Limbic Hyperactivity in Response to Emotionally Neutral Stimuli in Schizophrenia: A Neuroimaging Meta-Analysis of the Hypervigilant Mind
Abstract
Objective:
It has long been assumed that paranoid ideation may stem from an aberrant limbic response to threatening stimuli. However, results from functional neuroimaging studies using negative emotional stimuli have failed to confirm this assumption. One of the potential reasons for the lack of effect is that study participants with psychosis may display aberrant brain responses to neutral material rather than to threatening stimuli. The authors conducted a functional neuroimaging meta-analysis to test this hypothesis.
Methods:
A literature search was performed with PubMed, Google Scholar, and Embase to identify functional neuroimaging studies examining brain responses to neutral material in patients with psychosis. A total of 23 studies involving schizophrenia patients were retrieved. Using t-maps of peak coordinates to calculate effect sizes, a random-effects model meta-analysis was performed with the anisotropic effect-size version of Seed-based d Mapping software.
Results:
In schizophrenia patients relative to healthy control subjects, increased activations were observed in the left and right amygdala and parahippocampus and the left putamen, hippocampus, and insula in response to neutral stimuli.
Conclusions:
Given that several limbic regions were found to be more activated in schizophrenia patients than in control subjects, the results of this meta-analysis strongly suggest that these patients confer aberrant emotional significance to nonthreatening stimuli. In theory, this abnormal brain reactivity may fuel delusional thoughts. Studies are needed in individuals at risk of psychosis to determine whether aberrant limbic reactivity to neutral stimuli is an early neurofunctional marker of psychosis vulnerability.
Schizophrenia is a heterogeneous disorder associated with complex emotional disturbances that are known to negatively interfere with patients’ functioning and quality of life (1, 2). Psychopathological research has shown that emotional disturbances in schizophrenia encompass anxiety, depression, guilt, and irritability; conversely, blunting of affect has been observed in a subgroup of patients (1, 2). In recent decades, several laboratory studies have investigated the nature of emotional disturbances in schizophrenia. Using facial emotional expression paradigms, it has been shown that facial emotion recognition is significantly impaired in schizophrenia patients (3). Several studies have also investigated emotion experience in schizophrenia using emotion induction paradigms (e.g., film clips, affective pictures, and faces). These studies have shown that relative to control subjects, schizophrenia patients report greater negative emotions (aversion) and greater arousal levels in response to neutral stimuli (for meta-analyses, see references 4, 5). Echoing these results, studies using ecological momentary assessment or the experience sampling method have shown that schizophrenia patients consistently report more negative emotion than healthy volunteers in their daily lives (6).
The importance, range, and functional significance of emotional disturbances in schizophrenia have led investigators to conduct several functional neuroimaging studies on the topic. Despite the heterogeneity of findings, meta-analyses of these studies have shown that schizophrenia patients display reduced activations in limbic regions, such as the amygdala and the parahippocampal gyrus, during the processing of negative emotional stimuli (7–9). Importantly, the main results of these meta-analyses seem to be observed in studies using affective faces or pictures, as well as studies using implicit (e.g., gender discrimination) or explicit (e.g., emotion recognition) task instructions. These neuroimaging results have been interpreted in light of the well-known blunting of affect and/or impaired emotion recognition abilities associated with schizophrenia.
One factor that has received insufficient attention in the field is that the reduced limbic activations observed during emotion processing in schizophrenia may be explained by the brain activity observed during the processing of neutral stimuli (e.g., control condition). In 2010, Anticevic et al. (10) performed a seminal meta-analysis of 35 functional neuroimaging studies on emotion processing in schizophrenia that used the amygdala as a region of interest because of its well-known role in fear processing (11). As observed by other research teams, the meta-analysis showed small reductions in amygdala activity in schizophrenia patients relative to healthy control subjects during emotion processing. More importantly, a subanalysis showed that this reduced amygdala activity is only present in studies examining the “negative emotion minus neutral” contrast. In the studies examining the “negative emotion only” condition, there were no significant differences in amygdala activations between schizophrenia patients and control subjects. This latter finding indirectly raised the possibility that schizophrenia patients actually display hyperactivations in response to neutral stimuli, which could explain (at least partially) the limbic hypoactivations that are usually reported during negative emotion processing in this population. In theory, it has been proposed that such aberrant amygdala reactivity to nonthreatening stimuli could fuel paranoid ideation in schizophrenia (12). Consistent with this idea, a recent study using arterial spin labeling showed that amygdala activity at rest is elevated in patients with paranoid schizophrenia (13). Likewise, positron emission tomography studies have shown increased tonic amygdala activity in schizophrenia patients regardless of experimental conditions (14, 15).
Over the years, a growing number of investigators studying the neural correlates of emotion processing in schizophrenia have begun to report how schizophrenia patients specifically respond to neutral stimuli. Thus far, most studies have shown that limbic reactivity is increased in schizophrenia during the processing of neutral material, especially the reactivity of the amygdala (12, 16, 17). However, a minority of studies have failed to show this aberrant limbic reactivity to neutral material in schizophrenia (18). It also remains to be determined whether or not the presumed aberrant limbic reactivity to nonthreatening stimuli in schizophrenia patients is restricted to the amygdala.
Our objectives in this study were twofold. First, we aimed to perform a meta-analysis of functional neuroimaging studies examining the brain reactivity to neutral stimuli in schizophrenia patients while taking into account moderator variables such as psychotic symptoms, antipsychotic dosage, experimental stimuli, task instructions, and neuroimaging acquisition and processing parameters. Second, in order to test the aberrant salience hypothesis, we aimed to assess the brain reactivity of schizophrenia patients in response to emotionally negative stimuli in comparison to neutral stimuli. We hypothesized that schizophrenia patients would show increased activity in limbic regions such as the amygdala in response to emotionally neutral material as well as reduced or normal activity in response to emotionally negative stimuli.
Methods
Selection Procedures
Search strategies.
A systematic search strategy was employed to identify relevant studies for the meta-analysis. A search of the literature up to December 2018 was performed independently by two researchers (N.B., J.R.D.) using three search engines—PubMed, Google Scholar, and Embase. The following search terms were used: (“schizophrenia” or “psychosis”) AND (“emotion” or “negative emotion” or “neutral emotions”) AND (“PET” (positron emission tomography) or “fMRI” (functional magnetic resonance imaging)). Also, a cross-referencing method was used by manually examining reference lists of the articles selected for the meta-analysis.
Selection criteria.
Articles were included in the meta-analysis if they were reported in an original article in a peer-reviewed journal, included patients with schizophrenia and/or schizoaffective disorder and/or individuals at risk of psychotic disorders, included a control group of healthy subjects for group comparisons, employed an emotional fMRI task that included “emotionally neutral stimuli,” and reported fMRI results from group comparisons (schizophrenia patients compared with control subjects) relative to neutral stimuli. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to achieve a high reporting standard (19) (see Table S1 in the online supplement).
Meta-Analysis
The meta-analysis was performed by using the anisotropic effect-size version of Seed-based d Mapping (AES-SDM), using software from the SDM Project (20, 21). Briefly, in contrast to the activation likelihood estimation method, AES-SDM uses peak coordinates and effect sizes to recreate, for each study, an effect size map of contrast results. A standard random-effects variance-weighted meta-analysis for each voxel was then executed. Default AES-SDM kernel size and thresholds were used (full width at half maximum [FWHM], 20 mm, voxel p=0.005, peak height Z=1, cluster extent, 10 voxels) (20, 21). To minimize the detection of spurious results, we increased the cluster extent threshold to 100 voxels. To assess the robustness of the results, we examined residual heterogeneity and performed jackknife and subanalyses. Jackknife sensitivity analyses consisted of repeating the meta-analysis iteratively by removing one study at a time to assess the replicability of the results (20, 21). Furthermore, we assessed whether the main findings had been driven by a small subset of studies or studies with small sample sizes. Finally, publication bias was assessed by Egger’s test (22) for asymmetry of the funnel plots.
Several additional subanalyses were conducted to examine the robustness of the results. We assessed whether the results were partially driven by studies using 1) whole brain versus region-of-interest methodology; 2) facial versus other emotionally neutral stimuli; 3) implicit versus explicit emotional tasks (implicit processing refers to passive viewing of images or instructions regarding characteristics other than emotional ones, such as gender, whereas explicit processing refers to emotion recognition or emotional rating of stimuli); and 4) an FWHM of the smoothing kernel size of ≤8 mm versus >8 mm, since heterogeneous results could arise from different FWHM of the smoothing kernel size (23). Meta-regression analyses were performed on peak regions derived from the main analysis, using the repetition time of functional volumes, the positive symptom subscale of the Positive and Negative Syndrome Scale (PANSS) (24), and antipsychotic dosage in chlorpromazine equivalents.
Results
Twenty-three studies met inclusion criteria for the meta-analysis (12, 16–18, 25–43) (Figure 1). The study characteristics are summarized in Table 1. The studies included 474 individuals with schizophrenia spectrum disorders who were compared with 472 healthy control subjects. The mean age of the schizophrenia patients was 35.3 years, and 70.8% of the sample were male. Patients had a mean antipsychotic dosage of 510.11 mg/day of chlorpromazine equivalents, and they had a mean score of 14.1 on the positive symptom subscale of the PANSS. Of the 23 studies, 14 used a whole-brain methodology and 17 used a smoothing kernel size of ≤8 mm. Eleven studies used an explicit emotional task, and 12 used only facial stimuli. Further details on the tasks, stimuli, and experimental contrasts used in the included studies are presented in Table S2 in the online supplement.
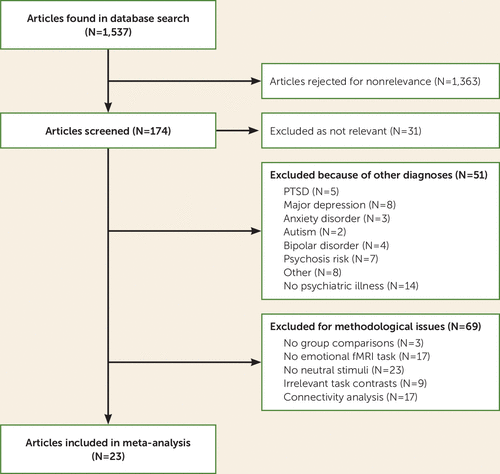
FIGURE 1. Flow chart of study selection for a neuroimaging meta-analysis of limbic hyperactivity in response to emotionally neutral stimuli in schizophrenia
| First Author, Year (Reference Number) | Patients(N) | Control Subjects (N) | Whole Brain or ROI | Mean Age (years) | Male (%) | Mean Antipsychotic Dosage (mg/day CPZ) | FWHM (mm) | Explicit/ Implicit | Facial Expressions | Positive Symptom Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Dowd, 2010 (18) | 40 | 32 | WB | 36.8 | 65.0 | 452.2 | 6 | Explicit | No | NA |
| Ferri, 2014 (25) | 22 | 22 | WB | 27.5 | 63.6 | 480.0 | 8 | Implicit | No | 12.5 |
| Habel, 2010 (26) | 17 | 17 | WB | 34.4 | NA | NA | 10 | Explicit | Yes | 18.0 |
| Hall, 2008 (12) | 19 | 24 | ROI | 37.7 | 63.2 | 496.0 | 8 | Implicit | Yes | 12.3 |
| Holt, 2006 (16) | 15 | 16 | ROI | 47.7 | 100.0 | 424.5 | 8 | Explicit | Yes | NA |
| Jensen, 2008 (27) | 13 | 13 | ROI | 37.6 | 77.0 | 313.5 | 10 | Implicit | No | 14.1 |
| Lakis, 2013 (28) | 27 | 37 | ROI | 32.5 | 51.0 | 613.9 | 12 | Explicit | No | 18.8 |
| Lee, 2014 (29) | 15 | 14 | WB | 31.7 | 53.3 | 489.1 | 8 | Explicit | No | 13.4 |
| Mier, 2010 (30) | 16 | 16 | ROI | 34.3 | 68.8 | 901.6 | 8 | Implicit | Yes | NA |
| Modinos, 2015 (17) | 18 | 22 | WB | 27.9 | 73.3 | 124.4 | 8 | Explicit | No | 13.9 |
| Mothersill, 2014 (31) | 25 | 21 | WB | 42.9 | 80.0 | 377.5 | 10 | Explicit | Yes | NA |
| Pankow, 2013 (32) | 35 | 36 | WB | 31.1 | 62.9 | NA | 8 | Explicit | No | 19.4 |
| Rauch, 2010 (33) | 12 | 12 | ROI | 27.7 | 75.0 | 902.1 | 6 | Explicit | Yes | 14.4 |
| Reske, 2009 (34) | 18 | 18 | WB | 31.9 | 55.6 | NA | 10 | Explicit | Yes | 8.0 |
| Shin, 2015 (35) | 16 | 16 | WB | 32.0 | 100.0 | NA | 8 | Implicit | Yes | 12.1 |
| Spilka, 2015 (36) | 28 | 27 | WB | 41.1 | 53.6 | NA | 7 | Implicit | Yes | 14.5 |
| Surguladze, 2006 (37) | 15 | 11 | WB | 43.1 | 100.0 | 487.1 | 7.2 | Implicit | Yes | NA |
| Suslow, 2013 (38) | 30 | 35 | ROI | 30.9 | 56.7 | NA | 8 | Explicit | Yes | NA |
| Taylor, 2002 (39) | 14 | 13 | WB | 36.4 | 71.4 | NA | 14 | Explicit | No | NA |
| Taylor, 2007 (40) | 23 | 15 | ROI | 39.1 | 73.9 | 569.5 | 6 | Explicit | No | NA |
| Taylor, 2011 (41) | 21 | 21 | WB | 40.7 | 66.7 | NA | 6 | Implicit | Yes | NA |
| Ursu, 2011 (42) | 20 | 20 | WB | 28.8 | 75.0 | NA | 8 | Explicit | No | NA |
| Whalley, 2009 (43) | 15 | 14 | ROI | 38.4 | 73.3 | NA | 6 | Explicit | No | 11.9 |
TABLE 1. Description of the studies included in the meta-analysis (N=23)a
Among the 23 studies (12, 16–18, 25–43), no residual heterogeneity was observed between studies reporting increased activations (τ=0.0001, Q=11.57, df=11, p=0.397) or between studies reporting decreased activations (τ=0.034, Q=13.77, df=11, p=0.245) in schizophrenia patients compared with healthy subjects during emotionally neutral stimuli.
Responses to Emotionally Neutral Stimuli in Patients With Schizophrenia
During neutral stimuli, schizophrenia patients showed significantly increased activations relative to healthy control subjects in large and robust limbic clusters bilaterally. Voxels from the left limbic cluster encompassed the putamen, the hippocampus, the amygdala, and the insula as well as the parahippocampal gyrus and the superior temporal gyrus (Z=1.90, cluster size=1,787, p<0.001). The right limbic cluster included the amygdala, the parahippocampus, and the middle temporal gyrus (Z=1.57, cluster size=776, p<0.001). Moreover, schizophrenia patients showed reduced activations, in comparison to healthy subjects, in the right middle frontal gyrus (Z=−1.16, cluster size=354, p<0.001), extending to the inferior frontal gyrus and the precentral gyrus (see Table 2 and Figure 2).
| Main Peaks | MNI Coordinates | SDM z-valueb | pc | Voxels (N)d | Breakdown | |
|---|---|---|---|---|---|---|
| Structure | Voxels (N)d | |||||
| Increased activations | ||||||
| Left putamen | –28, –6, –12 | 1.90 | 0.00004 | 1,787 | Left putamen | 234 |
| Left hippocampus | 203 | |||||
| Left amygdala | 188 | |||||
| Left insula | 105 | |||||
| Left parahippocampal gyrus | 80 | |||||
| Left superior temporal gyrus | 33 | |||||
| Right amygdala | 32, 0, –24 | 1.57 | 0.00006 | 776 | Right amygdala | 110 |
| Right parahippocampal gyrus | 95 | |||||
| Right superior temporal gyrus | 145 | |||||
| Right middle temporal gyrus | 49 | |||||
| Decreased activations | ||||||
| Right middle frontal gyrus | 48, 16, 34 | –1.16 | 0.0003 | 354 | Right inferior frontal gyrus, opercular | 260 |
| Right middle frontal gyrus | 76 | |||||
| Right precentral gyrus | 65 | |||||
| Right inferior frontal gyrus, triangular | 35 | |||||
TABLE 2. Brain responses to emotionally neutral stimuli in individuals with schizophrenia compared with healthy control subjects in studies included in the meta-analysis (N=23)a
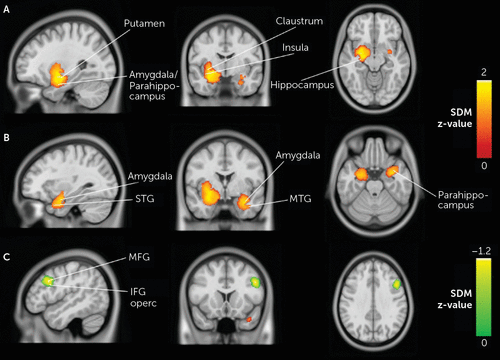
FIGURE 2. Overlay of brain regions with significantly increased or decreased activity during neutral stimuli in individuals with schizophrenia compared with healthy control subjectsa
a Rows A, B, and C represent the results from the three main peaks. Columns represent (in order) sagittal (x), coronal (y), and axial (z) slices of the main peak result (rows). Row A shows significant increased activation in the left limbic cluster (x, y, z: −28, −6, −12); row B shows significant increased activation in the right limbic cluster (32, 0, −24); row C shows significant decreased activation in the middle frontal gyrus (48, 16, 34). These blobs were generated using the SDM uncorrected p-value threshold of 0.005 derived from the original analysis. IFG operc=opercular part of inferior frontal gyrus; MFG=middle frontal gyrus; MTG=middle temporal gyrus; SDM=seed-based d mapping; STG=superior temporal gyrus.
As shown by the jackknife analyses (see Table S3 in the online supplement), the bilateral limbic clusters were highly replicable. Moreover, funnel plots and the Egger’s test suggested that these results were unlikely to be driven by small or noisy studies or publication bias (left peak: p=0.330; right peak: p=0.885). However, the result from the right inferior frontal gyrus was less replicable, as shown by the jackknife analyses (see Table S3 in the online supplement). In fact, when the Ferri et al. study (25) or the Ursu et al. study (42) were excluded from the analysis, no significant peak was observed in this region. Although Egger’s test was nonsignificant (p=0.776), the funnel plot suggested that the effect observed in this particular region may have been driven by these two studies.
Subanalyses of emotionally neutral stimuli in patients with schizophrenia.
To enhance our understanding of the brain responses to emotionally neutral stimuli in individuals with schizophrenia, we performed several subanalyses.
First, when we restricted analyses to studies using only whole-brain analyses (N=14), we obtained results similar to those reported above: increased activations were observed in the schizophrenia group in the left putamen, left amygdala, left hippocampus, and left insula. However, we also observed a small but significant increase in activation in the left postcentral gyrus (Z=1.05, cluster size=212, p=0.0012) and decreased activation in the right fusiform gyrus (Z=−1.15, cluster size=300, p=0.0006) in schizophrenia patients in comparison to healthy subjects (see Table S4 in the online supplement). Conversely, in region-of-interest studies, we observed increased activations in the right amygdala (Z=1.65, cluster size=636, p=0.0004), encompassing the right parahippocampus, right middle temporal gyrus (Z=1.65, cluster size=424, p=0.0004), and left parahippocampal gyrus (Z=1.59, cluster size=269, p=0.0006), and decreased activations in right precentral gyrus (Z=−1.22, cluster size=363, p=0.0005), in schizophrenia patients compared with healthy subjects (see Table S5 in the online supplement).
Second, when we restricted the analyses to studies using facial stimuli (N=12), we also replicated the results on bilateral limbic activations as reported above (e.g., left and right putamen, left amygdala, left hippocampus, left parahippocampus, and left and right insula). Also, we observed a small but significant decreased activation in the left lingual gyrus (Z=−1.06, cluster size=142, p<0.001) (see Table S6 in the online supplement). Conversely, studies using stimuli other than facial expressions reported increased activations in the right angular gyrus (Z=1.13, cluster size=309, p=0.0006) and right amygdala (Z=1.06, cluster size=183, p=0.0009), as well as decreased activation in the opercular part of the right inferior frontal gyrus (Z=−1.72, cluster size=1072, p=0.00007), in patients compared with healthy control subjects (see Table S7 in the online supplement).
Third, when we restricted the analyses to studies using an explicit emotional task (N=11), we observed increased activation in the left putamen (Z=1.29, cluster size=601, p=0.00014) and decreased activation in the right fusiform gyrus (Z=−1.29, cluster size=688, p=0.0003) in schizophrenia patients in comparison to healthy subjects (see Table S8 in the online supplement). Conversely, studies using implicit task instructions (N=12) reported increased activations in the right uncus (Z=1.85, cluster size=1529, p=0.00004), encompassing the right amygdala and right parahippocampus, the left amygdala (Z=2.33, cluster size=1181, p<0.000001), encompassing the left hippocampus and left parahippocampus, as well as decreased activation in the right precentral gyrus (Z=−1.32, cluster size=976, p=0.0003) (see Table S9 in the online supplement).
Fourth, since there were too few studies using larger smoothing size kernel (N=6), no further subanalyses were performed. However, when we performed a subanalysis within studies using a smoothing kernel of ≤8 mm (N=17), we observed results similar to those from the main analysis (e.g., left putamen, left and right amygdala, left hippocampus, left and right parahippocampus, and left insula) (see Table S10 in the online supplement). Finally, meta-regressions revealed no significant relationships between results from the main analysis and score on the positive symptom subscale of the PANSS (p values, 0.443–0.970), antipsychotic dosage in chlorpromazine equivalents (p values, 0.495–0.914), and repetition time of functional volumes (p values, 0.303–0.515).
Responses to Emotionally Negative Versus Neutral Stimuli in Schizophrenia Patients
Of the 23 studies included in the meta-analysis, 16 reported responses to emotionally negative stimuli versus neutral stimuli in schizophrenia patients compared with healthy control subjects (for detailed information about the studies, see Table S11 in the online supplement).
No residual heterogeneity was observed between studies (positive peaks: τ=0.075, Q=7.35, df=5, p=0.196; negative peaks: τ=0.043, Q=4.28, df=3, p=0.233). Results of the meta-analysis (N=16) revealed that there were no significant between-group differences in activations between schizophrenia patients and healthy subjects in response to emotionally negative stimuli.
Discussion
To our knowledge, this is the first functional neuroimaging meta-analysis on the processing of emotionally neutral material in schizophrenia. Whereas the meta-analysis of Anticevic et al. (10) inferred that the amygdala may be overactivated in schizophrenia during the neutral condition, here we were able to directly test this hypothesis using data from 23 functional neuroimaging studies. Results showed increased activations in schizophrenia patients relative to healthy control subjects in the left and right amygdala, the left putamen, the left hippocampus, the left and right parahippocampal gyrus, and the left insula. Conversely, reduced activations were observed in the right middle frontal gyrus, a region involved in emotion regulation (44). Importantly, no differences in brain activity were observed between groups when examining the emotion minus neutral contrast. Moreover, the limbic results were homogeneous and robust. In the case of the middle frontal gyrus, results were mostly driven by the influence of only two studies. Subanalyses and meta-regression analyses revealed no noticeable impact on results of temporal resolution and smoothing level. On the other hand, the type of stimuli (e.g., faces) and the type of task instruction (e.g., explicit processing) had a significant impact on results, as the most widespread limbic hyperactivations in schizophrenia patients were observed in studies using facial stimuli and studies using implicit task instructions having a lower cognitive load (45). Finally, the increased activations observed in the right amygdala and the left and right parahippocampal gyrus were mostly driven by studies adopting a region-of-interest approach, since no differences between schizophrenia patients and control subjects were observed in these two brain regions in the set of studies that performed whole-brain analyses.
The most important finding of this meta-analysis is the confirmation of the hypothesis of increased activity of the left amygdala specifically in response to emotionally neutral stimuli in schizophrenia patients. Given the key role of the amygdala in threat detection (11), this result strongly suggests that schizophrenia patients confer aberrant emotional significance to nonthreatening stimuli. In theory, this neurophysiologic process could fuel delusional thinking in schizophrenia. Another contribution of this meta-analysis is that it showed that other important subcortical regions (e.g., putamen, hippocampus, insula) are also more activated in schizophrenia patients, apart from the amygdala. The finding of increased activity in the left putamen in schizophrenia patients in our meta-analysis is consistent with the dopaminergic hypothesis of psychosis. Using PET imaging, several studies have indeed shown that striatal dopamine function is increased in schizophrenia and, more precisely, that dopaminergic alterations are more prominent in the associative rather than the ventral striatum (46). As for the (left) hippocampus, this region is well known for playing a key role in memory formation and associative learning (47). The increased activations observed in this region in schizophrenia during the viewing of emotionally neutral stimuli may therefore be interpreted as a difficulty in making relevant associations between emotionally neutral stimuli and their context. As for the (left) insula, it plays a key role in interoceptive awareness, and as such, the increased activations observed in this region suggest that irrelevant stimuli are inappropriately associated with somatic changes in schizophrenia. Taken together with the normal activity observed in schizophrenia during emotion processing, these complex neuroimaging results are clearly consistent with the aberrant salience hypothesis, which proposes that psychosis arises from an aberrant attribution of motivational or emotional significance to irrelevant stimuli that are out of context (48). In this model, delusions arise from a top-down cognitive effort of the patient to make sense of the aberrant salience experiences. An indirect implication of the model is that aberrant salience experiences may give rise to psychiatric symptoms other than delusions, depending on how these experiences are apprehended. In that regard, no study has examined the relationship between the aberrant limbic reactivity to neutral stimuli and anxious-depressive symptoms in schizophrenia. However, preliminary fMRI results have shown that the limbic reactivity to emotionally neutral faces is increased in mood and anxiety disorders (49).
To better understand the meaning of the increased limbic reactivity observed in schizophrenia during the processing of nonthreatening stimuli, the use of more sophisticated neuroimaging analyses, such as multivoxel pattern analyses or independent component analyses, would be required. Perhaps more importantly, careful attention will need to be paid to the experimental paradigms used to study this phenomenon. In that regard, two particular avenues seem promising. As the studies included in our meta-analysis all included an emotional condition and a neutral condition, a first possibility is that neutral stimuli may acquire aberrant significance in schizophrenia via impaired associative learning. This is a likely possibility given that we found increased activations in the hippocampus. For instance, in one of the studies included in the meta-analysis, the investigators used an aversive Pavlovian conditioning paradigm and showed increased activations in schizophrenia patients in limbic regions (e.g., ventral striatum and hippocampus) in response to conditioned stimuli paired to unconditioned neutral stimuli (27). Another relevant possibility has to do with the growing body of work in social neuroscience showing that faces that are neutral in terms of expressed emotion are not necessarily neutral in every other aspect. Indeed, emotionally neutral faces convey subtle information (e.g., physiognomic features) implicitly processed by the brain. When watching neutral faces, people make several spontaneous judgments (implicit or explicit) about the face’s level of attractiveness, introversion/extraversion, and trustworthiness. In the present meta-analysis, the most prominent limbic effects were observed in studies using facial stimuli. In theory, schizophrenia patients may underestimate the trustworthiness of others’ faces, and this bias could fuel suspicious thoughts. Interestingly, preliminary fMRI studies have shown limbic alterations in schizophrenia patients who were asked to make trustworthiness judgments about emotionally neutral faces (13). Despite the promise of this social neuroscience approach, it must be remembered that the physiognomic features of faces judged as being untrustworthy are not entirely independent from the physiognomic features of faces expressing anger (50).
In the past decade or so, preliminary studies have begun to pay attention to brain reactivity to neutral stimuli in individuals at risk for psychosis. Notably, a neuroimaging study examining emotion processing in youths at risk for psychosis (51) showed that the aberrant limbic reactivity to neutral stimuli (e.g., dynamic faces) is detectable as early as age 14. However, some studies have failed to observe this effect (regardless of the brain region) (52, 53). One of the critical factors that may compromise the acquisition of consistent findings here is the wide range of definitions of psychosis risk. Future studies will need to pay greater attention to this population using well-defined inclusion criteria.
Our study has some limitations that need to be acknowledged. First, some studies included in the meta-analysis used a region-of-interest approach, and used the amygdala as the seed region, for instance. The choice of this approach may have inflated results in the chosen regions of interest and underestimated effects outside these regions. Because of this concern, we performed a subanalysis and found that only the results regarding the right amygdala and the left and right parahippocampal gyrus were driven by the region-of-interest approach. The effects seen in the left amygdala, the left putamen, the left hippocampus, and the left insula were not confounded by this factor.
Another limitation of our meta-analysis is that the vast majority of patients were on antipsychotic treatment when they underwent scanning. However, antipsychotics all block dopamine D2 receptors and are thought to dampen aberrant salience, not to stimulate it (46). Moreover, we performed a meta-regression analysis using antipsychotic dosage in chlorpromazine equivalents and found no association with any of our main results.
A third limitation of our meta-analysis is that the results may have been influenced by publication bias. Since it is not common to report activations during the neutral condition, it is possible that investigators who decided to do so were those who found significant differences between patients and control subjects. Aware of this possibility, we performed analyses of publication bias and found that the results regarding the middle frontal gyrus were driven by a minority of studies. Importantly, however, none of the findings related to the limbic system (e.g., amygdala, putamen, hippocampus, parahippocampal gyrus, and insula) were influenced by publication bias.
A fourth limitation of the meta-analysis is that the subanalysis on negative emotions was based on the 16 studies (of the 23 studies in the analysis) that reported relevant data. In previous meta-analyses that had a primary objective of examining negative emotions, more studies were included, and their aggregation showed reduced limbic activations during the processing of negative emotions in schizophrenia (7, 10).
Finally, no correlation was found between the limbic regions observed to be more activated in schizophrenia patients in response to neutral stimuli and the positive symptoms of the disorder. If the increased brain reactivity to neutral stimuli is involved in the generation of paranoid or delusional ideas, we would have expected to observe a correlation across studies. One factor that may have compromised our ability to detect this expected association is that the meta-regression analysis was performed with the PANSS positive symptom score, which measures four symptoms (e.g., hallucinations, disorganization, hostility, excitement) other than delusional thinking. Moreover, the mean PANSS positive symptom score was rather low across studies.
Conclusions
The results of this meta-analysis strongly suggest that schizophrenia is associated with an aberrant reactivity of the limbic system to nonthreatening stimuli. At the very least, these results should encourage investigators to systematically report activations during the neutral condition in future task-based fMRI studies on emotion processing in schizophrenia or to make the relevant data accessible to investigators who are interested in the question. Investigations using more complex paradigms and more complex analytic strategies are required. Such investigations should favor the use of facial stimuli and implicit task instructions. Finally, more studies on the limbic reactivity to neutral stimuli in attenuated psychosis syndrome and psychiatric disorders other than schizophrenia are warranted. In the meantime, the results presented here add another significant pillar to the influential aberrant salience hypothesis of psychosis.
1 : Anxiety and depression in psychosis: a systematic review of associations with positive psychotic symptoms. Acta Psychiatr Scand 2013; 128:327–346Crossref, Medline, Google Scholar
2 : The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr Scand 2018; 137:380–390Crossref, Medline, Google Scholar
3 : Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull 2010; 36:1009–1019Crossref, Medline, Google Scholar
4 : Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull 2010; 36:143–150Crossref, Medline, Google Scholar
5 : Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr Res 2012; 142:65–70Crossref, Medline, Google Scholar
6 : Do people with schizophrenia experience more negative emotion and less positive emotion in their daily lives? A meta-analysis of experience sampling studies. Schizophr Res 2017; 183:49–55Crossref, Medline, Google Scholar
7 : Abnormal brain activation during threatening face processing in schizophrenia: a meta-analysis of functional neuroimaging studies. Schizophr Res 2017; 197:200–208Crossref, Medline, Google Scholar
8 : Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry 2012; 71:136–145Crossref, Medline, Google Scholar
9 : Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull 2010; 36:1029–1039Crossref, Medline, Google Scholar
10 : Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull 2012; 38:608–621Crossref, Medline, Google Scholar
11 : Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005; 48:175–187Crossref, Medline, Google Scholar
12 : Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry 2008; 64:70–73Crossref, Medline, Google Scholar
13 : Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry 2015; 172:784–792Link, Google Scholar
14 : 18FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2010; 260:69–76Crossref, Medline, Google Scholar
15 : Neural response to emotional salience in schizophrenia. Neuropsychopharmacology 2005; 30:984–995Crossref, Medline, Google Scholar
16 : Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res 2006; 82:153–162Crossref, Medline, Google Scholar
17 : Neural correlates of aberrant emotional salience predict psychotic symptoms and global functioning in high-risk and first-episode psychosis. Soc Cogn Affect Neurosci 2015; 10:1429–1436Crossref, Medline, Google Scholar
18 : Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry 2010; 67:902–911Crossref, Medline, Google Scholar
19 : Reprint: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Phys Ther 2009; 89:873–880Medline, Google Scholar
20 : Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev 2012; 36:2325–2333Crossref, Medline, Google Scholar
21 : A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry 2012; 27:605–611Crossref, Medline, Google Scholar
22 : Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634Crossref, Medline, Google Scholar
23 : Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage 2013; 66:270–277Crossref, Medline, Google Scholar
24 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
25 : Binding action and emotion in first-episode schizophrenia. Psychopathology 2014; 47:394–407Crossref, Medline, Google Scholar
26 : Neural correlates of emotion recognition in schizophrenia. Schizophr Res 2010; 122:113–123Crossref, Medline, Google Scholar
27 : The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology 2008; 33:473–479Crossref, Medline, Google Scholar
28 : Individuals diagnosed with schizophrenia assign emotional importance to neutral stimuli: an fMRI study. ISRN Psychiatry 2013; 2013:
29 : Abnormal neural processing during emotional salience attribution of affective asymmetry in patients with schizophrenia. PLoS One 2014; 9:
30 : Neuronal correlates of affective theory of mind in schizophrenia out-patients: evidence for a baseline deficit. Psychol Med 2010; 40:1607–1617Crossref, Medline, Google Scholar
31 : Altered medial prefrontal activity during dynamic face processing in schizophrenia spectrum patients. Schizophr Res 2014; 157:225–230Crossref, Medline, Google Scholar
32 : Altered amygdala activation in schizophrenia patients during emotion processing. Schizophr Res 2013; 150:101–106Crossref, Medline, Google Scholar
33 : Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatry Res 2010; 182:200–206Crossref, Medline, Google Scholar
34 : Differential brain activation during facial emotion discrimination in first-episode schizophrenia. J Psychiatr Res 2009; 43:592–599Crossref, Medline, Google Scholar
35 : Effects of oxytocin on neural response to facial expressions in patients with schizophrenia. Neuropsychopharmacology 2015; 40:1919–1927Crossref, Medline, Google Scholar
36 : Functional activation abnormalities during facial emotion perception in schizophrenia patients and nonpsychotic relatives. Schizophr Res 2015; 168:330–337Crossref, Medline, Google Scholar
37 : A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biol Psychiatry 2006; 60:423–431Crossref, Medline, Google Scholar
38 : Automatic amygdala response to facial expression in schizophrenia: initial hyperresponsivity followed by hyporesponsivity. BMC Neurosci 2013; 14:140Crossref, Medline, Google Scholar
39 : A functional anatomic study of emotion in schizophrenia. Schizophr Res 2002; 58:159–172Crossref, Medline, Google Scholar
40 : Medial frontal hyperactivity in reality distortion. Biol Psychiatry 2007; 61:1171–1178Crossref, Medline, Google Scholar
41 : Social appraisal in chronic psychosis: role of medial frontal and occipital networks. J Psychiatr Res 2011; 45:526–538Crossref, Medline, Google Scholar
42 : Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am J Psychiatry 2011; 168:276–285Link, Google Scholar
43 : Functional imaging of emotional memory in bipolar disorder and schizophrenia. Bipolar Disord 2009; 11:840–856Crossref, Medline, Google Scholar
44 : Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev 2014; 45:202–211Crossref, Medline, Google Scholar
45 : Cerebral differences in explicit and implicit emotional processing: an fMRI study. Neuropsychobiology 2007; 56:32–39Crossref, Medline, Google Scholar
46 : The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 2012; 69:776–786Crossref, Medline, Google Scholar
47 : Adult hippocampal neurogenesis modulates fear learning through associative and nonassociative mechanisms. J Neurosci 2015; 35:11330–11345Crossref, Medline, Google Scholar
48 : The dopamine hypothesis of schizophrenia: version III: the final common pathway. Schizophr Bull 2009; 35:549–562Crossref, Medline, Google Scholar
49 : Rethinking the use of neutral faces as a baseline in fMRI neuroimaging studies of axis I psychiatric disorders. J Neuroimaging 2017; 27:281–291Crossref, Medline, Google Scholar
50 : Evaluating face trustworthiness: a model based approach. Soc Cogn Affect Neurosci 2008; 3:119–127Crossref, Medline, Google Scholar
51 : Functional neuroimaging predictors of self-reported psychotic symptoms in adolescents. Am J Psychiatry 2017; 174:566–575Link, Google Scholar
52 : Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophr Res 2010; 118:88–97Crossref, Medline, Google Scholar
53 : Exaggerated brain activation during emotion processing in unaffected siblings of patients with schizophrenia. Biol Psychiatry 2011; 70:81–87Crossref, Medline, Google Scholar



