Brain Reward System Dysfunction in Adolescence: Current, Cumulative, and Developmental Periods of Depression
Abstract
Objective:
Reward system dysfunction is a well-known correlate and predictor of depression in adults and adolescents, with depressed individuals showing blunted (hyporeactive) striatal response to monetary rewards. Furthermore, studies of remitted depression suggest network-wide hyporeactivity of striatal (caudate, putamen, nucleus accumbens) and cortical (insula, anterior cingulate cortex [ACC]) regions even in the absence of current symptoms. Thus, it remains unclear which patterns of hyporeactivity represent a trait-like indicator of depression and which represent a current depressed state. The authors examined the relationships between regions of a cortico-striatal circuit supporting reward processing and both current depression and cumulative depression history.
Methods:
Using a functional MRI monetary reward task, the authors measured brain response to monetary gains and losses in a longitudinal sample of adolescents (N=131) who had been annually assessed for psychiatric symptoms since ages 3–5 years.
Results:
Current depression severity was associated with hyporeactivity exclusively in the nucleus accumbens in response to the anticipation of a reward, while cumulative depression severity was associated with blunted response to anticipation across a cortico-striatal circuit (striatum, ACC, insula). Follow-up analyses investigating the effects of depression on reward processing at different developmental stages revealed a similar pattern: recent depression severity during adolescence was associated with more focal hyporeactivity in the nucleus accumbens, while depression severity during early childhood (i.e., preschool) was associated with more global hyporeactivity across the cortico-striatal circuit.
Conclusions:
The study findings indicate important distinctions between disruptions in reward system neural circuitry associated with a history of depression (particularly early-onset depression) and current depression. These results have implications for understanding the etiology and treatment of reward processing deficits in depression.
Reward system dysfunction (deficits in reward learning and reduced response to gains in the reward network of the brain) appears to be concurrently and prospectively related to depression in adults and in adolescents. Blunted responses in the striatum (caudate, putamen, nucleus accumbens) to rewarding stimuli are found in depressed individuals (1, 2), predict later depressive episode (3–5), and are found in the offspring and first-degree relatives of depressed individuals (6, 7). This suggests that reward system dysfunction may serve as a candidate neural correlate or even risk factor for depression. Our goal in this study was to examine the relationships between regions of a cortico-striatal circuit supporting reward processing and both current depression and cumulative depression history in a sample of adolescents who have been participating since preschool in a longitudinal study of early-onset depression.
In addition to being present in currently depressed individuals, blunted reward responses have been reported in studies of patients in remission from depression, both behaviorally (8) and in the ventral striatum (9). However, brain responses to reward occur in regions beyond the striatum, engaging a broader cortico-striatal circuit. Individuals in remission show blunted neural responses to reward in this broader circuit, including in the anterior cingulate cortex (ACC) (10–12) and the insula (13). Such findings suggest the possibility that hyporeactivity of a broader set of regions within the cortico-striatal circuit persists beyond the depressive episode, potentially putting patients at risk of recurrence, particularly with early-onset depression. Specifically, subcortical striatal function may be disrupted concurrently with depression. If so, such striatal disruption in early-onset depression may in turn contribute to disruption in the development of cortical regions and their functions. In later-onset depression, these cortical areas are more developed, and thus their function may be less disrupted by depression. Few studies have been able to simultaneously examine both current depression severity and past history of depression (particularly using prospective data) to test this hypothesis. Thus, our longitudinal sample presents a unique opportunity for testing the prediction that current depression is associated with a more focal blunted response to rewards in the striatum, while previous or cumulative depression (especially early-onset depression) is associated with a more global blunted response across the cortico-striatal circuit.
Furthermore, there is evidence that depression can be related to dysfunction in both reward anticipation and receipt. Blunted reactivity to rewarding cues (anticipation) and outcomes (receipt) has been associated with depression in adolescents (1, 2, 6, 14–16), suggesting deficits in the experience of rewards or hedonic tone. However, other studies have observed blunted responsivity only to reward anticipation (5, 7, 17, 18). Therefore, determining whether depression is more strongly related to reward anticipation or receipt will help clarify the neural and behavioral mechanisms that contribute to reward processing dysfunction related to depression.
We tested these predictions using functional MRI (fMRI) responses in regions of the brain’s reward system (e.g., cortico-striatal network) to monetary rewards and losses in a longitudinal sample of adolescents who have been annually assessed for psychiatric symptoms since early childhood. We examined neural responses related to both current depression severity and cumulative depression severity since early childhood. Because participants were followed longitudinally, we had measures of depression severity prospectively acquired at annual assessment waves using clinical interviews rather than based on retrospective report. Furthermore, while previous studies have typically compared healthy control subjects with depressed patients as a group (1, 2, 14, 16), in this study we used continuous measures of depression severity, which are more reliable than categorical measures of psychopathology (19) and are in line with the Research Domain Criteria framework (20, 21). We also tested the hypothesis that early onset of depression would be associated with blunting in a broader network of reward-responsive regions as compared with only current depression by comparing the relationship between cortico-striatal activation and depression symptoms reported at assessment waves during three different developmental periods: preschool, school age, and adolescence.
Methods
Participants
A total of 306 children, 3–6 years old at baseline, oversampled for symptoms of depression, were recruited in the St. Louis metropolitan area for participation in a study of preschool-onset depression. Details of recruitment have been reported previously (22, 23). An imaging phase of the study started when the sample reached school age (8–14 years old), at which time 216 children were eligible. An additional 42 children, 9–14 years old, with no history of psychopathology at the time of recruitment, were enrolled in the study to increase the sample size starting at the first imaging wave (see the online supplement for further information). Of these 258 children, 148 participated in the current, fourth wave of imaging. Of those, 131 had usable data (seven were excluded for unusable fMRI data, eight for excessive motion, and two for too few trials with responses). Table 1 summarizes the participants’ demographic and clinical characteristics (see the online supplement for the study timeline). Because some participants were recruited at later waves, results are described both for the full sample, consisting of 131 youths, and for a subsample of 109 participants with initial preschool-age assessments. Parental written consent and child assent were obtained before participation, and the Washington University School of Medicine Institutional Review Board approved all procedures.
| Sample and Characteristic | ||
|---|---|---|
| Full sample (N=131) | ||
| N | % | |
| Female | 67 | 51.1 |
| Race | ||
| Caucasian | 73 | 55.7 |
| African American | 51 | 38.9 |
| Other | 7 | 5.3 |
| Psychotropic medication use in past 48 hours | 15 | 11.5 |
| Mean | SD | |
| Age at scan (years) | 16.39 | 1.11 |
| Income-to-needs ratiob | 3.36 | 2.17 |
| Cumulative depression symptoms (AUC) | 1.90 | 1.24 |
| Childhood Depression Inventory, T-scorec | 48.48 | 8.27 |
| Preschool depression symptoms (AUC) | 2.32 | 1.62 |
| School-age depression symptoms (AUC) | 2.19 | 1.44 |
| Adolescent depression symptoms (AUC) | 1.62 | 1.45 |
| Subsample followed since preschool (N=109) | ||
| N | % | |
| Female | 57 | 51.8 |
| Race | ||
| Caucasian | 62 | 55.5 |
| African American | 42 | 38.2 |
| Other | 6 | 5.5 |
| Psychotropic medication use in past 48 hours | 15 | 13.6 |
| Mean | SD | |
| Age at scan (years) | 16.48 | 1.00 |
| Income-to-needs ratiob | 3.40 | 2.22 |
| Cumulative depression symptoms (AUC) | 2.07 | 1.21 |
| Childhood Depression Inventory, T-score | 49.05 | 8.54 |
| Preschool depression symptoms (AUC) | 2.34 | 1.62 |
| School-age depression symptoms (AUC) | 2.29 | 1.43 |
| Adolescent depression symptoms (AUC) | 1.77 | 1.47 |
TABLE 1. Demographic and clinical characteristics of the full sample and the subsample of youths followed since preschoola
Depression Severity Measures
Cumulative depression was measured as the area under the curve of symptoms of depression endorsed in a clinical interview over all available assessment waves. The area under the curve was calculated for each child by graphing the depression symptoms on the y-axis and days since initial assessment on the x-axis, yielding a trajectory depicting the number of depression symptoms endorsed by time in the study. The area below this curve was calculated and divided by the total number of days between the first and most recent assessment to account for individual differences in time in the study. When children were between the ages of 3 years and 7 years 11 months, the Preschool Age Psychiatric Assessment was administered to caregivers (24–26). When children were 8 years old or older, both child and caregiver reports of psychiatric symptoms were collected using the Child and Adolescent Psychiatric Assessment (27–29). At the current wave, the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) (30) was used. There were up to 14 possible assessment waves for participants recruited in preschool and up to four possible assessment waves for participants recruited at ages 9–14. See the online supplement for interrater reliability of depression. Current depression was measured as the average of the T-scores of child- and parent-reported symptoms on the Childhood Depression Inventory (CDI) (31) at time of scan. Raw scores were converted into T-scores, which reflect standardized scores based on the child’s gender and age, with a mean of 50 and a standard deviation of 10, thereby providing a more easily interpretable measure of depression severity. The CDI was used instead of number of symptoms reported on the K-SADS at the current assessment wave to increase intersubject variability and thus to increase power to detect meaningful individual differences. The CDI was not administered at the waves prior to imaging and thus was not used to measure cumulative depression severity. Area under the curve of depression symptoms endorsed in a clinical interview were calculated for three mutually exclusive developmental periods: preschool, <6 years old; school age, 6 years old to 10 years and 11 months; and adolescence, ≥11 years old.
Procedure
An event-related card-guessing task was used to assess neural reactivity to anticipation and receipt of reward feedback (7, 14, 15, 32), allowing us to estimate responses to cues (2,000 ms) indicating that they were likely to win (win cue), lose (lose cue), either win or lose (mixed cue), or get neutral feedback indicating no change (neutral cue) as well as feedback that they won (reward outcome), lost (loss outcome), or neither won nor lost (none outcome). See the online supplement for details.
fMRI Analyses
fMRI data were run though the Human Connectome Project minimal preprocessing pipelines (33–37) (see the online supplement for details). Individual-subject generalized linear models included eight regressors: presentation of each type of cue (win, lose, mixed, neutral), presentation of each possible outcome (reward, loss, none), and onset of each trial/prompt to guess whether the card will be greater than or less than 5. The generalized linear model assumed a hemodynamic response shape lasting 12 seconds, using a gamma variate basis function convolved with the hemodynamic response function provided in AFNI, where beta weights represent the peak height of the hemodynamic curve.
Region-of-Interest Analyses
A priori regions of interest were selected on the basis of the literature showing reactivity in the cortico-striatal circuit to monetary rewards in adulthood and adolescence (38). Six regions of interest were used: the caudate (defined as the caudate head), putamen, and nucleus accumbens (NAcc) from the TT Daemon atlas, and the insula, dorsal anterior cingulate cortex (dACC), and rostral anterior cingulate cortex (rACC) from the Destrieux atlas (39) (see the online supplement). Mean beta estimates were extracted across each region of interest for the a priori selected win>lose cue contrast and reward>loss outcome contrast, along with a composite average measure of activation across all regions of interest reflecting activation across the cortico-striatal circuit (a mean of means). When we refer to activation across the cortico-striatal circuit, we mean task-related blood-oxygen-level-dependent (BOLD) signal from the six regions of interest (i.e., a mean value computed across all regions of interest, with each region of interest defined as mean response across the anatomically defined region). This measure does not refer to connectivity.
First, using multiple regression models, mean activation across the cortico-striatal network was regressed onto measures of current and cumulative depression severity simultaneously (i.e., in the same model) as well as covariates including gender, race, age at scan, and income-to-needs ratio. These covariates were selected because they have been shown to be related to either depression severity or functioning of the brain’s reward system (40, 41). These covariates were controlled for in all analyses. Next, mean activation in each region of interest was regressed onto measures of current and cumulative depression severity. Second, activation in each region of interest was regressed onto each measure of depression during three distinct developmental periods. Third, these analyses were followed up with multiple regressions that included all three developmental periods as regressors, to test which developmental period accounted for the greatest variance in region-of-interest activity. Additional analyses covaried for psychotropic medication use in the past 48 hours, using a dichotomous variable (see the online supplement). Finally, complementary analyses testing cue type by current and cumulative depression interactions are presented in the online supplement.
Whole Brain Analysis
To identify significance thresholds, we conducted a nonparametric permutation test using the Clustsim option within 3dttest++ in AFNI, using a group-level brain mask where at least 70% of the participants had signal. The updated version of Clustsim generates cluster thresholds based on voxel-wise threshold and family-wise error corrected p value. Results indicated a voxel-wise threshold of p<0.005 with a minimum cluster of 448 voxels for cumulative depression severity and 520 voxels for current depression severity corresponding with a whole brain family-wise error corrected p of 0.05. Because such a large spatial extent may not be realistic for subcortical regions such as the striatum, whole brain analyses were also conducted with a newer equitable thresholding and clustering method, using the ETAC option within 3dttest++ in AFNI. ETAC has the “potential to detect both small, intense clusters (found using small p thresholds and small blurring) and large, weak clusters (found using large p thresholds and perhaps more blurring) within a single execution” (42). ETAC has recently been lauded as “eliminat[ing] the need for selection of a primary cluster-defining threshold by combining information from multiple simulations at a range of primary voxelwise thresholds, and then adjusting for multiple tests to control the overall false positive rate” (43).
To test whether the subsample followed since preschool showed a similar pattern of activation within the same clusters, clusters from the full sample were extracted and used as a mask in follow-up analyses. At the group level, we examined neural activity to the win>lose cue and reward>loss outcome contrasts. We used multiple regression models to examine the relationship between individual-level current depression and cumulative depression and activation to the win>lose cue and the reward>loss outcome contrasts while accounting for the same covariates as specified above.
We refer to the nucleus accumbens when discussing the results of the region-of-interest analyses, as it is a region of interest distinct from the caudate and putamen. We refer to the ventral striatum when discussing whole brain results because it is more difficult to determine whether the activity in these analyses is localized to the nucleus accumbens or is also present in ventral portions of the caudate and putamen.
Results
Group-Level Response to Reward Anticipation and Receipt
Participants showed significant BOLD response to reward anticipation and receipt across regions of the cortico-striatal circuit, including the dorsal and ventral striatum, dorsal and rostral anterior cingulate cortex (dACC and rACC, respectively), and insula, among other regions (p<0.01 corrected; see Figure S2 in the online supplement).
Depression Severity and Neural Response to Reward Receipt
Neither cumulative nor current depression was related to mean activation to reward receipt across the cortico-striatal circuit or in specific individual regions of interest (see Table S1 in the online supplement). Furthermore, whole brain analyses did not reveal any significant clusters of activation during reward receipt that were correlated with cumulative depression severity. Some clusters were correlated with current depression severity, although none were in the dorsal or ventral striatum (see Table S2 in the online supplement).
Depression Severity and Neural Response to Reward Anticipation
Multiple regression analyses that included both current and cumulative depression as simultaneous regressors showed that cumulative depression was related to mean activation in the cortico-striatal circuit (Table 2; Figure 1A), as well as the caudate, putamen, insula, dACC, and rACC. Current depression was related to activation in the nucleus accumbens (Figure 1B) and dACC (Table 2). The relationship with the nucleus accumbens was nominally significant but not after false discovery rate correction, although this relationship was significant when cumulative depression was excluded from the model and false-discovery-rate corrected (see Table S3 in the online supplement). The association with the dACC did not hold when cumulative depression severity was excluded from the model (see Table S3). Regressions with current and cumulative depression as separate regressors showed a similar pattern of results (see Table S3). Findings were consistent when the analyses accounted for psychotropic medication use and for the subsample followed since preschool (see Tables S5–S7, S9, and S10 in the online supplement). See the online supplement for complementary analyses using multivariate analyses of covariance with all four cue-type conditions.
| Region of Interest and Depression Severity | β | 95% CI | p (nominal) | pb (FDR corrected) |
|---|---|---|---|---|
| Cortico-striatal circuit | ||||
| Current | 0.026 | –0.176, 0.228 | 0.803 | |
| Cumulative | –0.300 | –0.501, –0.099 | 0.004 | |
| Nucleus accumbens | ||||
| Current | –0.219 | –0.424, –0.014 | 0.037 | 0.111 |
| Cumulative | –0.144 | –0.348, 0.060 | 0.164 | 0.164 |
| Caudate | ||||
| Current | –0.063 | –0.266, 0.141 | 0.544 | 0.653 |
| Cumulative | –0.220 | –0.422, –0.018 | 0.033 | 0.042 |
| Putamen | ||||
| Current | 0.039 | –0.165, 0.243 | 0.704 | 0.704 |
| Cumulative | –0.249 | –0.452, –0.046 | 0.016 | 0.038 |
| Insula | ||||
| Current | 0.120 | –0.092, 0.332 | 0.263 | 0.526 |
| Cumulative | –0.228 | –0.439, –0.017 | 0.035 | 0.042 |
| Dorsal ACC | ||||
| Current | 0.232 | 0.027, 0.437 | 0.027 | 0.111 |
| Cumulative | –0.371 | –0.574, –0.167 | 0.001 | 0.001 |
| Rostral ACC | ||||
| Current | 0.093 | –0.120, 0.306 | 0.389 | 0.584 |
| Cumulative | –0.253 | –0.465, –0.042 | 0.019 | 0.038 |
TABLE 2. Association between current and cumulative depression severity and BOLD response in a priori regions of interest to reward anticipation in the full sample (N=131)a
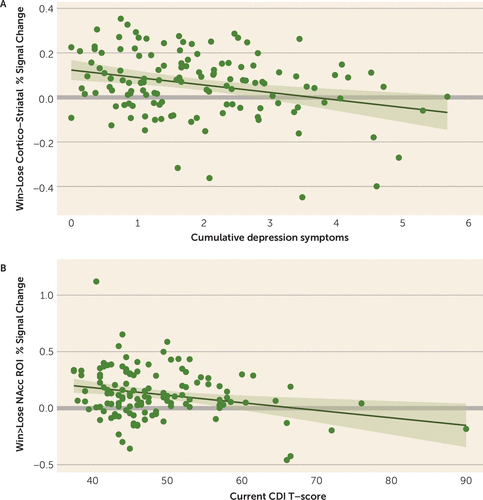
FIGURE 1. Associations of cumulative and current depression with BOLD response to reward anticipation in nucleus accumbens and cortico-striatal circuit regions of interesta
a Panel A shows the association between cumulative core depression symptoms (i.e., area under the curve of depression trajectory) and blood-oxygen-level-dependent (BOLD) response to win>lose cue across the cortico-striatal circuit (average mean activation across 12 a priori regions of interest). Panel B shows the association between parent and child Childhood Depression Inventory T-scores at time of scan and difference in BOLD response to win versus lose cues within the nucleus accumbens region of interest. Shaded bands surrounding regression lines indicate 95% confidence intervals. CDI=Childhood Depression Inventory; NAcc=nucleus accumbens; ROI=region of interest.
In whole brain analyses, consistent with the region-of-interest analyses, cumulative depression was negatively correlated with activity in the dorsal and ventral striatum as well as cortical regions including the left insula and left and right superior frontal gyrus (p<0.005, uncorrected for spatial extent) (Figure 2A, Table 3). Results from ETAC analyses confirm the significance of striatal regions of activity (see Figure S7 and Table S12 in the online supplement). The results did not meaningfully differ when the analyses accounted for psychotropic medication (see Figure S5B and Table S8 in the online supplement). For the subsample followed since preschool, a mask was applied using the clusters from the full sample, and analyses tested whether activation within this mask correlated with cumulative depression. All eight clusters were negatively correlated with cumulative depression, including clusters in the dorsal and ventral striatum (see Figure S6B and Table S11 in the online supplement).
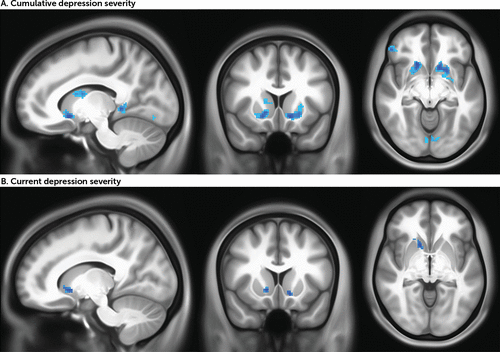
FIGURE 2. BOLD response to reward anticipation in the striatum associated with cumulative depression severity and current depression severity from whole brain analyses in the full samplea
a BOLD=blood-oxygen-level-dependent. Images in panel A are centered at Montreal Neurological Institute coordinates (x, y, z) −12, 14, −8, and images in panel B at 14, 14, −4.
| MNI Coordinates | ||||
|---|---|---|---|---|
| Brain Region | Cluster Size (number of 2 mm3 voxels) | x | y | z |
| Cumulative depression severityb | ||||
| Left insula | 405 | –50 | –26 | 20 |
| Left parahippocampal gyrus | 325 | –12 | –42 | 0 |
| Left ventral striatum | 274 | –12 | 14 | –8 |
| Right putamen | 263 | 18 | 20 | –6 |
| Right inferior frontal gyrus | 246 | 52 | 38 | –6 |
| Left caudate | 218 | –10 | 6 | 16 |
| Left superior frontal gyrus | 210 | –26 | 42 | 30 |
| Right superior frontal gyrus | 190 | 30 | 44 | 32 |
| Right lingual gyrus | 171 | 2 | –74 | –2 |
| Current depression severityc | ||||
| Right ventral striatum | 46 | 14 | 14 | –4 |
| Left middle occipital gyrusd | 37 | –16 | –104 | 16 |
| Left middle frontal gyrusd | 28 | –16 | –0 | 64 |
| Left ventral striatum | 19 | –12 | 12 | –8 |
| Right inferior temporal gyrusd | 18 | 58 | –32 | –22 |
| Left medial frontal gyrusd | 13 | –18 | –8 | 58 |
| Left cerebellar tonsil | 13 | –6 | –54 | –56 |
| Left pons | 11 | –8 | –20 | –44 |
TABLE 3. Cumulative and current depression severity associations with BOLD response to reward anticipation in whole brain analysesa
In whole brain analyses, consistent with the region-of-interest analyses, current depression was negatively correlated with activity in the ventral striatum in the full sample (p<0.005, uncorrected for spatial extent) (Figure 2B, Table 3). Four cortical clusters located in the left middle occipital gyrus, left middle frontal gyrus, right inferior temporal gyrus, and left medial frontal gyrus were also positively correlated with current depression. Results from ETAC analyses confirm the significance of striatal regions of activity (see Figure S7 and Table S12 in the online supplement). The results did not meaningfully differ when the analyses accounted for psychotropic medication (see Figure S5A and Table S8 in the online supplement). In the subsample followed since preschool, three clusters were negatively correlated with current depression, two in the striatum (the left and right ventral striatum) and one in the left ventral pons (see Figure S6A and Table S11 in the online supplement).
Depression Severity During Distinct Developmental Periods and Response to Reward Anticipation
As shown in Table S3 in the online supplement, after false discovery rate correction, regression analyses with each developmental period as a separate regressor indicated that preschool depression severity was related to reduced activity in the cortico-striatal circuit as well as in the caudate, putamen, insula, dACC, and rACC. School-age depression severity was related to reduced activity in the cortico-striatal circuit, caudate, and putamen. Adolescent depression severity was related to reduced activity in the cortico-striatal circuit and the nucleus accumbens. The results were mostly consistent when the analyses accounted for psychotropic medication use and for the subsample followed since preschool (see Tables S7 and S10 in the online supplement).
We then directly compared each developmental period by conducting multiple regression analyses that simultaneously included preschool, school-age, and adolescent depression severity as regressors. Because these models exclude participants for whom depression severity ratings were missing from any of the three developmental periods, only the subsample that was followed since preschool was used. As shown in Table S4 in the online supplement, preschool depression severity was related to reduced activation in the cortico-striatal circuit, putamen, and the rACC. In contrast, adolescent depression severity was related to reduced activation only in the nucleus accumbens. The results were consistent when the analyses accounted for psychotropic medication use (see Table S6 in the online supplement).
Discussion
We replicated previous findings (5, 18, 44) that greater current depression severity was related to reduced activity in the nucleus accumbens (i.e., ventral striatum) to reward anticipation, but not receipt, in both a priori region-of-interest and whole-brain analyses. A priori region-of-interest analyses further revealed that cumulative depression severity was related to blunting to reward anticipation across the cortico-striatal circuit, with whole brain analyses also showing that cumulative depression severity was related to blunted activity in both the dorsal and ventral striatum. Of note, the whole brain associations did not survive very conservative whole brain correction, primarily because of the large spatial extent threshold mandated by such corrections. Supplementary analyses using equitable thresholding and clustering were used to assess for the presence of smaller, more intense clusters. The strongest results were directly consistent with the region-of-interest-based analyses in showing a relationship between current depression severity and ventral striatum activity, with broader relationships of cumulative depression across the ventral and dorsal striatum. Additional analyses demonstrated that preschool depression severity was related to blunted response to anticipation in regions including the dorsal striatum (i.e., putamen) and rostral ACC, while adolescent depression severity was related to response in the ventral striatum (i.e., nucleus accumbens). Finally, we did not find support for any a priori relationships between depression severity and response to reward receipt.
Our finding that current depression severity was associated with focal dysfunction in the ventral striatum while cumulative depression severity was associated with global dysfunction of the cortico-striatal circuit has implications for future research seeking to identify risk factors and consequences of depression. First, global blunting of the cortico-striatal circuit could represent a risk factor for chronic or recurrent depression and may be associated with early onset and/or more severe forms of depression. This hypothesis is consistent with our finding that both cumulative depression severity and depression severity in the preschool period specifically were associated with reduced responsivity across the cortico-striatal network. This hypothesis would predict that children who go on to experience early onset of depression or more severe or chronic depression will show more widespread dysfunction of a cortico-striatal network related to reward anticipation even before the onset of depression.
A second possibility is that blunting across a broad cortico-striatal network represents a scar of chronic depression. That is, with repeated exposure to depressive symptoms, particularly early in life, the cortico-striatal circuit may not develop properly, giving rise to deficits in reward processing. Further, our findings also demonstrated that current depression severity was associated with hyporeactivity of the ventral striatum to anticipation of reward. If such an association between current depressed mood state and ventral striatal hyporeactivity to reward anticipation is present across development, repeated experience of depression that starts early in childhood could lead to downstream hyporeactivity of the entire circuit. An early onset of depression may disrupt this network as the child is developing and have a cascading and broader impact. This could be tested by longitudinal studies concurrently measuring depression and neural responses to rewards from early childhood to adolescence.
One intriguing possible explanation for these findings is that, over time, blunting to rewards transitions from the ventral to the dorsal striatum, becoming a “habit” of reduced reward response. This is analogous to the hypotheses of Everitt and Robbins et al. proposing stages of substance use. They proposed a shift in responsivity from the nucleus accumbens to the dorsolateral striatum as drug-seeking behavior transitions from instrumental (i.e., controlled or voluntary) to habitual, via striato-nigro-striatal ascending anatomical connections (45, 46), with ventral areas of the striatum innervating more dorsal areas via spiraling anatomical connections (47, 48). The authors further posit that the striatum may interact with cortical regions to drive negatively reinforced behaviors, resulting in anhedonia (45). The present findings lend some evidence to this theory’s generalizability to depression, with a greater accumulation of depression symptoms associated with more blunted activity in the dorsal striatum and cortical regions and acute depression more associated with blunting of the ventral striatum. If the findings are replicated, this hypothesis has implications for how we characterize and treat depression.
Finally, our findings revealed associations between depression and reward anticipation but not reward receipt, contrary to our hypotheses. While some studies have found blunted reactivity to both reward anticipation and receipt (1, 2), others have similarly found stronger relationships between depression and reward anticipation, sometimes to the exclusion of reward receipt (7, 17, 18), including a study of 1,576 adolescents (5). Moreover, anhedonia appears to be linked more strongly to blunted responses to reward anticipation than to reward receipt (49). This dissociation has interesting implications. While blunted reactivity to reward receipts may be representative of hedonic pleasure, blunted reactivity to reward anticipation may represent deficits in learning about cues that predict reward or in ability to represent future reward experience or information. Our findings suggest the possibility that rather than leading to failure to experience pleasure from rewards, chronic depression experienced over childhood affects motivation more than hedonic response in adolescence. One possible explanation is that reward anticipation is a developmental skill honed by learning and experience, whereas hedonic response to rewards is more automatic. If so, repeated exposure to depression during development may disrupt this trajectory, resulting not in impairments to hedonic response to rewards per se but rather in altered motivation and related aberrant reactivity of the striatum and broader regions. In fact, other studies have found that depression is related to reduced behavioral reward learning (50) and reward-related decision making (51). Further studies assessing reward learning and responsivity to reward cues longitudinally will be necessary to further test this hypothesis.
A positive relationship between current depression and medial/middle prefrontal regions appeared in exploratory whole brain analyses, as has been observed in previous studies (2). One possible explanation is that depressed individuals use cognitive resources to compensate for insufficient striatal resources in order to represent the reward that follows the cue (i.e., learn the cue). This would suggest coordination between the cortico-striatal circuit and frontal regions in learning and anticipating rewards.
Several limitations of this study should be noted. First, a subset of participants included in the full sample (N=20) were added at later waves to increase the sample size of healthy control subjects for the imaging waves and therefore were missing depressive measures before age 9. The findings did not meaningfully change when this subset was excluded. Second, because rewards in the fMRI task we used were based on chance, we were not able to compare neural response to reward with a behavioral measure of reward learning. At the same time, the task we used avoids potential confounders that can occur with such behavioral measures, such as motor preparation between the anticipatory cue and action or task anxiety over one’s performance (52). Third, our whole brain results were significant at a p value of 0.005 but did not meet the full spatial extent mandated by a conservative whole brain correction. However, for the striatum, such a large spatial extent may not be realistic. Whole brain results using equitable thresholding and clustering confirm the striatal results found in a priori region-of-interest-based results. Fourth, our a priori planned analyses focused on win>lose contrasts, and complementary multivariate analyses of covariance with additional cue type conditions were very consistent, but not every result replicated to the same significance level (see the online supplement). Fifth, we used mean BOLD signal to measure cortico-striatal circuit activity. An interesting extension of these findings will be to test whether the functional connectivity of these regions is related to depression severity. Finally, it is often difficult to disentangle chronicity from developmental effects. Children with early-onset depression are at greater risk of experiencing more chronic depression throughout childhood, making it difficult to know whether associations between cumulative depression and cortico-striatal function is the result of early-onset depression or of a chronic course. However, our finding that nucleus accumbens activity was most associated with adolescent depression severity provides some evidence for developmental specificity. Future studies comparing youths with depression exclusively in early childhood with those who experience depression exclusively later in life could inform such questions.
Conclusions
Our findings demonstrate a relationship between cumulative depression throughout childhood and brain responses to rewards. This study additionally distinguishes neural patterns of hyporeactivity associated with cumulative depression from those of current depression severity and between distinct developmental periods, showing that early and cumulative experiences of depression disrupt the cortico-striatal circuit in optimally responding to a rewarding cue, while acute experiences of depression occurring in adolescence exclusively disrupt the ventral striatum. Therefore, individuals with early and recent episodes of depression may both show blunted reactivity to rewards, but with differing neural contributions from concurrent and cumulative depression history. These findings suggest that unique neurodevelopmental processes may be at play. Understanding the differences in these mechanisms is integral to creating interventions aimed at alleviating depression.
1 : Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry 2018; 175:1111–1120Link, Google Scholar
2 : The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 2013; 151:531–539Crossref, Medline, Google Scholar
3 : Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis 2013; 52:66–74Crossref, Medline, Google Scholar
4 : Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. J Abnorm Psychol 2014; 123:298–309Crossref, Medline, Google Scholar
5 : The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry 2015; 172:1215–1223Link, Google Scholar
6 : Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. J Am Acad Child Adolesc Psychiatry 2016; 55:328–337Crossref, Medline, Google Scholar
7 : Reduced reward anticipation in youth at high-risk for unipolar depression: a preliminary study. Dev Cogn Neurosci 2014; 8:55–64Crossref, Medline, Google Scholar
8 : Blunted reward responsiveness in remitted depression. J Psychiatr Res 2013; 47:1864–1869Crossref, Medline, Google Scholar
9 : Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009; 205:667–677Crossref, Medline, Google Scholar
10 : Enhanced negative feedback responses in remitted depression. Neuroreport 2008; 19:1045–1048Crossref, Medline, Google Scholar
11 : Blunted response to feedback information in depressive illness. Brain 2007; 130:2367–2374Crossref, Medline, Google Scholar
12 : Blunted neural responses to reward in remitted major depression: a high-density event-related potential study. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1:87–95Crossref, Medline, Google Scholar
13 : Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord 2012; 136:1126–1134Crossref, Medline, Google Scholar
14 : Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry 2009; 166:64–73Link, Google Scholar
15 : Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry 2010; 49:162–172.e5Medline, Google Scholar
16 : fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord 2009; 118:69–78Crossref, Medline, Google Scholar
17 : Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol 2012; 26:677–688Crossref, Medline, Google Scholar
18 : Altered neural reward and loss processing and prediction error signalling in depression. Soc Cogn Affect Neurosci 2015; 10:1102–1112Crossref, Medline, Google Scholar
19 : The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychol Bull 2011; 137:856–879Crossref, Medline, Google Scholar
20 : The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 2014; 13:28–35Crossref, Medline, Google Scholar
21 : Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Link, Google Scholar
22 : Trajectories of preschool disorders to full DSM depression at school age and early adolescence: continuity of preschool depression. Am J Psychiatry 2014; 171:768–776Link, Google Scholar
23 : Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry 2009; 66:897–905Crossref, Medline, Google Scholar
24 : The Preschool Age Psychiatric Assessment: Version 1.4. Durham, NC, Duke University Medical Center, Department of Psychiatry and Behavioral Sciences, Center for Developmental Epidemiology, 2003Google Scholar
25 : Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA). J Am Acad Child Adolesc Psychiatry 2006; 45:538–549Crossref, Medline, Google Scholar
26 : The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord 2009; 112:111–119Crossref, Medline, Google Scholar
27 : The Child and Adolescent Psychiatric Assessment (CAPA). J Am Acad Child Adolesc Psychiatry 2000; 39:39–48Crossref, Medline, Google Scholar
28 : The Child and Adolescent Psychiatric Assessment (CAPA). Psychol Med 1995; 25:739–753Crossref, Medline, Google Scholar
29 : Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry 2016; 73:31–38Crossref, Medline, Google Scholar
30 : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
31 : Children’s Depression Inventory. Toronto, Multi-Health Systems, 1992Google Scholar
32 : Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci 2010; 10:107–118Crossref, Medline, Google Scholar
33 : Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 2013; 80:169–189Crossref, Medline, Google Scholar
34 : The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013; 80:105–124Crossref, Medline, Google Scholar
35 : Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med 2010; 63:1144–1153Crossref, Medline, Google Scholar
36 : Multiplexed echo planar imaging for sub-second whole brain fMRI and fast diffusion imaging. PLoS ONE 2010; 5:
37 : Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage 2013; 83:991–1001Crossref, Medline, Google Scholar
38 : Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage 2015; 122:427–439Crossref, Medline, Google Scholar
39 : Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010; 53:1–15Crossref, Medline, Google Scholar
40 : Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am J Psychiatry 2016; 173:625–634Link, Google Scholar
41 : The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr 2013; 167:1135–1142Crossref, Medline, Google Scholar
42 : Equitable thresholding and clustering: a novel method for functional magnetic resonance imaging clustering in AFNI. Brain Connect 2019; 9:529–538Crossref, Medline, Google Scholar
43 Pfeifer JH, Flournoy JC, Cheng TW, et al: Improving practices and inferences in developmental cognitive neuroscience [preprint]. PsyArXiv, September 15, 2019 (https://doi.org/10.31234/osf.io/ez5sf)Google Scholar
44 : Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology (Berl) 2015; 232:331–341Crossref, Medline, Google Scholar
45 : From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 2013; 37(9 Pt A):1946–1954Crossref, Medline, Google Scholar
46 : Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 2008; 363:3125–3135Crossref, Medline, Google Scholar
47 : Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 2000; 20:2369–2382Crossref, Medline, Google Scholar
48 : Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Brain Res Rev 2007; 56:27–78Crossref, Google Scholar
49 : Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 2012; 121:553–558Crossref, Medline, Google Scholar
50 : Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry 2013; 73:639–645Crossref, Medline, Google Scholar
51 : Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry 2007; 61:633–639Crossref, Medline, Google Scholar
52 : Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 2011; 35:537–555Crossref, Medline, Google Scholar



