Clozapine Intoxication in COVID-19
Clozapine is regarded as a very effective antipsychotic (1). For efficacy in schizophrenia, an expert guideline recommends trough steady-state clozapine concentrations of 350–600 μg/L (2).
Mr. A, a 46-year-old Caucasian nonsmoker, lives at an institution for people with intellectual disabilities. He is known to have a severe intellectual disability of unknown cause as well as schizophrenia, for which he takes 200 mg of clozapine twice daily. The clozapine trough level at steady state was 553 μg/L, the clozapine level/dose (C/D) ratio 1.4, and the clozapine/norclozapine (C/N) ratio 1.7 (Table 1).
Mr. A’s past medical history records several infections, including one episode of bacterial pneumonia, two of bronchitis, and an infection of the upper respiratory tract, not described in further detail (see Table 1). With the bronchitis episodes and the upper respiratory tract infection, the clozapine level did not rise further than 661 μg/L (C/D ratio, 1.7). With the bacterial pneumonia, the level rose to 1,183 μg/L (C/D ratio, 2.4). On several occasions, including during the bacterial pneumonia, halving the dosage of clozapine led to psychotic decompensation. On the basis of these clinical experiences, it was concluded that for this patient the dosage of clozapine could not be automatically halved when signs of inflammation appeared, given that in several cases respiratory tract infections had not resulted in a doubling of clozapine levels and halving the dosage resulted in lower, evidently subtherapeutic levels.
In April 2020, during the COVID-19 pandemic, Mr. A was found to have sonorous wheezes, audible without stethoscope, but no further clinical symptoms or abnormal test results (see Table 1 for the progression of the case). When COVID-19 was suspected (day 1), isolation measures were taken, a nasopharyngeal swab was collected, and a blood test was conducted. The clozapine level was in accordance with baseline. The rest of the blood tests showed no abnormalities, but the nasopharyngeal swab tested positive for COVID-19.
From day 4 onward, Mr. A became increasingly ill. He was sweating and his face was pale, with blue lips. His temperature rose to 38.4°C. He also showed possible signs of clozapine intoxication, namely, ataxia and tremors. He was given oxygen and paracetamol. Given his history of psychotic decompensation after his clozapine dosage was halved during fever, from day 4 onward the dose given depended on whether his temperature was ≥38°C. If it was, the dosage was reduced to 300 mg per day (days 4, 5, and 7); on day 6 the dosage went back to 400 mg/day (the patient’s normal daily dose). During days 4 to 6, Mr. A seemed lethargic, with sweating, echolalia, and reduced initiative. His temperature fluctuated between 37.1 and 38.7°C. On day 7, the patient’s condition deteriorated further, with a temperature of 39.4°C. On auscultation, crackles could be heard at the base of the right lung. Because bacterial pneumonia was suspected in addition to COVID-19, the patient was given amoxicillin/clavulanic acid 500/125 mg t.i.d. for 7 days and the blood tests were repeated. His co-medication in this period was atenolol, diazepam, esomeprazole, and lithium. The clozapine trough level turned out to be three times as high as usual: 1,814 μg/L. Because this was not a steady-state situation, a C/D ratio of 5 was calculated using the mean dosage over the past 5 days. The C/N ratio was 3.5. At this point clozapine was stopped altogether. From day 9 onward, the patient began to recover. At that point the clozapine level was 1,335 μg/L. From day 10 onward, after the clozapine had been discontinued for 48 hours, the clozapine dosage was cautiously increased again. On day 13, the clozapine level was 213 μg/L (dosage, 50 mg/day), and on day 19, 107 μg/L (dosage 100 mg/day). On days 30 and 37, the serum clozapine levels were normal, as were the C/D and C/N ratios; the patient had clinically recovered and the clozapine dosage had now been increased to 350 mg/day (Figure 1).
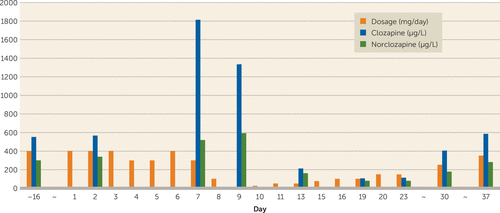
FIGURE 1. Clozapine dosage and serum levels of clozapine and norclozapine in a patient before and during a COVID-19 infection
It is well known that an inflammatory response associated with respiratory tract infections may cause increased clozapine serum levels, leading to symptoms of clozapine intoxication such as sedation and lethargy, sialorrhea, ataxia, convulsions, and ECG abnormalities (3–5). Infections cause relevant increases in clozapine levels only when there are systemic manifestations of fever with C-reactive protein (CRP) elevations and a significant cytokine release (6). Mild respiratory infections without fever or leukocytosis usually cause minimal elevations.
| Year or Day | Clinical | O2 Sat. | Respiratory Rate (min) | Temperature (°C) | CRP (mg/L) | WBC (×109 cells/L) | Neutrophils (×109 cells/L) | Clozapine dosage (mg/day)a | Clozapine level (ng/mL)b | Norclozapine level (ng/mL)b | C/Dc | C/Nd | Treatment Strategy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Past history (through April 2020) | |||||||||||||
| 2013 | Bronchitis | 36 | 300 | 210 | ? | 0.7 | ? | ||||||
| 2018 | Pneumonia | 37 | 22 | 500 | 1,183 | 490 | 2.4 | 2.4 | |||||
| 2019 | Bronchitis | 37 | 240 | 264 | 170 | 1.1 | 1.5 | ||||||
| 2019 | Pneumonia | 38.1 | 47 | 450 | 817 | 350 | 1.8 | 2.3 | |||||
| 2020 | Upper respiratory tract infection | 37.6 | 39 | 400 | 661 | 380 | 1.7 | 1.7 | |||||
| Current case (April 2020) | |||||||||||||
| Baselinee | 9 | 6.4 | 400 | 553 | 300 | 1.4 | 1.8 | ||||||
| 1 | Sonorous wheezes audible without stethoscope. Nasopharyngeal swab, PCR SARS-coronavirus-2 RNA: positive | 95%–96% | 14 | 36.9 | 400 | Home quarantine | |||||||
| 2 | Sedimentation rate, 2 mm/hour | 37.0–37.5 | 9 | 5.3 | 4.2 | 400 | 565 | 340 | 1.4 | 1.7 | |||
| 4 | Tremors in arms and legs, unsteady on feet, sweating, pale face, blue lips | 38.4 | 300 | Halve clozapine dosage per dose at temperatures ≥38°C. Start oxygen 1 liter/minute, start paracetamol 1,000 mg t.i.d. | |||||||||
| 5 | Clinical deterioration, loss of initiative, echolalia | 24 | 38.7 | 13 | 300 | Increase oxygen to 2 liters/minute | |||||||
| 6 | Clinical deterioration, increased fatigue | 93% | 24 | 37.1–38.1 | 400 | Increase oxygen to 3 liters/minute | |||||||
| 7 | Lungs: vesicular breath sounds, dubious basal right crackles. Gross tremors in arms and hands. Clinical deterioration. Working diagnosis: bacterial pneumonia in addition to COVID-19 | 90%–96% | 39.4 | Unknown (test failed) | 6 | 5.2 | 300 | 1,814 | 520 | 5.0 | 3.5 | Increase oxygen to 4 liters/minute. Start amoxicillin/clavulanic acid 500/125 mg t.i.d. for 7 days | |
| 8 | Clinical deterioration, increased fatigue | 98% | 20 | 100 | |||||||||
| 9 | Clinical improvement: more active | 95% | 20 | 38.4 | 2.1 | 1.3 | 0 | 1,335 | 590 | 2.2 | Reduce oxygen to 3 liters/minute | ||
| 10 | Clinical improvement | 95%–98% | 37.6–38.0 | 25 | |||||||||
| 11 | Clinical improvement | 93%–99% | 16 | 36.7–38.1 | 50 | ||||||||
| 12 | Clinical deterioration: fatigue, reduced alertness | 95%–97% | 20 | 37.3 | 50 | ||||||||
| 13 | Clinical deterioration: fatigue, dyspnea, drowsy | 88%–99% | 14 | 35.2 | 3.1 | 1.8 | 50 | 213 | 160 | 1.3 | |||
| 14 | Clinical improvement, but tired | 18 | 37.8 | 50 | Reduce oxygen to 2 liters/minute | ||||||||
| 15 | Clinical improvement | 97% | 16 | 36.3 | 75 | ||||||||
| 16 | Clinical improvement | 98% | 14 | 36.9 | 100 | Reduce oxygen to 1 liter/minute | |||||||
| 17, 18 | Clinical improvement | 100 | |||||||||||
| 19 | Clinical improvement | 95%–98% | 18 | 36.3 | 10 | 7.5 | 100 | 107 | 80 | 1.4 | 1.3 | No extra oxygen | |
| 20 | Clinical improvement | 99% | 36.1 | 150 | Stop extra oxygen. Continue home quarantine in accordance with guidelines. Clozapine titration schedule to usual dose of 400 mg/day, in accordance with Clozapine Plus Werkgroep guideline | ||||||||
| 23 | Clinical improvement | 6.8 | 4.2 | 150 | 112 | 80 | 1.1 | 1.4 | |||||
| 30 | Clinically recovered | 7.4 | 5.2 | 250 | 406 | 180 | 1.6 | 2.7 | |||||
| 37 | Clinically recovered | 6.7 | 4.3 | 350 | 583 | 280 | 1.7 | 2.0 | |||||
TABLE 1. Past history and case history of a patient on clozapine before and during a COVID-19 infection
Clozapine is primarily metabolized in the liver via the cytochrome P450 system (7, 8). About 70% is metabolized to norclozapine by CYP1A2. With inflammation, cytokines are increased, and interleukin 1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), interferon-α (IFN-α), and IFN-γ in particular seem to inhibit the activity of CYP1A2 (3, 7, 9). This results in an increase in the clozapine level. However, symptoms of intoxication are not observed in all cases of high clozapine levels. A possible explanation of this is that in plasma, 95% of clozapine is bound to the acute-phase protein alpha-1-acid glycoprotein (AGP), the concentration of which rises during an inflammatory response. It is thought that an increase in AGP leads to an elevation in the protein-bound concentration of clozapine, while the unbound concentration seems to be less affected (10). The AGP concentrations and the degree of increase during an infection vary depending on the individual and the situation (11).
Other factors that can cause a reduction in the metabolic activity of CYP1A2 are cessation of smoking or of inducing drugs and coadministration of CYP1A2-inhibiting drugs, including caffeine (12). All these factors were unchanged or absent in Mr. A.
The degree of increase in the clozapine level associated with inflammation varies across cases. The literature identifies a median clozapine level increase of 48% in inflammation (CRP ≥5 mg/L) (5). Based on this, it is recommended that if there are any signs of inflammation, the clozapine dosage should be halved and clozapine levels monitored (5). However, some patients with infections have normal levels, whereas in others the levels more than triple (3, 4). In our patient’s case, the clozapine level more than tripled in the presence of pneumonia and COVID-19. On this occasion, the level was considerably higher than with previous respiratory tract infections. Unfortunately, measurement of CRP failed during this episode, so the degree of inflammation cannot be compared with that in earlier episodes of respiratory tract infection in this patient.
The C/D ratio is a measure of clozapine clearance. For a reliable interpretation, a steady state is necessary. With changing clozapine dosages, the mean dosage over the 5 days before blood sampling has been proposed as an acceptable basis for calculating the C/D ratio (6). Because steady-state levels were not possible during infection, we used mean dosage levels calculated over the preceding 5 days (day 7 and day 19). A very low C/D ratio indicates a rapid metabolizer, while a very high C/D ratio indicates a poor metabolizer (average ranges, Caucasians, 0.6–1.2; Chinese, 1.2–2.4) (6–13). The C/D ratio at a steady state may help to assess whether and by how much the dosage should be reduced if a baseline C/D ratio is known. For this purpose, the current clozapine dosage is multiplied by the baseline C/D ratio and divided by the actual C/D ratio. This is the dosage for reaching the new steady state baseline level again. However, if the level is much higher or there are clinical symptoms of intoxication, clozapine treatment must first be temporarily suspended so that the concentration falls back to the desired level. It is advisable to wait at least until any symptoms of intoxication have clinically disappeared (4).
In Mr. A, an increase in the C/N ratio was also observed, which may indicate an inhibition of CYP1A2 (14) (see Table 1). However, a recent review found no evidence that the C/N ratio is a measure of CYP1A2 activity (13).
In this patient, we found a greater increase in the clozapine level with this respiratory tract infection than with previous respiratory tract infections. An increase in cytokines, including IL-1β, IL-6 and TNF-α, in COVID-19 has been reported, and this increase may develop into cytokine storm syndrome (15). The degree of cytokine increase correlates with the severity of the illness and the prognosis. SARS-CoV is accompanied by a greater cytokine increase than other viral infections, such as influenza and respiratory syncytial virus (16). In bacterial pneumonia, a cytokine storm is rare. This may explain why Mr. A’s clozapine level rose considerably more than with previous respiratory tract infections. The progression of his illness suggests that a COVID-19 infection may cause an extreme elevation in clozapine levels. If this is the case, halving the clozapine dosage in COVID-19 in accordance with the recent recommendation (17) will not be sufficient.
In summary, COVID-19 may be associated with hyperinflammation and extremely severe pneumonia. Our case illustrates that this can lead to an unexpectedly high increase in the clozapine level, with the danger of intoxication. The recommendation to reduce the dosage of clozapine by half is therefore probably not cautious enough. Frequent monitoring of the patient for any symptoms of intoxication and monitoring the clozapine levels may help to determine the dose day by day to avoid both underdosing, with the risk of psychotic relapse, and overdosing, with the risk of intoxication. Calculating the C/D ratio may help with this.
1 : Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019; 394:939–951Crossref, Medline, Google Scholar
2 : Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry 2018; 51:9–62Crossref, Medline, Google Scholar
3 : Respiratory infections rather than antibiotics may increase clozapine levels: a critical review of the literature. J Clin Psychiatry 2004; 65:1144–1145Crossref, Medline, Google Scholar
4 : Infection-associated clozapine toxicity. Clin Schizophr Relat Psychoses 2011; 5:159–160Crossref, Medline, Google Scholar
5 : Inflammation and psychotropic drugs: the relationship between C-reactive protein and antipsychotic drug levels. Psychopharmacology (Berl) 2016; 233:1695–1705Crossref, Medline, Google Scholar
6 : Around 3% of 1,300 levels were elevated during infections in a retrospective review of 131 Beijing hospital in-patients with more than 24,000 days of clozapine treatment. Psychother Psychosom 2020; 89:255–257Crossref, Medline, Google Scholar
7 : Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol 2006; 46:123–149Crossref, Medline, Google Scholar
8 : Personalizing dosing of risperidone, paliperidone, and clozapine using therapeutic drug monitoring and pharmacogenetics. Neuropharmacology 2020; 168:107656Crossref, Medline, Google Scholar
9 : Bacterial pneumonia can increase serum concentration of clozapine. Eur J Clin Pharmacol 2002; 58:321–322Crossref, Medline, Google Scholar
10 : Unbound fraction of clozapine significantly decreases with elevated plasma concentrations of the inflammatory acute-phase protein alpha-1-acid glycoprotein. Clin Pharmacokinet 2019; 58:1069–1075Crossref, Medline, Google Scholar
11 : Elevated clozapine levels associated with infection: a systematic review. Schizophr Res 2018; 192:50–56Crossref, Medline, Google Scholar
12 : Assessing drug-drug interactions through therapeutic drug monitoring when administering oral second-generation antipsychotics. Expert Opin Drug Metab Toxicol 2016; 12:407–422Crossref, Medline, Google Scholar
13 : A comprehensive review of the clinical utility of and a combined analysis of the clozapine/norclozapine ratio in therapeutic drug monitoring for adult patients. Expert Rev Clin Pharmacol 2019; 12:603–621Crossref, Medline, Google Scholar
14 : Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993–2007. Ther Drug Monit 2010; 32:438–447Crossref, Medline, Google Scholar
15 : The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect 2020; 80:607–613Crossref, Medline, Google Scholar
16 : Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol 2006; 78:417–424Crossref, Medline, Google Scholar
17 : Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci 2020; 45:222–223Crossref, Medline, Google Scholar



