Nociceptin Receptors Upregulated in Cocaine Use Disorder: A Positron Emission Tomography Imaging Study Using [11C]NOP-1A
Abstract
Objective:
Nociceptin/orphanin FQ (N/OFQ) is an antistress neuropeptide transmitter in the brain that counteracts corticotropin-releasing factor (CRF)-mediated stress and anxiety symptoms during drug and alcohol withdrawal. It also inhibits the release of a wide array of neurotransmitters, including dopamine and glutamate, which allows for it to block the rewarding properties of cocaine. Chronic cocaine administration in rodents has been shown to decrease N/OFQ and increase nociceptive opioid peptide (NOP) receptors in the nucleus accumbens. No previous studies have reported on the in vivo status of NOP in chronic cocaine-abusing humans.
Methods:
[11C]NOP-1A and positron emission tomography (PET) were used to measure in vivo NOP binding in 24 individuals with cocaine use disorder and 25 healthy control subjects matched for age, sex, and smoking status. Participants with cocaine use disorder with no comorbid psychiatric or medical disorders were scanned after 2 weeks of outpatient-monitored abstinence. [11C]NOP-1A distribution volume (VT) was measured with kinetic analysis using the arterial input function in brain regions that mediate reward and stress behaviors. Participants with cocaine use disorder were followed up for 12 weeks after PET scanning to document relapse and relate it to VT.
Results:
A significant increase in [11C]NOP-1A VT was observed in the cocaine use disorder group compared with the healthy control group. This increase, which was generalized across all regions of interest (approximately 10%), was most prominent in the midbrain, ventral striatum, and cerebellum. However, increased VT in these regions did not predict relapse.
Conclusions:
Increased NOP in cocaine use disorder suggests an adaptive response to decreased N/OFQ, or increased CRF transmission, or both. Future studies should examine the interactions between CRF and NOP to elucidate their role in negative reinforcement and relapse. NOP agonist medications to enhance N/OFQ should be explored as a therapeutic to treat cocaine use disorder.
The endogenous neuropeptide nociception/orphanin FQ (N/OFQ) and its target receptor, nociceptive opioid peptide (NOP) receptor, are structurally similar to dynorphin A and its target kappa opioid receptors (1, 2). However, N/OFQ has no affinity for the mu, kappa, or delta opioid receptors, and endogenous opioids such as endorphins, enkephalins, and dynorphin do not bind to NOP. N/OFQ is an antistress- and resilience-mediating neuropeptide in the brain that counteracts stress and anxiety, which is primarily mediated by corticotropin-releasing factor (CRF), norepinephrine, orexin, vasopressin, and dynorphin (3). N/OFQ blocks stress-induced analgesia, anorexia, and anxious behaviors in rodents (4–6). N/OFQ knockout mice show increased anxiety-like behaviors compared with wild-type mice (7). N/OFQ also blocks the rewarding properties of cocaine in behavioral paradigms, including the acquisition of conditioned place preference (8, 9).
In line with this, increases in the rewarding properties of cocaine have been observed in NOP knockout mice (10). Studies have also shown that N/OFQ can reverse the psychomotor sensitization response that develops following chronic exposure to cocaine (considered to be a rodent model for drug craving) (9, 11, 12). In addition, N/OFQ reduces cocaine-induced dopamine release in the nucleus accumbens (12–14). Two previous studies in rodents evaluated the effects of chronic cocaine on N/OFQ and NOP. In the first study, which used both radioimmunoassay and immuno-autoradiographic techniques, animals that were chronically administered cocaine showed decreased N/OFQ in the nucleus accumbens, substantia nigra/ventral tegmental area, and medial (but not lateral) caudate putamen compared with the control group (15). In a replication of this observation, the expression of N/OFQ precursor protein (pro-N/OFQ) was found to be decreased in the nucleus accumbens and in the lateral (but not medial) caudate putamen in rodents that were chronically administered cocaine (16). NOP gene expression (mRNA) examined in the same animals that were chronically exposed to cocaine was significantly increased in the nucleus accumbens (approximately 50%) but decreased in the caudate putamen (approximately 50%–100%), compared with the control group. In summary, the findings from several basic investigations suggest that N/OFQ and NOP alterations modulate drug-induced reward in cocaine use disorders. They also suggest a role for N/OFQ in balancing the functions of the stress-mediating neuropeptides, such as CRF, that promote negative reinforcement and lead to relapse (3).
Because of the lack of a radiotracer to image NOP, no previous in vivo studies, to our knowledge, have characterized the N/OFQ system in chronic cocaine-abusing humans. [11C]NOP-1A, first radiolabeled and validated by the National Institute of Mental Health Molecular Imaging Branch, has been successfully used to measure NOP in humans (17–19). In the present study, we used [11C]NOP-1A and positron emission tomography (PET) to measure the in vivo binding to NOP in 24 recently abstinent individuals with cocaine use disorder and 25 healthy control subjects. After the [11C]NOP-1A PET scan, participants with cocaine use disorder were enrolled in a follow-up protocol in which they were monitored three times per week for 12 weeks using a contingency management approach. The objective of the follow-up protocol was to document relapse and relate it to NOP. We hypothesized that there would be an increase in [11C]NOP-1A binding in participants with cocaine use disorder compared with healthy control subjects in brain regions that mediate reward and stress behaviors. Such a finding would support the notion that NOP is upregulated in response to decreased N/OFQ in cocaine use disorder.
Methods
Human Study Subjects
This study was approved by the University of Pittsburgh Human Research Protection Office and Radioactive Drug Research Committee. All study subjects provided written informed consent. Persons with cocaine use disorder who were interested in abstaining from cocaine were recruited through advertisements at addiction clinics, on buses, in newspapers, and on web sites. Study subjects were also recruited via a research registry administered by the University of Pittsburgh (Pitt+Me).
Inclusion criteria for the cocaine use disorder group were being 18 to 50 years of age, meeting DSM-5 criteria for cocaine use disorder as assessed by the Structured Clinical Interview for DSM-5 (SCID-5) (20), having no other current psychiatric or drug or alcohol use disorder diagnosis except cocaine use disorder (tobacco use disorder and recreational cannabis or alcohol use were not exclusionary), having no medical or neurological illness, not currently taking any medical or psychotropic medication, not currently pregnant, having no recent research or occupational radioactivity exposure, and having no contraindications for MRI. Inclusion criteria for healthy control subjects were the same as for the cocaine use disorder group except that they could not have cocaine use disorder or any present or past psychiatric and addictive disorders as assessed by the SCID-5.
Data on demographic characteristics, substance use, and family history of addiction were recorded as recommended by the National Institute on Drug Abuse in the substance abuse and addiction assessments core PhenX Toolkit (https://www.phenxtoolkit.org). Clinical assessments included the Substance Use Inventory, the Cocaine Craving Questionnaire, the Fagerström Test for Nicotine Dependence, the Perceived Stress Scale, the Hamilton Anxiety Rating Scale (HAM-A), and the Barratt Simplified Measure of Social Status. Participants with cocaine use disorder were monitored three times per week in the outpatient setting for abstinence from cocaine for a minimum of 10 days before the PET scans. These participants were scanned only if a urine drug screen confirmed the absence of all drugs of abuse, including cannabis, on the scan day. In addition, they were not permitted to use any nicotine products after their arrival to the PET facility (i.e., the last cigarette could not be <2 hours before the PET scan). Female participants underwent scanning irrespective of menstrual cycle phase or hormonal contraceptive use.
PET Image Acquisition and Analysis
Before PET imaging, a magnetization-prepared rapid gradient-echo structural MRI scan was obtained using a Siemens 3-T Trio scanner for region determination. [11C]NOP-1A PET imaging sessions were conducted with the Siemens Biograph64 mCT scanner using methods described elsewhere (17, 21–24). Following a low-dose CT scan of the brain that was acquired for attenuation correction, participants received an intravenous bolus injection of [11C]NOP-1A, and emission data were collected for 70 minutes. Metabolite-corrected arterial input function and plasma free fraction (fP) measurements were performed for all study subjects. PET data were reconstructed by filtered back-projection with the camera’s built-in software. The image analysis software PMOD, version 3.802 (PMOD Technologies, Zurich), was used to conduct frame-to-frame motion correction for head movement. MR-PET image alignment was performed with a normalized mutual information algorithm. Regions of interest were generated for each study subject using the built-in brain parcellation workflow within PMOD’s PNEURO Tool (25). Region generation was based on the Automated Anatomical Labeling volumes of interest atlas (26, 27). Regions of interest included the amygdala, hippocampus, midbrain, cerebellum, striatum (ventral striatum, caudate, and putamen), and prefrontal cortex (specifically the dorsolateral, orbital, medial, and anterior cingulate) subdivisions. These regions, which have been a focus of N/OFQ and NOP basic investigations, are the same regions we examined in a previous study of individuals with alcohol use disorder (24). All regions generated by the brain parcellation tool were visually inspected and adjusted as deemed necessary by an image analyst trained in manual region drawing. Regional volumes and time activity curves were also generated in PMOD. Derivation of [11C]NOP-1A volume of distribution (VT) expressed relative to total plasma concentration in the regions of interest were performed using a two-tissue compartment kinetic analysis with the arterial input function implemented in MATLAB (21, 22, 28). VT, which includes both receptor-bound specific and nonspecific binding, was used as the outcome measure (as opposed to the binding potential relative to nondisplaceable uptake, BPND) because there is no brain region that is devoid of specific binding to NOP (23).
Relapse Monitoring Protocol for Participants With Cocaine Use Disorder
To document relapse, participants in the cocaine use disorder group were enrolled in a 12-week follow-up protocol after the [11C]NOP-1A PET scan. This follow-up protocol was modeled after the contingency management protocol used in a previous [11C]raclopride-amphetamine PET study (29, 30). Briefly, participants in the cocaine use disorder group met with the research team three times per week for urine drug screens and earned voucher points on an escalating schedule for each negative urine drug screen. They earned bonus points for every three consecutive cocaine-free screens (1 week of abstinence). Missed appointments reset the voucher points to a value that was lower by 10 points. Participants had the potential to earn a maximum of $1,197.00 for providing cocaine-free urine drug screens on all of the scheduled monitoring visits. The money earned was disbursed to the participants on a weekly basis via a debit card. Participants were terminated from the follow-up protocol if they tested positive for cocaine three times (i.e., they were allowed only three distinct relapses), if they missed three consecutive scheduled appointments (i.e., if they were lost to follow-up for 1 week), or if they initiated any medications that influence N/OFQ (for example, buprenorphine). In addition, participants were monitored once a week for depression and suicidal ideation and were debriefed on their weekly progress pertaining to abstinence.
Statistical Analysis
All statistical analyses were conducted with IBM SPSS, version 25 (IBM, Armonk, N.Y.). Normality of data was examined with Kolmogorov-Smirnov and Shapiro-Wilk tests. Comparisons of group demographic and baseline scan parameters (such as injected dose, mass, and plasma clearance) were performed with unpaired t tests. The primary analysis examined overall group differences in [11C]NOP-1A VT, with a linear mixed-model analysis performed with regions of interest as a repeated measure and diagnostic group (cocaine use disorder, healthy control) as the fixed factor. The effect of sex and smoking status on VT and diagnostic group was subsequently examined in a second-level linear mixed-model analysis by including these variables as fixed factors in the model. Post hoc unpaired t tests in the individual regions of interest were also conducted. A two-tailed probability value of ≤0.05 was selected as the significance level for the primary analysis (linear mixed-model analysis that included all the regions of interest). A Benjamini-Hochberg false discovery rate correction with an alpha set at 0.05 was applied to correct for multiple comparisons in the individual regions of interest in the post hoc analyses.
Group comparison of regional VT in participants with cocaine use disorder who abstained, relapsed, or dropped out were evaluated with a linear mixed-model analysis (as described previously). Previous studies of cocaine use disorder have shown that early treatment response and motivation as reflected by clinic attendance in the first 2–3 weeks predict sustained abstinence during 12 weeks of contingency management treatment (29, 31). On the basis of these results, we also used a linear mixed-model analysis to compare VT in participants with cocaine use disorder who relapsed or dropped out within the first 2 weeks (out of a 12-week follow-up) with VT in participants who did not relapse or who continued in the protocol past 2 weeks. The amount of voucher money earned, which reflected abstinence, was used as a secondary outcome measure in a Pearson product moment correlation (only regions that survived false discovery rate correction were used for all correlational analyses). Lastly, a Cox proportional hazards model was used to determine the association between time to relapse and regional VT in the cocaine use disorder group. Time to relapse was defined as the time between the date of [11C]NOP-1A PET scan and the date of the first relapse to cocaine (positive cocaine urine drug screen) over the 12-week follow-up period. Data were censored if a study subject did not experience relapse by the end of the follow-up period, including study subjects lost to follow-up. Models were run with and without adjustment for potential confounding factors (such as sex, Perceived Stress Scale ratings, and Cocaine Craving Questionnaire ratings). The relationship between regional VT and other clinical measures of stress (Perceived Stress Scale), anxiety (HAM-A), craving (Cocaine Craving Questionnaire), and severity of cocaine abuse (cocaine use duration in years, frequency of use in days, and amount spent on cocaine in dollars) in the cocaine use disorder group was assessed with Pearson product moment correlation. A two-tailed probability value ≤0.05 was selected as the significance level for all analyses that involved clinical measures.
Results
Twenty-four individuals with cocaine use disorder were matched as closely as possible with 25 healthy control subjects on age, sex, ethnicity, and nicotine use status. The demographic characteristics and clinical variables of the study sample are summarized in Table 1.
| Characteristic | Cocaine Use Disorder Group (N=24) | Healthy Control Group (N=25) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Female | 10 | 42 | 11 | 44 |
| Male | 14 | 58 | 14 | 56 |
| Ethnicity | ||||
| African American | 10 | 42 | 5 | 20 |
| Caucasian | 12 | 50 | 18 | 72 |
| Asian | 0 | 0 | 1 | 4 |
| Hispanic | 1 | 4 | 0 | 0 |
| More than one race | 1 | 4 | 1 | 4 |
| Mean | SD | Mean | SD | |
| Age (years) | 40.0 | 9.0 | 38.0 | 9.0 |
| Barratt Simplified Measure of Social Status | ||||
| Education | 14.0 | 3.0 | 15.0 | 4.0 |
| Occupation | 21.0 | 10.0 | 27.0* | 9.0 |
| Total | 33.0 | 13.0 | 42.0* | 12.0 |
| N | % | N | % | |
| Tobacco use | 16 | 67 | 13 | 50 |
| >10 cigarettes per day | 7 | 29 | 4 | 15 |
| Cannabis use (recreational) | 4 | 17 | ||
| Mean | SD | Mean | SD | |
| Hamilton Anxiety Rating Scale (range, 0–56) | 8.5 | 7.5 | 2.1* | 3.8 |
| Perceived Stress Scale (range, 0–40) | 15.2 | 7.8 | 5.5* | 5.3 |
| Cocaine Craving Questionnaire (range, 0–70) | 26.0 | 15.0 | ||
| Age at first use of cocaine (years) | 23.0 | 8.0 | ||
| Duration of cocaine use (years) | 15.0 | 9.0 | ||
| Cocaine use frequency per week (days) | 2.0 | 2.0 | ||
| Amount spent on cocaine per week (dollars) | 126.0 | 64.0 | ||
| N | % | N | % | |
| 12-week follow-up protocol to monitor for relapse following PET scan, final outcome | ||||
| Abstinent | 6 | 25.0 | ||
| Relapsed (urine drug screen positive for cocaine) | 9 | 37.5 | ||
| Dropped out | 9 | 37.5 | ||
| Mean | SD | Mean | SD | |
| Amount of money earned in vouchers (dollars) | 415 | 476 | ||
| Time to an event (i.e., first relapse or dropout or completion) (days) | 35 | 34 | ||
| Time to first relapse (days) | 13 | 14 | ||
| Duration in follow-up protocol (days) | 43 | 35 | ||
TABLE 1. Demographic and clinical characteristics of participants with cocaine use disorder and healthy control subjectsa
[11C]NOP-1A Scan Parameters
No significant group differences were observed between participants with cocaine use disorder and healthy control subjects in any of the baseline scan parameters (Table 2). Consistent with previously reported findings, [11C]NOP-1A plasma free fraction (fP) measurements were not reliable, because the tracer showed relatively high retention to the filter in the saline buffer solution condition (24). No significant group differences were observed in the region-of-interest volumes determined from MRI scans.
| Cocaine Use Disorder Group (N=24) | Healthy Control Group (N=25) | |||
|---|---|---|---|---|
| Scan Parameter | Mean | SD | Mean | SD |
| Injected dose (mCi) | 12.4 | 0.7 | 12.3 | 1.0 |
| Specific activity (Ci/mmoles) | 2169 | 975 | 2230 | 558 |
| Injected mass (µg) | 2.8 | 1.0 | 2.5 | 0.6 |
| Plasma free fraction (fP, %)a | 14.5 | 3.2 | 13.2 | 2.3 |
| Plasma free fraction buffer (fP, %)a | 71.0 | 15.7 | 76.1 | 11.5 |
| Clearance (L/h) | 151.8 | 38.9 | 155.1 | 38.1 |
TABLE 2. [11C]NOP-1A scan parametersa
In Vivo Binding of [11C]NOP-1A (VT)
Regional [11C]NOP-1A VT was significantly higher in the cocaine use disorder group compared with the healthy control group (linear mixed-model analysis: effect of diagnosis [F=4.39, df=1, 47, p=0.042], effect of region [F=520.20, df=10, 470, p<0.001], region-by-diagnosis interaction [F=1.45, df=10, 470, p=0.156]).
[11C]NOP-1A VT in the regions of interest was on average 7%−12% lower among females compared with males (Figure 1). The inclusion of sex as a factor in the linear mixed-model analysis did not alter the significance of the result described above (effect of diagnosis: F=4.65, df=1, 45, p=0.037; effect of sex: F=4.45, df=1, 45, p=0.041; sex-by-diagnosis interaction: F=0.25, df=1, 45, p=0.622). However, the effect of diagnosis fell short of significance when tobacco use status was included as an additional factor in the linear mixed-model analysis (effect of diagnosis: F=3.88, df=1, 43, p=0.055; effect of smoking: F=0.03, df=1, 43, p=0.86; smoking-by-diagnosis interaction: F=0.11, df=1, 43, p=0.74). Excluding the four participants in the cocaine use disorder group who tested positive for cannabis during the outpatient monitoring before the PET scan did not change the results (cocaine use disorder group, N=20; healthy control group, N=25; linear mixed-model analysis: effect of diagnosis: F=5.65, df=1, 43, p=0.022; effect of region: F=500.02, df=10, 430, p<0.001; region-by-diagnosis interaction: F=1.97, df=10, 430, p=0.035).
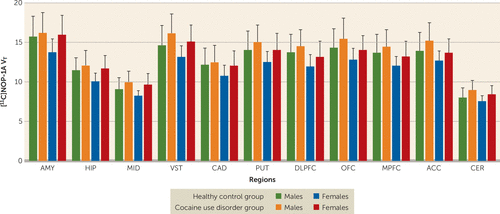
FIGURE 1. Female study subjects with significantly lower distribution volume (VT) values in the regions of interest compared with male study subjectsa
a Sex-based differences were not present with respect to the increase in VT in the cocaine use disorder group compared with the healthy control group (sex-by-diagnosis interaction, p=0.62). AMY=amygdala, ACC=anterior cingulate cortex, CAD=caudate, CER=cerebellum, DLPFC=dorsolateral prefrontal cortex, HIP=hippocampus, MID=midbrain, MPFC=medial prefrontal cortex, OFC=orbital frontal cortex, PUT=putamen, VST=ventral striatum.
Unpaired t tests conducted at the level of the individual regions of interest were significant in four of the 11 regions of interest (Table 3). Comparisons in the ventral striatum and cerebellum survived the false discovery rate correction.
| Cocaine Use Disorder Group (N=24) | Healthy Control Group (N=25) | Uncorrected p | |||
|---|---|---|---|---|---|
| Region of Interest | Mean | SD | Mean | SD | |
| Amygdala | 16.10 | 2.48 | 14.86 | 2.40 | 0.084 |
| Hippocampus | 11.93 | 1.76 | 10.85 | 1.53 | 0.027 |
| Midbrain | 9.81 | 1.40 | 8.72 | 1.22 | 0.0056 |
| Ventral striatum | 15.70 | 2.35 | 13.99 | 2.21 | 0.0118* |
| Caudate | 12.32 | 1.99 | 11.56 | 1.90 | 0.177 |
| Putamen | 14.60 | 2.18 | 13.37 | 2.13 | 0.051 |
| Dorsolateral prefrontal cortex | 13.97 | 2.09 | 12.98 | 2.11 | 0.105 |
| Orbital frontal cortex | 14.85 | 2.40 | 13.68 | 2.15 | 0.078 |
| Medial prefrontal cortex | 13.93 | 2.14 | 12.96 | 2.05 | 0.115 |
| Anterior cingulate cortex | 14.57 | 2.18 | 13.39 | 1.99 | 0.053 |
| Cerebellum | 8.73 | 1.21 | 7.81 | 1.03 | 0.0062* |
TABLE 3. [11C]NOP-1A volume of distribution expressed relative to total plasma concentration (distribution volume) in regions of interest
Relationship Between VT and Relapse to Cocaine During Follow-Up
There were no significant differences in VT between participants with cocaine use disorder who abstained compared with those who relapsed compared with those who dropped out during the follow-up period (linear mixed-model analysis: effect of final outcome status: F=0.13, df=2, 21, p=0.88; effect of region: F=230.65, df=10, 210, p<0.001; region-by-diagnosis interaction: F=0.49, df=20, 210, p=0.97) (Figure 2). Stratification of the participants with cocaine use disorder on the basis of whether they relapsed or dropped out within the first 2 weeks (<2 weeks, N=9; >2 weeks, N=15) also revealed no group differences in [11C]NOP-1A VT (linear mixed-model analysis: effect of duration: F=1.24, df=1, 22, p=0.28; effect of region: F=223.81, df=10, 220, p<0.001; time-by-region interaction: F=0.65, df=10, 220, p=0.77). In addition, there were no significant correlations between VT and the amount of voucher money earned during the follow-up period (midbrain, r=0.19, p=0.36; ventral striatum, r=0.18, p=0.41; and cerebellum, r=0.18, p=0.40). Lastly, VT in the regions of interest was not predictive of time to relapse to cocaine use (midbrain, Exp [B]=1.0, p=0.99; ventral striatum, Exp [B]=0.97, p=0.78; and cerebellum, Exp [B]=1.02, p=0.95). These results were unchanged when the models were adjusted for potential confounding factors such as sex, Perceived Stress Scale ratings, and Cocaine Craving Questionnaire ratings.
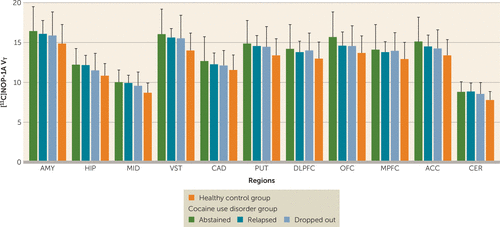
FIGURE 2. Abstinence (N=6), relapse (N=9), and dropout (N=9) among cocaine use disorder subjects during the 12-week follow-up period showing no significant differences in distribution volume (VT)a
a Baseline VT data for healthy control subjects (N=25) are also shown. AMY=amygdala, ACC=anterior cingulate cortex, CAD=caudate, CER=cerebellum, DLPFC=dorsolateral prefrontal cortex, HIP=hippocampus, MID=midbrain, MPFC=medial prefrontal cortex, OFC=orbital frontal cortex, PUT=putamen, VST=ventral striatum.
Higher levels of perceived stress on the Perceived Stress Scale were predictive of less time to relapse (Exp [B]=1.08, p=0.05). No such relationship was observed between cocaine cravings (Cocaine Craving Questionnaire) and time to relapse (Exp [B]=1.00, p=0.88).
Relationships Between VT and Clinical Measures
No significant relationships were observed between regional [11C]NOP-1A VT and any of the other clinical measures in the cocaine use disorder group, including cocaine use frequency and duration, amount of money spent, perceived stress, anxiety, and cocaine craving.
Relationship Between VT and Age
In the healthy control group, there was a negative relationship between age and VT in the medial prefrontal cortex (r=–0.39, p=0.06) and dorsolateral prefrontal cortex (r=–0.36, p=0.08) but not in other regions. No such relationship between age and VT was observed in any of the regions in the cocaine use disorder group.
Discussion
In this [11C]NOP-1A PET study, we found that cocaine abuse in humans was associated with a generalized (approximately 10%) increase in binding to NOP in brain regions that mediate reward and stress behaviors (the four regions that were significant included the midbrain, ventral striatum, hippocampus, and cerebellum), that females have less NOP binding compared with males, and that increased stress during cocaine withdrawal predicts early relapse. An important negative result was the lack of a relationship between VT and relapse.
Increased [11C]NOP-1A binding in cocaine users suggests a compensatory upregulation of NOP in response to decreased N/OFQ in the brain. This is supported by studies of rodents that were administered cocaine chronically, which showed decreases in N/OFQ in the nucleus accumbens, caudate-putamen, and substantia nigra/ventral tegmental area (15, 16). However, NOP’s regulatory response to decreased N/OFQ in these rodents was shown to be variable at the level of the striatum (i.e., increased in the nucleus accumbens [ventral striatum]) and decreased in the caudate-putamen (16). In the present study, PET data for the cocaine use disorder group, which showed an increase in NOP in the ventral striatum and putamen and no change in the caudate, was only partially consistent with these rodent data. The lack of an increase in NOP in the caudate, which has been observed in studies of both humans and rodents, is still noteworthy, because it may relate to the fact that the caudate is the only striatal subdivision in which dopamine transmission is not blunted in cocaine use disorder (32). Studies examining the relationship between NOP VT and amphetamine-induced dopamine release in people with cocaine use disorder might clarify this relationship. Behavioral paradigms that mimic chronic stress in rodents consistently show upregulation of NOP in the limbic brain regions (33–36). Thus, increased NOP in the cocaine use disorder group might also reflect an adaptive response to balance the effects of CRF, which increases stress and promotes relapse. Supportive of this hypothesis is a study in which an upregulation of NOP in the bed nucleus of the stria terminalis and the amygdala was observed following increases in CRF (33, 37). Similar interactions between NOP and other stress-promoting neuropeptides, such as dynorphin, have also been described in rodents administered cocaine chronically (16). Imaging studies with [11C]NOP-1A investigating NOP-CRF and NOP-dynorphin interactions in cocaine use disorder should be considered, because such studies might clarify the interplay between stress and antistress neuropeptides in negative reinforcement and relapse. N/OFQ stimulates NOP to inhibit calcium channels and activate potassium channels (38). This allows for N/OFQ to inhibit the release of multiple neurotransmitters, including dopamine, serotonin, acetylcholine, glutamate, and GABA (39). Increased N/OFQ has been shown to inhibit cocaine-induced dopamine release in the nucleus accumbens (12–14). This mechanism has therapeutic potential, because it can decrease the cocaine reward experience. It is likely that N/OFQ directly modulates midbrain dopamine neurons to affect cocaine-induced dopamine release, because 50%−90% of tyrosine hydroxylase positive cells (TH+) in the ventral tegmental area and substantia nigra express NOP (40, 41). However, an effect for presynaptic NOP on dopamine terminals cannot be ruled out, because administration of N/OFQ into the nucleus accumbens also blocks cocaine-induced dopamine release (13). In addition, increased N/OFQ has been shown to decrease glutamate transmission in the reward- and stress-mediating brain regions, such as the cortex, midbrain, amygdala, and cerebellum (42–44). This mechanism also has therapeutic potential, because increased glutamate in cocaine use disorder has been linked to relapse and reinstatement (45). These mechanistic investigations and the present PET study support clinical trials with a NOP agonist to increase N/OFQ transmission in cocaine use disorder. The inability to link NOP VT with relapse in the present study cannot be viewed as less supportive of this conceptual model, because it is not possible to quantitate endogenous N/OFQ levels from a baseline [11C]NOP-1A scan. [11C]NOP-1A imaging paradigms that measure the regulatory response of NOP to stress/CRF might be more successful in predicting relapse in cocaine use disorder.
Sex-based differences have been reported for stress-mediating neuropeptides and their receptors, including CRF and kappa opioid receptors (46, 47). To our knowledge, decreased NOP in females relative to males is the first report of sex-based differences in an antistress system in the brain. This unreported observation fell short of significance in a separate set of 15 healthy control subjects (females, N=5; males, N=10; 11 regions of interest; linear mixed-model analysis: effect of gender on VT [p=0.055], region [p<0.001], gender-by-region interaction [p=0.012]) in our previous [11C]NOP-1A alcohol study (19). Studies to understand the clinical relevance of decreased NOP in females are necessary, because they might explain previously described behavioral and physiological phenomena, such as females who report greater subjective levels of stress showing increased sensitivity to CRF. Such studies may also provide clues as to the reasons why females are at higher risk for certain chronic stress disorders, such as depression, anxiety, and chronic pain.
Sex had no effect on the increased NOP in cocaine use disorder (i.e., there was no sex-by-diagnosis interaction). However, the effect size of the difference in [11C]NOP-1A VT in the cocaine use disorder group compared with the healthy control group was much larger in females compared with males (Table 4). This is interesting, since rodent and human studies of chronic cocaine use have consistently reported more negative affect, withdrawal symptoms, and stress-induced relapse/reinstatement in females compared with males (48). It is tempting to speculate greater decreases in resilience-mediating N/OFQ or increases in stress-mediating CRF (both of which have been shown to upregulate NOP receptors in basic investigations) as reasons for more unpleasant symptoms and subsequent relapse in females with chronic cocaine abuse. Future [11C]NOP-1A PET studies should focus on these sex differences and also control for the fluctuations in sex hormones associated with menstrual cycle and hormonal contraceptive use.
| Cohen’s d | |||
|---|---|---|---|
| Regions of Interest | Male | Female | Effect Size Ratio (Female:Male) |
| Amygdala | 0.19 | 1.10 | 5.8 |
| Hippocampus | 0.37 | 1.27 | 3.4 |
| Midbrain | 0.62 | 1.33 | 2.1 |
| Ventral striatum | 0.62 | 1.12 | 1.8 |
| Caudate | 0.16 | 0.85 | 5.3 |
| Putamen | 0.44 | 0.89 | 2.0 |
| Dorsolateral prefrontal cortex | 0.38 | 0.73 | 1.9 |
| Orbital frontal cortex | 0.45 | 0.77 | 1.7 |
| Medial prefrontal cortex | 0.35 | 0.77 | 2.2 |
| Anterior cingulate cortex | 0.57 | 0.70 | 1.2 |
| Cerebellum | 0.81 | 0.96 | 1.2 |
TABLE 4. Effect size of the difference in [11C]NOP-1A distribution volume in participants with cocaine use disorder compared with healthy control subjects by sexa
The role of negative reinforcement in promoting stress and anxiety and leading to relapse to drug or alcohol use is well established in rodent models of addiction (3). Consistent with this model, we found that increased stress during cocaine withdrawal is predictive of less time to relapse. However, we found no relationship between perceived stress and anxiety and the antistress/resilience conferring NOP (VT). The study subjects were scanned after they abstained from cocaine for a minimum of 10 days, which may have limited the ability to detect such a relationship. PET studies focusing on the neurochemistry of withdrawal are warranted, because the behavioral symptoms experienced during this period predict relapse.
We were unable to exclude differences in [11C]NOP-1A nonspecific binding (VND) and plasma free fraction (fP) as contributors to VT. Reassuringly, previous [11C]NOP-1A-blocking studies in humans suggest that 50%−75% of VT represents specific binding to NOP (18). In summary, we demonstrated a significant increase in NOP in persons with cocaine use disorder. The data showed regionally selective increases in NOP that fell short of statistical significance and that were convincingly evidenced in the midbrain, ventral striatum, and cerebellum. Increased NOP in the cocaine use disorder group may be indicative of an adaptive response to decreased N/OFQ or increased CRF or both. The clinical development of NOP agonists to enhance N/OFQ transmission should be explored as a treatment for cocaine use disorder.
1 : Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 1995; 270:792–794Crossref, Medline, Google Scholar
2 : Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 1995; 377:532–535Crossref, Medline, Google Scholar
3 : A role for brain stress systems in addiction. Neuron 2008; 59:11–34Crossref, Medline, Google Scholar
4 : Orphanin FQ is a functional anti-opioid peptide. Neuroscience 1996; 75:333–337Crossref, Medline, Google Scholar
5 : Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA 1997; 94:14854–14858Crossref, Medline, Google Scholar
6 : Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacology (Berl) 2002; 161:113–119Crossref, Medline, Google Scholar
7 : Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci USA 1999; 96:10444–10449Crossref, Medline, Google Scholar
8 : Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 2004; 172:129–136Crossref, Medline, Google Scholar
9 : Orphanin FQ/nociceptin not only blocks but also reverses behavioral adaptive changes induced by repeated cocaine in mice. Biol Psychiatry 2010; 68:223–230Crossref, Medline, Google Scholar
10 : The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology 2008; 54:564–568Crossref, Medline, Google Scholar
11 : Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002; 164:168–176Crossref, Medline, Google Scholar
12 : Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001; 154:1–7Crossref, Medline, Google Scholar
13 : Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur J Pharmacol 2013; 699:200–206Crossref, Medline, Google Scholar
14 : Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 1996; 75:1–4Crossref, Medline, Google Scholar
15 : Chronic cocaine produces decreases in N/OFQ peptide levels in select rat brain regions. J Mol Neurosci 2007; 31:159–164Medline, Google Scholar
16 : Dynorphin/KOP and nociceptin/NOP gene expression and epigenetic changes by cocaine in rat striatum and nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry 2014; 49:36–46Crossref, Medline, Google Scholar
17 : Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J Med Chem 2011; 54:2687–2700Crossref, Medline, Google Scholar
18 : Occupancy of nociceptin/orphanin FQ peptide receptors by the antagonist LY2940094 in rats and healthy human subjects. Drug Metab Dispos 2016; 44:1536–1542Crossref, Medline, Google Scholar
19 : Nociceptin receptors in alcohol use disorders: a positron emission tomography study using [11C]NOP-1A. Biol Psychiatry 2018; 84:708–714Crossref, Medline, Google Scholar
20 : Structured Clinical Interview for DSM-5–Research Version. Washington, DC, American Psychiatric Association, 2015Google Scholar
21 : Retest imaging of [11C]NOP-1A binding to nociceptin/orphanin FQ peptide (NOP) receptors in the brain of healthy humans. Neuroimage 2014; 87:89–95Crossref, Medline, Google Scholar
22 : Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J Nucl Med 2012; 53:385–392Crossref, Medline, Google Scholar
23 : Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med 2011; 52:1638–1645Crossref, Medline, Google Scholar
24 : Nociceptin receptors in alcohol use disorders: a positron emission tomography study using [11C]NOP-1A. Biol Psychiatry 2018; 84:708–714Crossref, Medline, Google Scholar
25 : Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage 2006; 32:1562–1575Crossref, Medline, Google Scholar
26 : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289Crossref, Medline, Google Scholar
27 : Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging 1998; 17:463–468Crossref, Medline, Google Scholar
28 : Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27:1533–1539Crossref, Medline, Google Scholar
29 : Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 2011; 168:634–641Link, Google Scholar
30 : Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000; 68:64–72Crossref, Medline, Google Scholar
31 : Multimodal predictive modeling of individual treatment outcome in cocaine dependence with combined neuroimaging and behavioral predictors. Drug Alcohol Depend 2014; 143:29–35Crossref, Medline, Google Scholar
32 : Amphetamine-induced dopamine release is markedly blunted in cocaine dependent subjects and predictive of the choice to self administer cocaine. Am J Psychiatry 2007; 164:622–629Link, Google Scholar
33 : Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci 2014; 34:363–372Crossref, Medline, Google Scholar
34 : Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides 2009; 43:507–514Crossref, Medline, Google Scholar
35 : Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav Brain Res 2007; 184:167–173Crossref, Medline, Google Scholar
36 : Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder. Br J Pharmacol 2015; 172:571–582Crossref, Medline, Google Scholar
37 : Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl) 2008; 196:523–531Crossref, Medline, Google Scholar
38 : Nociceptin/Orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev 2016; 68:419–457Crossref, Medline, Google Scholar
39 : Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides 2000; 21:1023–1029Crossref, Medline, Google Scholar
40 : Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport 2002; 13:1137–1140Crossref, Medline, Google Scholar
41 : Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol 2002; 444:358–368Crossref, Medline, Google Scholar
42 : Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology 2013Medline, Google Scholar
43 : Nocistatin reverses nociceptin inhibition of glutamate release from rat brain slices. Eur J Pharmacol 1998; 356:R1–R3Crossref, Medline, Google Scholar
44 : Nociceptin induced inhibition of K+ evoked glutamate release from rat cerebrocortical slices. Br J Pharmacol 1996; 119:1081–1083Crossref, Medline, Google Scholar
45 : The good and bad news about glutamate in drug addiction. J Psychopharmacol 2016; 30:1095–1098Crossref, Medline, Google Scholar
46 : PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am J Nucl Med Mol Imaging 2016; 6:205–214Medline, Google Scholar
47 : Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab Clin North Am 2013; 42:201–225Crossref, Medline, Google Scholar
48 : Sex dfferences in animal models: focus on addiction. Pharmacol Rev 2016; 68:242–263Crossref, Medline, Google Scholar



