) and pharmacological (40) interventions in transdiagnostic affective disorder populations. We would expect a conjunction of the results of our meta-analyses if an interoceptive processing locus was altered by one of the treatment types.
Methods
We conducted a meta-analysis of neuroimaging studies comparing patients with psychiatric disorders with healthy control subjects to identify brain regions exhibiting convergent disrupted activation during interoception.
Inclusion Criteria and Search Protocol
The meta-analysis protocol was preregistered (Prospero ID, CRD42020176791). Records were identified through bibliographic (PubMed, MEDLINE, PsycINFO, and EMBASE), neuroimaging (NeuroSynth, BrainMap Sleuth, and Brainspell), and preprint databases (PsyArXiv, bioRxiv), supplemented by reference tracing. Two raters screened all records. Experiments were eligible if they included an interoceptive probe during neuroimaging data acquisition (interoceptive domains and contrasts are presented in Table S1 in the online supplement), included at least two groups differentiated according to a psychiatric criterion (current or past diagnosis or scores on a psychiatric dimension scale; clinical groups are presented in Table S1 in the online supplement), used a whole-brain sequence, and reported whole-brain coordinates in a defined stereotaxic space (e.g., Talairach or Montreal Neurological Institute [MNI]). Our initial analysis included adolescents (ages ≥13), although we confirmed our results in an adult-only sample.
We defined interoception according to previously published criteria: a sensing of the physiological condition or state of the body, including tickle, itch, skin temperature, hunger, thirst, heat, pain, and organ integrity. Following established neuroimaging meta-analysis guidelines (41), we included only one contrast per study. If more than one contrast was reported, we selected the one that was most specific to interoception, to minimize the contribution of other neural systems to our results (typically the primary contrast in the study; for example, the contrast interoceptive attention > exteroceptive attention is more specific to interoception than interoceptive focus > fixation, because a fixation baseline does not control for attention-related activation [42]). If multiple contrasts were equally relevant to interoception, we used the first contrast reported. Specific contrasts are presented in Table S1 in the online supplement. Note that some studies involved assessment of brain activation during top-down interoceptive attention to specific organ systems, while others involved assessment of brain activation during bottom-up perturbation of specific sensory signals, such as pain or hunger.
In line with the transdiagnostic motivation behind our analysis, our second criterion (clinical group) was intended to capture an inclusive array of mental health problems and included, for example, patients diagnosed with psychiatric disorders, patients with high levels of a clinically significant trait (e.g., problem substance use, high anxiety), and recovered or weight-restored patients with anorexia nervosa (for search terms, criteria, and specific diagnoses, see the Materials section and Table S1 in the online supplement).
Data (peak voxel coordinates for whole-brain between-group comparisons or group interaction effects during interoception, sample size, demographic characteristics, and contrast information) were extracted from eligible records, and all coordinates were double-checked by a second rater for accuracy. We obtained unreported whole-brain data via corresponding authors. All coordinates reported in Talairach space were converted to MNI space (43).
With respect to inclusion and exclusion, 637 of 642 (99.2%) record abstracts were classed concordantly between the two raters (intraclass correlation coefficient=0.969, 95% CI=0.964, 0.974, p<0.001). Discrepancies were resolved by discussion.
Activation-Likelihood Estimation (ALE) Meta-Analysis
We implemented the revised ALE algorithm, which compares the convergence of reported coordinates with those expected under random spatial association (44–46). The ALE algorithm treats foci as three-dimensional Gaussian probability distributions centered at the given coordinates and scaled according to the sample size and performs random-effect inference, testing for above-chance clustering between experiments (44–46). ALE results were thresholded at a cluster-level family-wise-error (FWE) corrected threshold p value <0.05 (cluster-forming threshold at voxel-level p<0.001; 1,000 threshold permutations). Conjunction analyses were set at a minimum volume of 50 mm3 (p=0.05; 1,000 p-value permutations).
Maps were overlaid onto a standard brain in MNI space (Colin 27, a stereotaxic average of 27 single-subject anatomical scans, skull stripped) using the Mango software package (http://ric.uthscsa.edu/mango).
Primary Analysis
The resulting cluster-corrected ALE map identified transdiagnostic patterns of disrupted activation during interoception for patients with psychiatric disorders compared with control subjects (36) (our primary, preregistered analysis).
Follow-Up Analyses
We quantitatively compared the cluster-corrected map of disrupted activation in interoception with results from three previous meta-analyses, as described below.
We conducted a conjunction and contrast ALE analysis testing convergence between disrupted activation and core affect circuitry (38) from a previous meta-analysis (data were obtained through correspondence with the authors). This sample (N=3,587; 216 studies; 3,867 individual foci) consisted of whole-brain neuroimaging tasks of valenced emotion processing (compared with neutral conditions) measured using functional MRI (fMRI) or positron emission tomography. After thresholding the initial ALE map of these meta-analysis data (p<0.05 FWE cluster corrected; cluster-forming threshold, p<0.001; 1,000 threshold permutations), we conducted a conjunction/contrast analysis of this map and the cluster-corrected map of disrupted activation during interoception (minimum volume, 50 mm3; p=0.05, 1,000 p-value permutations). For further details, see the Materials section in the online supplement.
We also conducted a conjunction and contrast ALE analysis testing convergence between disrupted activation during interoception and regions altered during antidepressant treatment (N=343; 24 studies; 200 foci) (40) and psychological therapy (N=276; 17 studies; 200 foci) (39) for affective disorders (data were obtained through correspondence with the authors). The study samples included patients with an affective disorder diagnosis (depression, anxiety, social phobia/anxiety, posttraumatic stress disorder, obsessive-compulsive disorder, and panic disorder) treated with either selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors or treated with psychological therapy (most commonly cognitive-behavioral therapy), with neural activation measured using valence processing tasks (see Tables S7 and S9 in the online supplement). Again, we ran individual meta-analyses on each data set (p<0.05 FWE cluster corrected; cluster-forming threshold, p<0.001; 1,000 threshold permutations) before conducting a conjunction/contrast analysis of this map and the cluster-corrected map of disrupted activation during interoception (minimum volume, 50 mm3; p=0.05, 1,000 p-value permutations).
For further details, see the Materials section in the online supplement.
Results
Thirty-three eligible records were identified (Figure 1) containing data from 33 separate experiments (306 foci). Approximately 17–20 experimental data sets are needed for ALE to be adequately powered to detect small effects and to ensure that results are not driven by single experiments (47). Our sample included 626 patients and 610 control subjects (see the Materials section and Table S1 in the online supplement). fMRI was used in all 33 included experiments. Mean ages ranged from 16.1 to 43.2 years for the psychiatric group and 16.5 to 43.6 years for the control group. The majority of experiments included psychiatric participants not currently taking psychotropic medication (K=25/33; one study did not report medication status), and most studies screened for past psychiatric disorders in the healthy control group (K=27/33; six studies did not report diagnostic interviews).
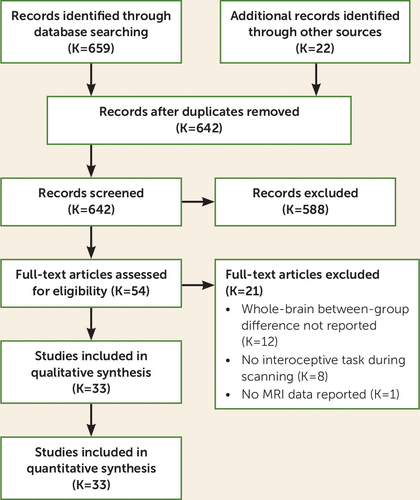
FIGURE 1. Flow diagram of study selection
Tasks in the final experiment set either measured top-down assessments of interoceptive attention (including heartbeat counting [K=1], as well as general attentional focus on specific organs [K=4]) or bottom-up perturbations of sensory signals (breathing load [K=10], pain [K=8], affective touch [K=5], and hunger [K=5]). No studies incorporated interoceptive accuracy (or any other interoceptive behavioral measure) into their neuroimaging analyses.
Transdiagnostic Disrupted Activation
Synthesizing across all studies revealed a single cluster of disrupted activation in the left dorsal mid-insula between patients with psychiatric disorders and control subjects (Z=4.47, p=0.0000038; peak coordinates: −36, −2, 14; volume: 928 mm3) (Figure 2; see also Table S2 in the online supplement). Studies falling within the cluster included patients with unipolar and bipolar depression, anxiety, remitted anorexia, and schizophrenia and used tasks assessing heartbeat detection, hunger, pain, and interoceptive focus. The same cluster was apparent in the subsample comprising only adult participants (Z=4.58, p=0.0000024; volume: 1,088 mm3; see also Table S2 in the online supplement). For clusters apparent at the uncorrected threshold (p<0.001), including right dorsal mid-insula clusters, see Table S3 in the online supplement.
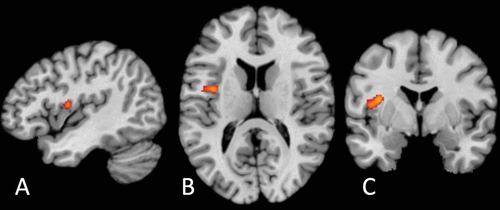
FIGURE 2. Transdiagnostic disrupted activation between patients with psychiatric disorders and control subjects during interoceptive processing derived from activation-likelihood estimation (ALE) meta-analysis (k=33)a
a A single significant cluster was found (p<0.05 family-wise-error-corrected; cluster-forming threshold, p<0.001; 1,000 threshold permutations) in the left dorsal mid-insula (Z=4.47, p=0.0000038; peak coordinates: −36, −2, 14; volume: 928 mm3) viewed in sagittal (panel A), axial (panel B), and coronal (panel C) sections.
For exploratory analyses split by disorder grouping and hyperactivation (patients > control subjects) compared with hypoactivation (control subjects > patients), see the Materials section and Tables S4 and S5 in the online supplement; these analyses are underpowered (41) and are included only for completeness. A small number of the included studies (K=4) used tasks involving verbal probes (e.g., the word “stomach”), which could have contributed to the laterality of this result (for a list of studies involving verbal probes, see Table S1 in the online supplement).
Comparison of Transdiagnostic Disrupted Activation and Affect Circuitry
We performed a conjunction analysis to assess whether there was any overlap or significant differences in convergence between our results and results obtained from the largest database of affect task-based neuroimaging studies. We extracted whole-brain contrasts across all participants with a neutral affect baseline from this database (for details on the protocol, see the Materials section in the online supplement). ALE analysis was performed on 3,867 foci originating from 249 experiments.
The conjunction analysis revealed no regions of significant overlap (Figure 3). However, the left dorsal mid-insula (Z=2.75, p=0.003, peak coordinates: −20, −18, −20; volume: 872 mm3) and the left entorhinal/perirhinal cortex (Z=3.29, p<0.001, peak coordinates: −34, −4, 16; volume: 272 mm3) were preferentially activated in disrupted interoceptive activation, compared with general affect processing. Conversely, a number of regions were preferentially activated during affect processing compared with disrupted interoception (see Table S6 in the online supplement), most notably a very large cluster in the left hemisphere that included a peak in the left anterior insula (Z=3.29, p<0.001; insula peak coordinates=−33.7, 25.7, −7.7; volume: 30,064 mm3). This indicates that our identified transdiagnostic neural locus of differential interoceptive processing was distinct from brain regions implicated in affective processing.
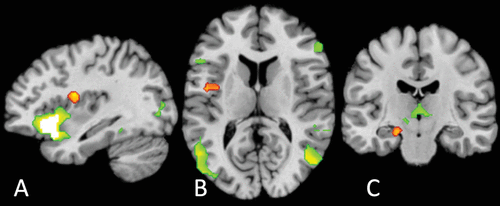
FIGURE 3. Conjunction analyses indicating significant differences in convergence between disrupted transdiagnostic interoceptive activation and general affect circuitrya
a Disrupted interoceptive activation differed significantly from general affect circuitry in the left dorsal mid-insula (panels A and B) (Z=3.29, p<0.001; peak coordinates: −34, −4, 16; volume: 872 mm3) and the left entorhinal/perirhinal cortex (panel C) (Z=2.75, p=0.003, peak coordinates: −20, −18, −20; volume: 272 mm3) (orange). General affect circuitry preferentially activated a large cluster including the left anterior insula (panel A) and left and right inferior frontal gyri, occipito-temporal regions (panel B), and thalamus (panel C). There were no regions of significant overlap (for a full list of regions, see Table S5 in the online supplement).
Comparison of Transdiagnostic Disrupted Activation With Neural Changes Following Evidence-Based Treatments
We performed a conjunction analysis to assess whether there was any overlap or significant differences in convergence between our results and regions modified by antidepressant medication treatment (40) (data from 24 experiments measuring activation during processing of affective material before and after antidepressant treatment; 200 foci; see the Materials section and Table S7 in the online supplement). We found no regions of overlap. However, the cluster in the left dorsal mid-insula (Z=2.33, p=0.010; peak coordinates: −42, 2, 10; volume: 408 mm3) was preferentially activated in disrupted interoception compared with neural changes following antidepressant treatment (Figure 4), while changes following antidepressant treatment preferentially converged on the amygdala bilaterally (right: Z=1.87, p=0.031; peak coordinates: 34, −6, −22; volume: 256 mm3; left: Z=2.23, p=0.013; peak coordinates: −22, 2, −22; volume: 256 mm3) and the medial globus pallidus (Z=2.23, p=0.013; peak coordinates: −15, −6, −10.5; volume: 408 mm3) (see Table S8 in the online supplement).
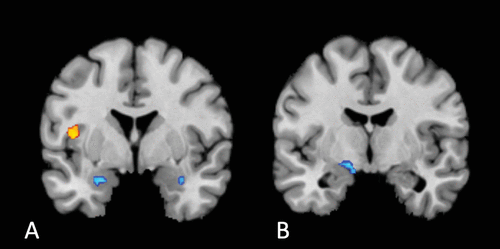
FIGURE 4. Conjunction analysis revealing significant differences in convergence between disrupted interoceptive activation and regions of neural change following treatment with antidepressant medicationa
a Disrupted interoceptive activation differed significantly from the neural changes associated with antidepressant medication treatment in the left dorsal mid-insula (orange cluster, panel A) (Z=2.33, p=0.01; peak coordinates: −42, 2, 10; volume: 408 mm3). Changes following antidepressant treatment preferentially converged on clusters in the amygdala bilaterally (right: Z=1.87, p=0.031; peak coordinates: 34, −6, −22; volume: 256 mm3; left: Z=2.23, p=0.013; peak coordinates: −22, 2, −22; volume: 256 mm3) (blue cluster; panel A) and the medial globus pallidus (Z=2.23, p=0.013; peak coordinates: −15, −6, −10.5; volume: 408 mm3) (blue cluster; panel B). There were no significantly overlapping regions.
A conjunction analysis comparing our interoception data with regions modified by psychological therapy (39) (data from 17 experiments measuring task-based or resting-state activation before and after psychological therapy in mood and anxiety disorders; 120 foci; see the Materials section and Table S9 in the online supplement) showed neither significant overlap nor differential convergence between disrupted interoceptive activation and changes following psychological therapy (see Table S10 in the online supplement for uncorrected [p<0.001] results; there was no significant convergence, but at the uncorrected threshold, the dorsal mid-insula was preferentially involved in disrupted interoception compared with psychological therapy).
Discussion
Influential theories propose a role for disrupted interoception across multiple psychiatric disorders (3, 19, 20, 48). Identifying common neural disruptions in interoception across traditional diagnostic categories could identify targets for novel transdiagnostic treatments. We used ALE meta-analysis to map the convergence of disrupted neural processing during interoception across psychiatric disorders and a variety of interoceptive probes. This revealed a transdiagnostic, domain-general convergence of disrupted activation in the left dorsal mid-insula. Studies comparing patients with unipolar and bipolar depression, anxiety, anorexia, and schizophrenia with healthy control subjects all showed differences that fell within this mid-insular cluster. Our follow-up conjunction and contrast analyses demonstrated that this cluster is spatially distinct from general affect circuitry and regions of neural change following current evidence-based psychological and pharmacological treatments. Altered processing of interoceptive information in the dorsal mid-insula may therefore represent a hitherto unidentified transdiagnostic common locus of disruption (19).
The locus of transdiagnostic differential neural activation we report is located on the precentral gyrus of the mid-insula (49). Nonhuman primate studies have established that the insula comprises three cytoarchitectonic subregions: a ventral-anterior agranular area, a mid-insular dysgranular zone, and a dorsal-posterior granular area (27, 50, 51). Human in vivo probabilistic tractography (52) and anatomical tracing studies in macaques (27) have revealed a graduated pattern of connectivity: agranular anterior insula projects to inferior frontal regions and some anterior temporal areas, while granular posterior insula projects primarily to posterior superior and middle temporal areas (52). In contrast, the mid-insular dysgranular zone possesses a hybrid connectivity pattern: the precentral insular gyrus projects to both frontal and temporal regions (27, 52), and its afferent inputs likewise originate from a hybrid of regions projecting to anterior and posterior insula (53). Moreover, while anterior and posterior insula show dense within-subregion connectivity (52), the mid-insula region identified here, in particular the precentral insular gyrus, projects to (27, 52) and receives input from (53) both anterior and posterior insula. This makes the mid-insula well placed to serve as an intermediary between the processing of sensory representation of bodily state in the posterior insula (31) and the abstract representation of affective state in the anterior insula (34), where the latter, as demonstrated empirically in our conjunction analysis, appears to be spatially distinct from our identified region of interoceptive dysfunction (31, 34, 38).
This functional anatomy of the precentral insular gyrus makes it an ideal candidate for the locus of processing of interoceptive prediction errors, which occur following a mismatch between generative expectations of physiological state and incoming signals from the body (19, 23, 25, 26). Influential theories have suggested that expectations of physiological state are communicated via projections from the ventral-anterior insula and fronto-cingulate regions to the mid-insula and posterior insula, with the mid- and posterior insula encoding any mismatch between these prior expectations and signals from the body (i.e., interoceptive prediction errors) (19, 23, 25, 26).
The dorsal mid-insula could represent a common locus of disruption emerging from other (domain-specific or pathology-specific) interoceptive changes. Some pathologies may originate from a primary dysfunction in interosensory signaling (e.g., an increased sensitivity to interoceptive stimuli, resulting in a higher weighting of prediction errors [54]), while others may stem from increased precision of prediction models, at the expense of prediction error signals (thought to be encoded by neuromodulators [55] or local GABAergic mechanisms [56]). We speculate that the unique hybrid architecture of the dysgranular dorsal mid-insula makes it a possible anatomical candidate for encoding of interoceptive prediction errors. This hypothesis requires testing in future studies employing tasks that manipulate the expectancy of interoceptive associations and fit learning models to trial-by-trial prediction errors (a paradigm commonly employed in exteroceptive predictive processing studies, e.g., 57). This approach could delineate the putative role of the dysgranular mid-insula in interoceptive predictive processing in psychiatric populations.
Two aspects of the location of our common cluster are surprising. The first is its laterality: previous findings strongly suggest a right lateralization of interoception (1). While our uncorrected results do include multiple smaller clusters in the right insula and claustrum, it is possible that this general lateralization of function is not reflected in right lateralization of disrupted function or that low power in the included studies resulted in weaker clustering on the right. In addition, it is surprising that our result does not overlap with the extensive affect network, given the strong overlap of interoceptive-frontotemporal regions subserving interoception and emotion in general (32, 58). We suggest that this originates from our locus occurring lower in the interoceptive hierarchy than might have been expected: the affect circuitry results clearly show a large, highly significant cluster in the anterior insula but not the mid- or posterior insula.
Limitations and Future Directions
The locus of disruption we report appears common across several disorders in our analysis: patients with unipolar and bipolar depression, anxiety, anorexia, and schizophrenia all fell within the cluster identified. Nevertheless, there may still exist anatomically distinct processing alterations in discrete disorders or specific transdiagnostic dimensions. The current literature does not contain sufficient data to provide the statistical power necessary for robust disorder-specific subanalyses. In addition, given the nature of our meta-analytic approach, it was not possible to conduct meta-regression analyses to examine the influence of sex and age on interoceptive differences in psychiatric disorders (59). This is because ALE tests for convergence of activation between studies, with no incorporation of different effect sizes (e.g., fMRI Z-score), a prerequisite for meta-regression (59). The exploration of latent variables that underlie our activation differences is an important avenue for future research. Although we conducted exploratory subgroup analyses of different symptom domains (see the Materials section in the online supplement), these subgroup analyses (as well as any future subgroup analyses testing effects of gender or age) will require a larger number of included studies to achieve sufficient statistical power and therefore remain a key question for future studies.
In addition, conclusions from the existing literature are limited by the fact that certain interoceptive domains are measured significantly more in some disorders than in others: disorder-specific results in the literature may partially be driven by task differences. By aggregating across studies that measure different interoceptive functions (nociception, respiration, cardiac attention, sensory touch, etc.), our meta-analysis is unable to disentangle the contribution of these task differences themselves. We did not have the statistical power to analyze convergent disrupted activation within specific interoceptive domains, despite the strong likelihood that different interoceptive channels are not necessarily integrated (42, 60). Therefore, we were unable to identify how the functional role of the insula (or any other region) in psychiatric disorders might differ across different features of interoception. Our result represents only a transdiagnostic, cross-domain alteration; in reality, transdiagnostic alterations could be observed within specific interoceptive domains. For example, in the respiratory domain, animal work has identified acid-sensing ion channels in the basolateral amygdala and bed nucleus of the stria terminalis that drive carbon-dioxide-evoked fear behavior; genetic variations in the sensitivity of these ion channels in humans relate to susceptibility to carbon dioxide challenge and, potentially, panic attacks (reviewed in reference 61).
Future neuroimaging work could better elucidate how interoceptive processing in the mid-insula (and elsewhere in interoceptive brain circuitry) might differ between particular (clusters of) disorders or symptom dimensions, consistent with recent dimensional neuroimaging approaches (62, 63). This would enable future neurocognitive treatment development focused on interoceptive loci and designed to modulate disrupted interoceptive processing, similar to the application of novel brain stimulation interventions to target particular transdiagnostic neurocognitive mechanisms (64, 65). This potential is further supported by our finding that neither antidepressant medication nor psychological therapy appears to evoke activation changes in this mid-insular region, albeit using mostly noninteroceptive tasks. This highlights the need for treatment studies employing interoceptive probes. However, existing interoceptive measures are limited in many respects: tasks often employ explicit interoceptive manipulations (e.g., 66), while interoception itself is usually unconscious (67), requiring invasive perturbation approaches (e.g., 68), and the timescales of interoceptive signals range vastly between systems (e.g., respiratory systems compared with gastric systems). Sophisticated computational approaches go some way toward parameterizing disruptions in interoceptive signaling (19) but are still subject to many of the above limitations and also usually require extremely lengthy procedures to estimate computational parameters.
Interoceptive processing is multidimensional. A recent consensus definition identified eight aspects of interoception: interoceptive attention (observing one’s internal bodily sensations), detection (reporting the presence or absence of a consciously reported sensation), magnitude (perceived intensity of a sensation), discrimination (localizing a sensation to a particular interoceptive channel), accuracy (how correct is one’s monitoring of sensations), insight (metacognitive evaluation of one’s interoceptive performance, i.e., the correspondence between accuracy and confidence), sensibility (a trait measure, the self-assessed tendency to focus on interoceptive stimuli), and self-report (assessed with psychometric questionnaires that can be state or trait assessments). These dimensions share some common features but likely have relatively distinct neural mappings (2, 69). All studies in this meta-analysis probed brain activation either by using bottom-up perturbations of sensory signals (e.g., aversive breathing load, affective touch, hunger, or pain) or by using top-down interoceptive attention instructions (e.g., “focus on your bladder”). No studies incorporated interoceptive accuracy, discrimination, or any other interoceptive behavioral measure into the neuroimaging analyses; future studies will need to establish how the neural representation of specific interoceptive dimensions differs in psychiatric disorders.
Additionally, in our meta-analysis, clinical sampling was limited to psychiatric disorders and related symptoms. The statistical constraints of our meta-analysis approach, which required studies on group differences in order to construct a map of convergent different activation between groups (41, 45), required us to exclude the large number of studies on interoception in healthy individuals alone, which has been formative in the field’s mechanistic understanding of interoceptive processes (reviewed in reference 42). We also did not include, for example, pain disorders (70, 71), functional gastrointestinal disorders (72), functional neurological disorders (16), or connective tissue conditions (73), although, unsurprisingly, interoception also differs across these and other conditions involving a markedly different bodily experience. As such, our analysis cannot adjudicate whether differential activation in the mid-insula represents a common locus for all disorders where interoceptive differences are implicated or whether it is specific to the psychiatric conditions we examined here. That said, in chronic pain disorders, pain-related mental health indices predict quality of life over and above physical pain variables (74, 75), suggesting (20) that the role of interoceptive disruption in disorders not classically considered psychiatric may not be as distinct from the present data as one might assume. Nevertheless, the degree to which this role is distinct or overlaps with the role of disrupted interoception in psychiatric disorders remains to be determined.
Lastly, but crucially, many studies included in this meta-analysis, as in the wider neuroscience field (76, 77), were underpowered to detect all but relatively large effect sizes. This increases the likelihood that at least some of the coordinates from studies included in our meta-analysis were false positives. However, the cluster-level thresholding we employed helps to mitigate the potential contribution of false positives: a simulation of 120,000 ALE meta-analyses shows that this technique can control for excessive contribution of single experiments as long as 17 or more experiments are included (47). However, small sample sizes in contributing studies does mean that true effects could be excluded or underrepresented in our meta-analysis as a result of lack of power in the original studies (i.e., increasing the likelihood of false negatives in our study). This can only be remedied by future large-scale neuroimaging studies characterizing interoception in patient groups.
Conclusions
Empirical work and theoretical models have proposed a core disruption in interoceptive neural processing across psychiatric disorders. Most previous neuroimaging work consists of studies of discrete diagnostic groups using a single interoceptive probe, an approach that is poorly suited to identifying common loci of disruption. Here, we reported on a transdiagnostic domain-general locus of disruption in the dorsal mid-insula. We proposed, in the interoceptive predictive coding framework, that mid-insular convergence could reflect a disruption in interoceptive prediction error signaling that represents a common pathway of interoceptive dysfunction across disorders with distinct pathologies. Other computational frameworks have identified similar regions in the neural computation of punishment or loss magnitude (78), a process also implicated in many neuropsychiatric disorders (e.g., 79–83). The particular convergence of activation differences we identified in the dorsal mid-insula projects to both frontal and temporal regions, making it a putative intermediary between posterior insula representation of bodily state and anterior insula representation of affective state (31, 32). This common pathway almost certainly represents only part of the neural changes underpinning disrupted interoception in psychiatric disorders. A fuller understanding of the complex psychopathology and domain-specific changes in interoceptive processing will require robust, well-powered assessments of multiple interoceptive domains of psychiatric dimensions and, ideally, incorporation of these measures into trials of current and novel treatment approaches.
1. : Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 2003; 13:500–505Crossref, Medline, Google Scholar
2. : Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:501–513Crossref, Medline, Google Scholar
3. : Interoception and drug addiction. Neuropharmacology 2014; 76(pt B):342–350Crossref, Medline, Google Scholar
4. : Decreased interoceptive awareness in patients with substance use disorders. J Subst Use 2017; 22:60–65Crossref, Google Scholar
5. : Heartbeat perception in depression. Behav Res Ther 2007; 45:1921–1930Crossref, Medline, Google Scholar
6. : Differential effects of anxiety and depression on interoceptive accuracy. Depress Anxiety 2009; 26:167–173Crossref, Medline, Google Scholar
7. : Clinical, psychopathological and personality correlates of interoceptive awareness in anorexia nervosa, bulimia nervosa and obesity. Psychopathology 2004; 37:168–174Crossref, Medline, Google Scholar
8. : Interoceptive sensitivity deficits in women recovered from bulimia nervosa. Eat Behav 2013; 14:488–492Crossref, Medline, Google Scholar
9. : Reduced perception of bodily signals in anorexia nervosa. Eat Behav 2008; 9:381–388Crossref, Medline, Google Scholar
10. : Atypical self-focus effect on interoceptive accuracy in anorexia nervosa. Front Hum Neurosci 2016; 10:484Crossref, Medline, Google Scholar
11. : Altered interoceptive awareness in anorexia nervosa: effects of meal anticipation, consumption and bodily arousal. Int J Eat Disord 2015; 48:889–897Crossref, Medline, Google Scholar
12. : Accurate heartbeat perception in panic disorder: fact and artefact. J Affect Disord 1997; 43:121–130Crossref, Medline, Google Scholar
13. : Interoceptive accuracy and panic. Behav Res Ther 1999; 37:1141–1158Crossref, Medline, Google Scholar
14. : Interoception and panic disorder. Adv Behav Res Ther 1993; 15:3–21Crossref, Google Scholar
15. : The roles of interoceptive sensitivity and metacognitive interoception in panic. Behav Brain Funct 2015; 11:14Crossref, Medline, Google Scholar
16. : Interoceptive awareness in patients with functional neurological symptoms. Biol Psychol 2016; 113:68–74Crossref, Medline, Google Scholar
17. : Autonomic imbalance is associated with reduced facial recognition in somatoform disorders. J Psychosom Res 2011; 71:232–239Crossref, Medline, Google Scholar
18. : Is interoceptive awareness really altered in somatoform disorders? testing competing theories with two paradigms of heartbeat perception. J Abnorm Psychol 2012; 121:719–724Crossref, Medline, Google Scholar
19. : Computational psychosomatics and computational psychiatry: toward a joint framework for differential diagnosis. Biol Psychiatry 2017; 82:421–430Crossref, Medline, Google Scholar
20. : Allostatic self-efficacy: a metacognitive theory of dyshomeostasis-induced fatigue and depression. Front Hum Neurosci 2016; 10:550Crossref, Medline, Google Scholar
21. : The neurobiology of interoception in health and disease. Ann N Y Acad Sci 2018; 1428:112–128Crossref, Medline, Google Scholar
22. : Charting the landscape of priority problems in psychiatry, part 1: classification and diagnosis. Lancet Psychiatry 2016; 3:77–83Crossref, Medline, Google Scholar
23. : Extending predictive processing to the body: emotion as interoceptive inference. Behav Brain Sci 2013; 36:227–228Crossref, Medline, Google Scholar
24. : Active inference, homeostatic regulation and adaptive behavioural control. Prog Neurobiol 2015; 134:17–35Crossref, Medline, Google Scholar
25. : Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 2013; 17:565–573Crossref, Medline, Google Scholar
26. : Interoceptive predictions in the brain. Nat Rev Neurosci 2015; 16:419–429Crossref, Medline, Google Scholar
27. : Insula of the old world monkey, I: architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol 1982; 212:1–22Crossref, Medline, Google Scholar
28. : Transdiagnostic approaches to mental health problems: current status and future directions. J Consult Clin Psychol 2020; 88:179–195Crossref, Medline, Google Scholar
29. : Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Link, Google Scholar
30. : Transdiagnostic expression of interoceptive abnormalities in psychiatric conditions. https://www.medrxiv.org/content/10.1101/19012393v1Google Scholar
31. : How do you feel? interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666Crossref, Medline, Google Scholar
32. : Interoception and emotion. Curr Opin Psychol 2017; 17:7–14Crossref, Medline, Google Scholar
33. : On the embodiment of emotion regulation: interoceptive awareness facilitates reappraisal. Soc Cogn Affect Neurosci 2013; 8:911–917Crossref, Medline, Google Scholar
34. : Anterior insular cortex and emotional awareness. J Comp Neurol 2013; 521:3371–3388Crossref, Medline, Google Scholar
35. : Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry 2020; 177:411–421Link, Google Scholar
36. : Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry 2017; 174:676–685Link, Google Scholar
37. : Taking time to feel our body: steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology 2017; 54:469–482Crossref, Medline, Google Scholar
38. : The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb Cortex 2016; 26:1910–1922Crossref, Medline, Google Scholar
39. : Meta-analyses of the neural mechanisms and predictors of response to psychotherapy in depression and anxiety. Neurosci Biobehav Rev 2018; 95:61–72Crossref, Medline, Google Scholar
40. : Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry 2015; 20:311–319Crossref, Medline, Google Scholar
41. : Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev 2018; 84:151–161Crossref, Medline, Google Scholar
42. : Neural correlates of heart-focused interoception: a functional magnetic resonance imaging meta-analysis. Philos Trans R Soc Lond B Biol Sci 2016; 371:20160018Crossref, Medline, Google Scholar
43. : Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007; 28:1194–1205Crossref, Medline, Google Scholar
44. : Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009; 30:2907–2926Crossref, Medline, Google Scholar
45. : Activation likelihood estimation meta-analysis revisited. Neuroimage 2012; 59:2349–2361Crossref, Medline, Google Scholar
46. : Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 2012; 33:1–13Crossref, Medline, Google Scholar
47. : Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 2016; 137:70–85Crossref, Medline, Google Scholar
48. : Interoception in anxiety and depression. Brain Struct Funct 2010; 214:451–463Crossref, Medline, Google Scholar
49. : MRI-based definition of a stereotactic two-dimensional template of the human insula. Stereotact Funct Neurosurg 2009; 87:385–394Crossref, Medline, Google Scholar
50. : Anatomy of the insula functional and clinical correlates. Aphasiology 1999; 13:55–78Crossref, Google Scholar
51. : Microstructural organization of human insula is linked to its macrofunctional circuitry and predicts cognitive control. eLife 2020; 9:e53470Crossref, Medline, Google Scholar
52. : The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. Neuroimage 2012; 59:3514–3521Crossref, Medline, Google Scholar
53. : Insula of the old world monkey, II: afferent cortical input and comments on the claustrum. J Comp Neurol 1982; 212:23–37Crossref, Medline, Google Scholar
54. : Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. J Psychiatry Neurosci 2016; 41:304–311Crossref, Medline, Google Scholar
55. : Free energy, precision and learning: the role of cholinergic neuromodulation. J Neurosci 2013; 33:8227–8236Crossref, Medline, Google Scholar
56. : Neural circuitry of reward prediction error. Annu Rev Neurosci 2017; 40:373–394Crossref, Medline, Google Scholar
57. : Adults with autism overestimate the volatility of the sensory environment. Nat Neurosci 2017; 20:1293–1299Crossref, Medline, Google Scholar
58. : Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex 2017; 88:124–142Crossref, Medline, Google Scholar
59. : Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 2011; 127:46–57Crossref, Medline, Google Scholar
60. : Multichannel investigation of interoception: sensitivity is not a generalizable feature. Front Hum Neurosci 2018; 12:223Crossref, Medline, Google Scholar
61. : Interoception, conditioning, and fear: the panic threesome. Psychophysiology 2019; 56:e13421Crossref, Medline, Google Scholar
62. : Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry 2015; 20:345–352Crossref, Medline, Google Scholar
63. : The neurochemical substrates of habitual and goal-directed control. Transl Psychiatry 2020; 10:84Crossref, Medline, Google Scholar
64. : Cortical paired associative stimulation influences response inhibition: cortico-cortical and cortico-subcortical networks. Biol Psychiatry 2018; 85:355–363Crossref, Medline, Google Scholar
65. : The effect of frontoparietal paired associative stimulation on decision-making and working memory. Cortex 2019; 117:266–276Crossref, Medline, Google Scholar
66. : Internal focus of attention in anxiety-sensitive females up-regulates amygdale activity: an fMRI study. J Neural Transm (Vienna) 2014; 121:1417–1428Crossref, Medline, Google Scholar
67. : The observable unconscious and the inferable conscious in current Soviet psychophysiology: interoceptive conditioning, semantic conditioning, and the orienting reflex. Psychol Rev 1961; 68:1–147Crossref, Medline, Google Scholar
68. : Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychol Med 2018; 48:142–154Crossref, Medline, Google Scholar
69. : Brain responses and self-reported indices of interoception: heartbeat evoked potentials are inversely associated with worrying about body sensations. Physiol Behav 2017; 180:1–7Crossref, Medline, Google Scholar
70. : Anhedonia to gentle touch in fibromyalgia: normal sensory processing but abnormal evaluation. Brain Sci 2020; 10:306Crossref, Google Scholar
71. : Pain in the body—altered interoception in chronic pain conditions: a systematic review. Neurosci Biobehav Rev 2016; 71:328–341Crossref, Medline, Google Scholar
72. : Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 2003; 103:99–110Crossref, Medline, Google Scholar
73. : Neuroimaging and psychophysiological investigation of the link between anxiety, enhanced affective reactivity and interoception in people with joint hypermobility. Front Psychol 2014; 5:1162Medline, Google Scholar
74. : Self efficacy as a mediator of the relationship between pain intensity, disability and depression in chronic pain patients. Pain 1999; 80:483–491Crossref, Medline, Google Scholar
75. : Musculoskeletal pain in primary health care: subgroups based on pain intensity, disability, self-efficacy, and fear-avoidance variables. J Pain 2007; 8:67–74Crossref, Medline, Google Scholar
76. : Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14:365–376Crossref, Medline, Google Scholar
77. : Power-up: a reanalysis of “power failure” in neuroscience using mixture modelling. J Neurosci 2017; 37:8051–8061Crossref, Medline, Google Scholar
78. : Critical roles for anterior insula and dorsal striatum in punishment-based avoidance learning. Neuron 2012; 76:998–1009Crossref, Medline, Google Scholar
79. : Sensitivity to reward and punishment in eating disorders. Psychiatry Res 2010; 177:1–11Crossref, Medline, Google Scholar
80. : Exaggerated loss-specific Pavlovian-instrumental transfer in unmedicated major depression. Sci Rep 2018; 8:1–10Medline, Google Scholar
81. : Disrupted habenula function in major depression. Mol Psychiatry 2017; 22:202–208Crossref, Medline, Google Scholar
82. : Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry 2014; 75:631–638Crossref, Medline, Google Scholar
83. : Carrots and sticks fail to change behavior in cocaine addiction. Science 2016; 352:1468–1471Crossref, Medline, Google Scholar



