Prenatal Primary Prevention of Mental Illness by Micronutrient Supplements in Pregnancy
Abstract
Genes, infection, malnutrition, and other factors affecting fetal brain development are a major component of risk for a child’s emotional development and later mental illnesses, including schizophrenia, bipolar disorder, and autism. Prenatal interventions to ameliorate that risk have yet to be established for clinical use. A systematic review of prenatal nutrients and childhood emotional development and later mental illness was performed. Randomized trials of folic acid, phosphatidylcholine, and omega-3 fatty acid supplements assess effects of doses beyond those adequate to remedy deficiencies to promote normal fetal development despite genetic and environmental risks. Folic acid to prevent neural tube defects is an example. Vitamins A and D are currently recommended at maximum levels, but women’s incomplete compliance permits observational studies of their effects. Folic acid and phosphatidylcholine supplements have shown evidence for improving childhood emotional development associated with later mental illnesses. Vitamins A and D decreased the risk for schizophrenia and autism in retrospective observations. Omega-3 fatty acid supplementation during early pregnancy increased the risk for schizophrenia and increased symptoms of attention deficit hyperactivity disorder, but in later pregnancy it decreased childhood wheezing and premature birth. Studies are complicated by the length of time between birth and the emergence of mental illnesses like schizophrenia, compared with anomalies like facial clefts identified at birth. As part of comprehensive maternal and fetal care, prenatal nutrient interventions should be further considered as uniquely effective first steps in decreasing risk for future psychiatric and other illnesses in newborn children.
[AJP at 175: Remembering Our Past As We Envision Our Future
July 1959: Longitudinal Observations of Biological Deviations in a Schizophrenic Infant
Barbara Fish described the course of an infant born with fluctuating motor problems who developed schizophrenia. (Am J Psychiatry 1959; 116:25–31)]
Fetal brain development, the combined effect of the genotype and the environment in the womb, influenced by the mother’s nutrition, infection, psychiatric status, and substance abuse, is the earliest developmental step in risk for mental illness. The odds ratio for the offspring developing schizophrenia after severe maternal malnutrition is 2.7 (1). The odds ratio for the offspring developing schizophrenia after maternal infection ranges from 2.1 for common respiratory infections to 5.0 for genital-reproductive infections (2). There is a substantial genetic component as well. Children with family histories of schizophrenia have increased risk for later mental illnesses (odds ratio=4.2) (3). Many genes associated with mental illnesses are expressed in the fetus before birth, some at considerably higher levels than in adult life, consistent with their role in fetal brain development (4, 5). Maternal depression, anxiety and stress, smoking, and alcohol abuse are other risk factors for subsequent mental illness in the child (6–9). No single determinant inevitably causes mental illness, and frequently several factors each with small effect size combine, such as infection and genetic risk (10). The resulting abnormalities in fetal brain development have been demonstrated as the first sign of risk for future illness by retrospective studies of abnormalities in newborns who later develop mental illness in adulthood (11).
The unique period of fetal brain development has not received much attention in clinical practice as a time for specific interventions, beyond good prenatal care, to ameliorate future risk for mental illness. Maternal psychiatric treatments are directed to the pregnant woman’s psychopathology, and the possibility that they might also affect the fetus positively is less frequently considered, despite evidence that maternal depression increases the risk for the child’s development of mental illness. The initial concern has been that antidepressants’ toxic effects might include increased risk for mental illness. For example, maternal antidepressants were identified as a risk factor for autism, a finding later challenged by studies controlling for the maternal psychopathology that prompted antidepressant prescription (12, 13). Good nutrition is an obvious recommendation, but the possibility that specific micronutrients might be increased to prevent future mental illness is just emerging. This approach has had dramatic effects with folic acid supplementation on a wide range of fetal developmental disorders, including spina bifida, microcephaly, and cleft palate (14, 15).
The Developmental Context of Prenatal Prevention
Prospective studies of the development of mental disorders have largely focused on newborns who are deemed to be at high risk either because one of their parents has a mental disorder or because the infant is showing early signs of abnormal development, first characterized in a 1959 American Journal of Psychiatry article by Barbara Fish as fluctuating delays in motor development (16, 17). Fish reported that one such child whom she identified in infancy as high risk later developed schizophrenia. Elaine Walker was among the first to observe early developmental signs in retrospective studies of home movies of infants who developed schizophrenia as adults. She proposed that defects in early motor movement, such as asymmetrical limb movements during crawling, and in the early expression of social features like smiling, were manifestations of problems in fetal brain development that later led to mental illness (11, 18).
Regardless of risk, no baby is born with schizophrenia or bipolar disorder or autism spectrum disorder (ASD). The developmental expression of risks whose neurobiology occurred earlier (e.g., in fetal brain development) interacts with additional risk factors throughout childhood and adolescence, both genetic and environmental, that contribute to the final expression of illness in adulthood (4, 19). Poor attachment to the mother is a cause as well as an early sign of later psychopathology (20). Other signs of future psychopathology emerge during subsequent development. For schizophrenia, early attentional and social deficits have been observed retrospectively in children as young as 3–4 years of age (21, 22). These early childhood symptoms, while not inevitably predictive of later illness, nonetheless indicate the developmental track of an individual toward possible illness.
Possible Strategies for Prenatal Prevention and their Investigation
The strategies that led to the adoption of folic acid supplementation as a universal primary prevention for midline developmental defects are instructive. Observational studies of children born with these defects identified possible folic acid deficiency in mothers. Small randomized controlled trials provided proof of efficacy. The British Medical Research Council then funded large multisite trials (14, 16). Results showed that the folic acid supplement plus multivitamins robustly reduced the incidence of cleft palate, spina bifida, and some forms of microcephaly, with little difference for whether the woman’s diet provided adequate amounts. Study of micronutrient interventions for mental illness follows similar lines. Most reports are observational, based on maternal choice of which nutrients to take and when to take them and on prenatal serum levels. The only prenatal nutrient other than folic acid to reach the stage of large multisite randomized trials with longer-term follow-up is omega-3 fatty acids, with trials for prevention of childhood wheezing and the development of cognition (23, 24). Prospective trials assess the development of emotional and behavioral problems and cognitive deficits associated with future mental illness as their outcome. Effects on incidence of mental illness itself, notably schizophrenia, have only been ascertained retrospectively with banked sera from several decades earlier because of the long interval between birth and appearance of illness in early adulthood.
The experience with folic acid is that an effective intervention ameliorates several different developmental abnormalities. The overlapping genetic and environmental risk factors for major mental illnesses suggest that a similar broad range of outcomes may occur with the nutrients discussed here, including illnesses with common features such as the affective and schizophrenia spectrum psychoses and autism, as well as behavioral and cognitive abnormalities that do not reach criterion for an illness.
Method
A MEDLINE search for the keyword “micronutrients” combined with “pregnancy” or “fetal development” from 1990 through 2017 was conducted for human studies. Seventy-nine articles were identified, and their references were used to identify 34 additional articles. Thirty-five human studies and trials of individual nutrients were identified with reports of their results on subsequent child behavior, emotion, or cognition in one or more articles. We reviewed the resultant total of 45 articles, and an additional nine were selected as representative of somatic outcomes. Four supplements were identified: folic acid, fish oil omega-3 fatty acids, phosphatidylcholine, and vitamins D and A (Figure 1, Table 1). Preferred studies for discussion were retrospective epidemiological studies of disease prevalence, randomized prospective clinical trials, and large prospective observational studies. The metric used in each study, odds ratio, effect size (d′), or regression coefficient (β), is cited if it was statistically significant. Although animal model studies were not the focus of this review, a summary of translational and mechanistic studies is included to describe possible mechanisms responsible for the nutrients’ effects (Table 2; see also the data supplement that accompanies the online edition of this review).
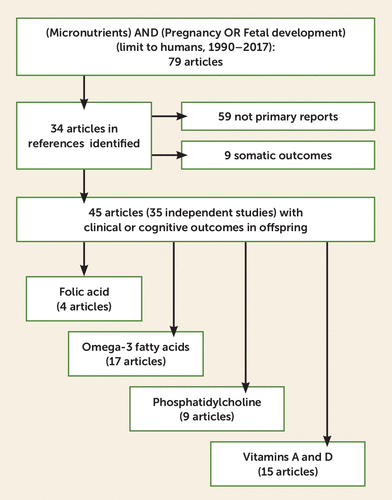
FIGURE 1. Selection of Articles for a Review of Prenatal Micronutrient Supplementation for the Prevention of Mental Illness
| Type of Trial | Subjects | Region or Country | Dose or Level and First Timing During Gestation | Effect | Limitations |
|---|---|---|---|---|---|
| Folic acid | |||||
| Prospective, randomized (25) | 311 women | Europe | 400 μg of 5-methyltetrahydrofolate compared with placebo at 20 weeks | Decreased reaction time with distractor at 8.5 years (d′=0.32) | 130 of 311 children studied; no clinical ratings |
| Prospective, observational (26, 27) | 3,210 women | Netherlands | ≥400 μg of folic acid within first 10 weeks’ gestation compared with no or later use | CBCL problems at 18 and 36 months; top 17th percentile (odds ratio=1.45, 95% CI=1.14–1.84) | Groups differ in sociodemographics; folic acid neonates 200 g heavier |
| Prospective, observational (14) | 573 infants with facial clefts, 763 controls | Norway | ≥400 μg of folic acid within 9 weeks | Decreased facial clefts (odds ratio=0.61, 95% CI=0.39–0.96) | Not effective in cleft palate without cleft lip |
| Prospective, randomized (15) | 1,817 women, prior neural tube defect | U.K., Europe, Israel, Australia, Canada, U.S.S.R. | 4 mg of folic acid at preconception until 12 weeks | Decreased neural tube defects (odds ratio=0.28, 95% CI=0.12–0.71) | No follow-up of other developmental traits |
| Retrospective, observational (28) | 104,428 women | United States | 400 μg of folic acid at first trimester | Increased asthma at 4.5–6 years (odds ratio=1.2, 95% CI=1.1–1.3) | Data only from Medicaid claims |
| Phosphatidylcholine | |||||
| Prospective, randomized (29, 30) | 100 women | United States | 6,300 mg of phosphatidylcholine at 15 weeks | Increased 1-month EEG sensory gating; decreased 3.5-year CBCL attention (d′=0.59) and social (d′=0.79) problems | 50% attrition; parental CBCL reports only |
| Prospective, observational (31) | 154 women | Canada | Plasma choline at 16 weeks’ gestation | Increased Bayley cognition score at 18 months (β=6.054, SE=2.283) | Healthy women in uncontrolled study |
| Prospective, randomized (32) | 24 women | Canada | 550 mg compared with 100 mg of choline supplements in third trimester | Lower placental sFLT1, angiogenic factor in preeclampsia (d′=0.2) | Small study with no clinical outcome |
| Prospective, observational (33) | 817 adults 33–55 years old | United States | Plasma trimethyl amine oxide, a choline bacterial metabolite | No increase in cardiac disease (odds ratio=1.03, 95% CI=0.71–1.52) | Patients were not supplemented |
| Omega-3 fatty acids | |||||
| Retrospective, observational (34) | 57 adult cases and 95 controls | United States | DHA acid >1.45% of fatty acids in second or third trimester | Increased schizophrenia spectrum (odds ratio=2.38, 95% CI=1.19–4.76) | Mercury from high fish diet not tested |
| Prospective, randomized (24) | 543 women | Australia | 800 mg of fish oil at 20 weeks | Increased 7-year Conners ADHD score (d′=0.1) | Small effect in large sample |
| Prospective, randomized (23) | 736 women and children | Denmark | 2.4 g of fish oil at 24–26 weeks | Decreased wheezing at 3–5 years (odds ratio=0.69, 95% CI=0.49–0.97) | Trend suggests vitamin D equally effective |
| Prospective, randomized (35) | 2,399 women | Australia | 800 mg of fish oil at 20 weeks | Fewer gestations at <34 weeks (odds ratio=0.49, 95% CI=0.25–0.94) | More DHA induction (odds ratio=1.28, 95% CI=1.06–1.54) |
| Vitamins A and D | |||||
| Retrospective, observational (36) | 430 adult cases, 430 controls | Denmark | 25(OH)D3 <19.7 nM compared with >51 nM, low versus high quintiles | Increased schizophrenia (odds ratio=2.1, 95% CI=1.3–3.5) | Confounded with summer birth in high quintile |
| Retrospective, observational (37) | 51 male adult cases, 4,616 in cohort | Finland | 2,000 IU of vitamin D in first year of life | Decreased schizophrenia (odds ratio=0.23, 95% CI=0.06–0.95) | Effects only in males |
| Prospective, observational (38) | 68 cases in birth cohort of 4,334 | Netherlands | <25 nmol/L of vitamin D at 20 weeks | Increased autism spectrum at 6–9 years (odds ratio=2.42, 95% CI=1.09–5.07) | Small number of cases |
| Retrospective, observational (39) | 186 cases in cohort of 1,194 | New Zealand | Vitamin D intake >724 IU (highest quartile) | Decreased recurrent wheeze at 3 years (odds ratio=0.39, 95% CI=0.25–0.62) | Infection-related wheeze, not new incident asthma |
| Retrospective, observational (40) | 55 cases, 106 controls | United States | <30 μg/dL of vitamin A at second trimester | Increased schizophrenia (odds ratio=3.05, 95% CI=1.06–8.79) | Higher levels in more educated women |
TABLE 1. Major Studies of Prenatal Dietary Supplements to Enhance Fetal Developmenta
| Supplement | Effect | Model Mechanism |
|---|---|---|
| Folic acid | Increased neuronal development | Deficiency lowers Stat3, allowing increased neurite outgrowth (41) |
| Imprinting of maternal genes | Biochemical role in one-carbon metabolism and methylation of DNA (42) | |
| Phosphatidylcholine | Development of inhibitory neurotransmission | Activation of α7-nicotinic receptors induces the chloride transporter NKCC1, establishing a chloride gradient for GABA inhibition (43); effects blocked in CHRNA7 null mice (43, 44) |
| Omega-3 fatty acids | Antiseizure effect in ketogenic diet but could also affect synapse formation in development | Accelerates inactivation and retards recovery of sodium and calcium channels in vitro, which decreases neuronal excitation (45) |
| Reduces preterm birth | Reduces prostaglandin E2 and F2α in uterine decidual cells (46) | |
| Vitamin D | Neuronal development | Interacts with Nurr1 to support development of dopamine neurons (47); activates low voltage (l-type) calcium channels to increase neurofilament phosphorylation (48) |
TABLE 2. Mechanistic Studies of Prenatal Interventions
Results
Folic Acid and the Baby’s Risk for Mental Illness
Prospective assessment of the behavioral development of infants whose mothers took folic acid supplements in the Rotterdam Generation R study relied on the incomplete compliance of women with folic acid supplementation for cleft palate and spina bifida (23). Mothers were grouped into those who began folic acid within 10 weeks of conception, those who began after 10 weeks, and those who did not use supplements at all. The Child Behavior Checklist (CBCL) for ages 1.5–5 years, a standard instrument for parental report of infant behavior, was administered at 18 months and 3 years after birth (23, 24). The CBCL contains 99 items from which various axes are derived. This study reported two axes: emotional problems (emotionally reactive, anxious or depressed, somatic complaints, and social withdrawal) and behavioral problems (attention and aggression). The highest 17% of scores were considered to be significantly elevated because previous studies showed that children referred to treatment by parents and teachers are more likely to have scores at or beyond this level (49). At 18 months, children whose mothers did not take folic acid before 10 weeks’ gestation more commonly had elevated problems as measured by the CBCL, specifically in social withdrawal, attention, and aggression (odds ratio=1.4, 95% CI=1.1–1.9). At 3 years of age, the children of mothers who did not take folic acid before 10 weeks’ gestation continued to have emotional problems (odds ratio=1.45, 95% CI=1.14–1.84). Maternal folic acid plasma levels <7 nM at 13 weeks’ gestation similarly predicted emotional problems (odds ratio=1.57, 95% CI=1.03–2.38). At 6 years of age, the children were assessed for autistic traits, defined as the highest 3% on the Pervasive Development Problems subscale of the CBCL and the highest 5% on the Social Responsive Scale. Folic acid supplementation at any time during pregnancy was associated with these higher scores on the two scales in a logistic regression analysis (β=−0.042, 95% CI=−0.068 to −0.017) (50).
All studies of infant outcomes from pregnancy have many confounding effects, especially in observational studies like the Generation R study, as opposed to trials with randomized treatment. Infants with folic acid deficiency weighed 200 g less. Mothers were younger, less educated, less likely to have planned their pregnancy, and less likely to be married, all of which may explain their failure to begin folic acid supplements before 10 weeks’ gestation. The women were also more likely to be non-Western immigrants. They were more likely to smoke but less likely to have more than one alcoholic drink per week. Only 4% of the women who did not take folic acid and 28% of the women who took folic acid also took multivitamins. The mother’s initiative to take folic acid meant that the timing of supplementation was confounded by these sociodemographic differences, but it also provided for groups of women who began the supplementation at different times in gestation. Differences between the women might also confound the assessment of outcome, which is based on maternal reports of children’s behavior.
A trial involving 311 women randomized the women at 20 weeks’ gestation to four groups: one group received 400 μg of methyltetrahydrofolate, the biologically active derivative of folic acid; one group received the folate with fish oil containing 650 mg of omega-3 fatty acids; one group received fish oil alone; and one group received placebos (25). The children of women who received only methyltetrahydrofolate had decreased time in the presence of a distractor at 8.5 years of age, compared with children in the other groups (d′=0.32). This trait is associated with schizophrenia and other mental illnesses, but only 130 children were studied, and no other laboratory or clinical ratings were performed.
Folic acid supplementation is generally recommended at 400–800 μg preconception, or at any point in early gestation that the pregnant woman can start, as a standard part of prenatal regimens worldwide because of its striking effect of decreasing facial clefts (odds ratio=0.61, 95% CI=0.12–0.71) (14). Supplements of up to 4 mg before 12 weeks’ gestation have been found to be safe and effective in preventing open neural tube defects in women at high risk because of a previous child with a defect (odds ratio=0.28, 95% CI=0.12–0.71) (15). No follow-up of emotional or behavioral outcomes in childhood was performed on these children. The only adverse effect reported from standard folic acid supplementation is increased childhood asthma at 4.5–6 years (odds ratio=1.2, 95% CI=1.1–1.3) (28).
There are no retrospective studies showing that higher maternal folate levels are associated with a decreased incidence of schizophrenia. The large margin of safety of folic acid would support randomized testing of doses of up to at least 4 mg compared with the standard 400–800 μg.
Omega-3 Fatty Acids (Fish Oil)
The omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid were studied in pregnancy following initial reports of higher levels of cognitive function in children whose mothers followed the Mediterranean diet during pregnancy (51). The Mediterranean diet differs from Western diets in many aspects, but fish oil and, specifically, omega-3 fatty acids are important differences. However, mixed effects of higher maternal omega-3 fatty acids have been found. For example, an observational study found that a lower ratio of omega-3 to omega-6 in the mother during pregnancy was associated with more autistic traits in the offspring, but the effect was primarily driven by higher levels of omega-6 as opposed to deficient levels of omega-3 (52).
Omega-3 fatty acid supplementation has had the longest double-blind clinical trial follow-up of any of the prospective interventions. The results have been disappointing. At 20 weeks’ gestation, 543 women were randomized to 800 mg of fish oil or to placebo. The 7-year follow-up, through parental reports, showed a small but significant increase with fish oil in behavioral problems in attention deficit hyperactivity disorder (ADHD) (d′=0.1), consistent with results that had been observed at 4 years of age (25, 28, 50). A retrospective observational study in a Kaiser Permanente group found that mothers in the highest tertile of levels of the omega-3 fatty acid DHA had children with a twofold elevation of adult schizophrenia spectrum disorders (odds ratio=2.38, 95% CI=1.19–4.76) (52). Mothers in the lowest tertile did not have elevated risk.
A number of trials of DHA supplementation in pregnancy have shown positive effect on attention and neurological signs in the first 5 years of life (53–57), but other trials have not shown differences (24, 35, 58, 59). The positive effects of fish oil in one observational trial disappeared when the socioeconomic differences between the mothers were considered (60). Another study found that the effect of fish was compromised by the mercury content (61). A meta-analysis of 11 studies concluded that the positive effects were biased by two of the trials (62). DHA supplementation postpartum is particularly effective for preterm female infants with birth weight less than 1,250 g. The supplement, six 500 mg capsules of DHA containing tuna oil, or the equivalent in formula, was given to the lactating mother in a randomized controlled trial. At 18 months of age, corrected for gestational age at birth, there was a significant improvement in the cognitive development of the infants who received supplements. Full-term and higher birth weight preterm infants did not show a significant effect (63, 64).
Other benefits from fish oil supplementation have been more consistently observed. The same randomized trial that later found increased ADHD symptoms at 7 years of age had previously found a decreased incidence of premature gestation at <34 weeks with fish oil supplementation (odds ratio=0.49, 95% CI=0.25–0.94), fewer babies with birth weight less than 2,500 g, and less admission of babies for neonatal intensive care (35). A meta-analysis of 21 controlled studies found a 5.8-day increase in gestational age of the newborn, a 22% reduced risk for early preterm delivery, higher infantile birth weight (51.23 g), and a 23% lower risk of low birth weight (65). Although the cognitive and behavioral findings in trials of omega-3 fatty acid do not consistently support the benefit of these improvements in birth outcomes, they are associated with enhanced fetal development, including brain development. For example, infants who are born prematurely have an increased odds ratio (2.6) of developing ADHD (66).
A placebo-controlled trial found that 2.4 g of fish oil supplementation, beginning at 24–26 weeks’ gestation, had a striking effect on the incidence of childhood wheezing at age 3–4 years (odds ratio=0.69, 95% CI=0.49–0.97) (21). The trial indicated a decreased effectiveness in women with higher maternal vitamin D levels, which the investigators interpreted as caused by the two interventions targeting the same mechanism. There was no assessment of behavioral symptoms in the children.
An assessment of how omega-3 fatty acids can be administered to protect against premature birth and infant wheezing, while minimizing the risk for mental illness, would seem to be a next step. Translational studies have identified a putative mechanism of the beneficial effect on premature birth, but the mechanisms of effects on brain function are far from established (see the online data supplement). Increased understanding of the mechanism of effects on childhood behavior and risk for mental illness could guide clinical strategies to retain the considerable clinical benefit of omega-3 fatty acids and diminish any risk for future mental illness.
Phosphatidylcholine
Choline supplementation is only now being recommended as a standard prenatal regimen (67). Phosphatidylcholine is the preferred dietary supplement because it is impervious to most colonic bacteria. It is converted in the blood to choline (68). Dietary choline itself is metabolized by colonic bacteria to trimethylurea, which imparts a fishy odor, or trimethylamineoxide, which is atherogenic (33). These metabolites are not increased by phosphatidylcholine supplements (68). A survey showed that fewer than half of pregnant women reach an adequate level through normal diet, the equivalent of 450 mg of choline daily (69). The dietary recommendation of 450 mg by the Institute of Medicine of the National Academy of Sciences was based on the level in adults that prevents increase in liver transaminase, an early indication of choline deficiency. For pregnant women, the amount was increased based on the observation that levels are elevated 10-fold in the placenta, amniotic fluid, and in the fetus itself (42). A case report noted very low levels of choline in a mother with bipolar disorder treated with lithium, but this finding has not been replicated (70). The Institute of Medicine recommends an upper limit of phosphatidylcholine of 24,000 mg/day for pregnant women older than 18 years, equivalent to 3,500 mg of choline, which indicates a large margin of safety for supplements (42). The upper limit is half the amount observed to cause hypotension and diarrhea.
An observational study measured plasma choline at 16 weeks’ gestation and found significant correlation with infant cognitive scores at 18 months of age as measured on the Bayley Scales of Infant Development (β=6.054, SE=2.283), with no effect on folic acid or vitamin B12 levels (31).
The first double-blind controlled trial administered 5,000 mg of phosphatidylcholine containing 750 mg of choline, a 200% increase over the 360 mg estimated from diet (71). The maternal supplement was begun by 18 weeks of gestation and continued through the first month of lactation. Plasma choline levels were twice the mean for the placebo-treated women. The trial found no effect on cognition as measured at 1 year of age by the infant’s ability to find an object concealed by the investigator after different delays, up to 24 hours later. The strength of the trial is the complete measurement of choline and its metabolites in the mother’s plasma. A weakness is that the primary cognitive task undergoes linear improvement from 6 to 24 months, which means that infants’ performance was still developing at a substantial rate at the time of testing (72). Forty-six percent of infants could not complete the testing protocol, in addition to 39% who dropped out of the study before assessment.
In a second double-blind clinical trial, which is from our group, 50 women took 6,300 mg of phosphatidylcholine daily from week 17 of gestation though delivery, a 250% increase in choline levels over their normal diet. After birth, the newborns received 100 mg of liquid phosphatidylcholine from 2 weeks postbirth until 3 months of age. Fifty women and their newborns were given placebos. Because of the newborns’ limited behavioral repertoire, the principal outcome measure was P50 auditory evoked potential inhibitory sensory gating at 1 month of age. This measure was previously shown to be abnormal in infants whose parents had psychosis or whose mothers smoked or were depressed, all risk factors associated with later schizophrenia in the offspring (73). Abnormality was defined as inhibition below the 95th percentile for subjects who had no known mental illness (74). At 1 month of age, the group who received phosphatidylcholine supplementation had significantly fewer infants with abnormal P50 inhibitory sensory gating (d′=0.7) (29).
The same cohort was reexamined at 3.5 years of age with the CBCL. Children from phosphatidylcholine-supplemented pregnancies, now on regular diets, had significantly fewer problems noted in attention and social interaction (30). This effect was related to their P50 sensory gating at 1 month of age. The magnitude of improvement in CBCL ratings at 3.5 years for the phosphatidylcholine-supplemented children, compared with a control group of children who received placebo (for attention problems, d′=0.59; for social problems, d′=0.79), was similar to the magnitude of decline found in a retrospective study of adults with schizophrenia compared with a control group, whose parents had rated them on the CBCL based on their recall of their child at 3–4 years of age (21). The intervention was found to be equally effective for infants who have a mother with schizophrenia, as well as for mothers who experienced an infection during pregnancy (30, 75). The findings were unchanged when some infants were reassessed at age 4, but none of the subjects have been seen later in childhood or adolescence to determine if they eventually became clinically ill. However, 23% of males and 15% of females in the placebo group had CBCL ratings of attention or social problems in the range of children who are referred by parents or schools for clinical intervention, compared with 7% of males and 11% of females in the phosphatidylcholine-treated group (49). Strengths of this study are the assessment of emotional behavior and its relation to an early neurobiological marker and a CHRNA7 genotypic effect in the infants. Effects of phosphatidylcholine supplementation on both electrophysiology and behavior, compared with placebo, were more marked in children who have a CHRNA7 promoter variant associated with schizophrenia. Weaknesses are the study’s small size and the 50% attrition of the groups by 4 years of age as mothers were lost to contact.
A randomized clinical trial of third-trimester choline supplementation (550 mg compared with 100 mg of choline chloride added to a controlled diet containing 480 mg) found increased placental methylation of the genes for corticotropin-releasing hormone and the glucocorticoid receptor (NR3C1), which moderate the cortisol reaction that can damage the placenta and fetus (7, 76). Cord blood cortisol was reduced by 30% in the newborns of mothers who received the higher dose of choline. A smaller randomized trial of the same levels of supplementation found lower placental sFLT1, an angiogenic factor associated with preeclampsia (d′=0.2) (32). None of the studies of phosphatidylcholine in the literature found significant adverse effects for mother or infant.
Most trials find that phosphatidylcholine supplements protect against maternal risk factors associated with schizophrenia and other mental conditions in the offspring and decrease the development of traits associated with later mental illness in adulthood, but as for folic acid, there are no retrospective studies showing that maternal choline lowers the incidence of schizophrenia itself.
Vitamins D and A
Vitamins D3 and A1 were initially assessed in retrospective observation of banked serum (40, 77). Part of the motivation for investigating these vitamins was the well-known effect of season of birth on risk for schizophrenia. In the largest study of vitamin D levels and schizophrenia, neonatal blood samples from 430 Danish case-control pairs were studied (36). The levels of 25(OH)D3, the circulating form of vitamin D that is produced by liver metabolism, were divided into quintiles based on levels in the control subjects. Neonates in the fourth quintile (40.5–50.9 nmol/L) were the least likely to develop schizophrenia. Those above and below this level were more likely to do so (odds ratio=2.1, 95% CI=1.3–3.5). Forty-four percent of the sample was in the fourth quintile, mostly individuals born from June through September, because of the effect of sunlight on vitamin D levels. For infants to be born in other seasons, the dose of vitamin D supplementation that might be recommended is unknown, beyond standard prenatal multivitamin preparations. In a separate observational postnatal cohort from Finland, male infants who were given 2,000 IU daily of vitamin D in the first year of life had less than 25% of the risk for schizophrenia compared with the rate found for those who did not receive such supplementation (odds ratio=0.23, 95% CI=0.06–0.95). There was no difference for females (37).
Vitamin D has subsequently been studied prospectively in observational studies. In the Dutch Generation R study, lower levels of midgestation maternal and cord blood 25(OH)D3 were associated with higher scores on the Social Responsiveness Scale used to measure autism-related traits (78). In this cohort, ASD itself was more likely to occur in individuals whose mothers had midgestational vitamin D deficiency (odds ratio=2.42, 95% CI=1.09–5.07) (38).
As in all observational studies, whether prospective or retrospective, the fundamental causative factor cannot be easily isolated from possible confounding effects. For the Danish study, the infants with lower vitamin D levels were more likely to be children of immigrants. The effect from season of birth could be ascribed to higher rates of maternal infection, with gestation occurring in the winter cold and flu season. However, the investigators point out that higher maternal vitamin D levels are associated with lower risk for maternal infection. The vitamin D effect on mental illness has been replicated in observational studies worldwide (39, 79–85). Vitamin D has also been found to reduce infant wheezing related to infection (odds ratio=0.39, 95% CI=0.25–0.62) and to reduce the risk of preterm birth <37 weeks (86, 87).
Vitamin A levels during the second trimester were assessed in 12,000 pregnant women enrolled in the Kaiser Permanente health plan. The 55 offspring who developed schizophrenia spectrum disorders were compared with control subjects. Maternal plasma levels below 30 μg/dL, the level generally considered as deficient, were associated with increased risk of schizophrenia in the offspring (odds ratio=3.05, 95% CI=1.06–8.79) (77).
Unlike the other nutrients, vitamins A and D have upper limits defined by their potential toxicity. Therefore, supplementation should not be greater than the amount in standard multivitamins. The daily recommendation in the United States for vitamin D is 600 IU for pregnant women and 400 IU for infants. Upper tolerable limits are 4,000 IU for pregnant women and 1,000 IU for infants. However, these recommendations assume adequate calcium intake and exposure to sunlight and may differ, particularly in winter seasons in countries with limited sunlight (88). An association with teratogenicity has limited the recommendation in the United States for vitamin A in pregnancy to 8,000 units from diet and supplements combined (89). Most supplements contain 2,500 units.
Discussion
This review identified studies of four types of prenatal maternal dietary interventions to promote fetal brain development and decrease subsequent risk for mental illness. The key findings are summarized in Table 3. Vitamins A and D and folic acid are already in common clinical use. Higher serum levels of vitamins A and D appear to promote brain development and to decrease risk for schizophrenia, but their potential toxicity limits their use to currently recommended amounts. Folic acid is also currently in prenatal vitamins but at levels far below those shown to be safe and effective for neural tube defects. Folic acid has benefits for the development of the fetal brain and subsequent child behavior and cognition, but it has not been shown specifically to prevent schizophrenia. Omega-3 fatty acids increase the risk for later schizophrenia and modestly increase childhood ADHD symptoms, but they also substantively decrease the risk of both premature birth and childhood wheezing. Phosphatidylcholine supplements have been more recently studied prospectively and have generally been found to promote the development of the fetal brain and subsequent childhood behavior, but no retrospective epidemiological studies have been performed.
| Intervention | Current Recommended Daily Allowance | Study Dose, Timing | Major Developmental Finding | Other Conditions | Known Adverse Effects |
|---|---|---|---|---|---|
| Folic acid | 400–800 μgb plus 200 μg from diet | 400 μg at preconception to 10 weeks’ gestation (27) | Decreased emotional problems (child) | Decrease in cleft lip, spina bifida | Infant wheezing increased |
| Phosphatidylcholine | 3,150 mg (450 mg of choline) from diet | 6,300 mg at preconception to 16 weeks’ gestation (30) | Decreased social and attention problems (child) | Possible decrease in preeclampsia | Trimethylamine N-oxide cardiotoxicity (in men with cardiac disease) |
| Omega-3 fatty acids (fish oil) | Up to 300 g of low mercury–containing fish per week | 2.4 g of fish oil daily at 24–26 weeks’ gestation for wheeze (23) | Increased risk for schizophrenia spectrum (adult) | Premature birth and infant wheezing decreased | Increased ADHD symptoms at 7 years |
| Vitamins A and D | Supplement of 2,567 IUb of vitamin A and 600 IUb of vitamin D, plus amounts from diet and sunlight | 1,000 IU of vitamin D3 for infants up to 1 year of age, 2,000 IU in northern countries (37) | Decreased schizophrenia (adult) with both vitamins A and D | Infant wheezing decreased | Teratogenicity with vitamin A intake >8,000 IU (diet plus supplement) |
TABLE 3. Summary of Findings for Nutrients With Possible Prenatal Preventive Effecta
Optimal dosages and timing should consider not only the fetal brain effect but the effect on other systems as well. For example, higher omega-3 fatty acid levels before 20 weeks’ gestation were found to increase the risk of schizophrenia, but administration after 24 weeks’ gestation decreased the risk of childhood wheezing (23, 34). Vitamin D effects on premature birth are related to administration in the third trimester, and effects on risk for schizophrenia have been observed with postnatal administration (37, 87). Phosphatidylcholine is probably best given preconception because levels in the first trimester are much lower than in the second trimester or at delivery (90). Dose can be affected by genotype. African people who live in countries with diets low in phosphatidylcholine nonetheless have normal choline levels in pregnancy, perhaps because they do not have variants in PEMT, the gene for the enzyme phosphatidyl-ethanolamine methyl transferase that regulates choline levels, whereas in Chinese people, who have PEMT variants, these variants are associated with risk for schizophrenia (91, 92). Environmental factors are also important, particularly for vitamins A and D. In Nordic individuals, deficiency is highly related to the low sunlight in winter (36). Nordic women with darker skin require higher levels, if their exposure to sunlight is poor (93). Future clinical use of the nutrients can be designed to consider all these modulators of effectiveness to maximize benefits and minimize any adverse effects.
Interventions can be directed to pregnancies considered at risk or, like folic acid, to the general population. Children of mothers who have schizophrenia are more likely to have abnormal fetal brain development than children of other mothers, but only 10% of ill individuals have children with schizophrenia (94). Thus, the criterion of having an ill parent does not identify who will inevitably become ill. Many women may consider themselves to be at low risk for transmitting mental illness because it is not present in their family, but common, unpredictable maternal infections during pregnancy, including upper respiratory viruses and urinary tract infections, transform the pregnancy to higher risk for the development of the fetus (2).
Public health is an important component of the research agenda. Only 50% of pregnancies are planned, and for some interventions, such as folic acid, preconception use is more effective. Furthermore, fewer than 50% of women currently take folic acid and other vitamins at any time during pregnancy (95). Food and Drug Administration requirements for additives to common foods, like vitamin D to milk, may be helpful, but for folic acid they are not as effective as preconception use of supplements. Dietary sources for phosphatidylcholine are also insufficient (69). Innovation and assessment of better ways to ensure use of evidence-based recommendations for women of childbearing age are needed.
It is unlikely that any single prenatal intervention will prevent mental illnesses in all individuals. The effect size for some of the interventions approaches the effect size for common respiratory infection (odds ratio=2.1), but none approach the effect size that might fully overcome genetic risk (odds ratio=4.2) for a parent or sibling with schizophrenia. However, exposure to respiratory infection during pregnancy is more common than family history of schizophrenia in a close relative. The combinations of nutrients that would further increase effectiveness have not been assessed. Nutrients are only part of good maternal care to promote fetal brain development and to ameliorate risk for mental illness in the offspring. Treatment of maternal psychiatric illnesses during gestation, a significant risk factor for the infant’s later mental illness, is an intervention that psychiatrists, obstetricians, and primary care physicians already provide (see the online data supplement). A model for the public health role of prenatal prevention of mental illness may be cardiac disease. The decline in heart attack deaths occurs not from a single fully effective intervention but from a combination of improved diet and exercise beginning early in childhood; antismoking campaigns in adolescence; statins, antihypertensives, and aspirin in older adults; and then advances in interventions for heart attacks themselves (96).
In the usual course of medical research, interventions are well substantiated before being released for general medical use. Surely no period of life should be better protected than gestation, which mandates even a higher standard of evidence. There are only 35 studies on which to base conclusions, only five of which are randomized controlled trials. Only three ongoing studies were found in registries, and their full results will not be known until the offspring grow to ages when effects can be measured (Table 4). There are no U.S. government–regulated standards for prenatal vitamins, but various bodies are now making specific recommendations that may be helpful in guiding families and their doctor. The American Medical Association (AMA) in June 2017, in response to an initiative from the National Medical Association, advocated for a change in the manufacture of prenatal vitamins to incorporate increased levels of choline (67). AMA delegates observed that many pregnant women do not achieve even the minimum dietary amount previously recommended by the National Academy of Medicine (450 mg). The AMA’s deliberation also noted the positive effects observed in the clinical trial using the equivalent of 900 mg reviewed in the present work (97). The resulting resolution advises that an “evidence-based” amount be included in the prenatal supplement.
| Principal Investigator, Country | Title | Type of Trial, Dose, Timing | Principal Outcome | Registry and Date |
|---|---|---|---|---|
| C. Grant, Australia | Randomized placebo-controlled study of vitamin D during pregnancy and infancy | Randomized; mothers receive 2,000 IU of vitamin D and infants receive 800 IU, compared with 1,000 IU and 400 IU for each | Infant 25(OH) vitamin D >75 nM at 6 months | Australian New Zealand Clinical Trials Registry, ACTRN12610000483055; 2010 |
| M.C. Hoffman, United States | Choline supplementation during pregnancy: impact on attention and social withdrawal | Randomized; 9 g of phosphatidylcholine at 15 weeks | Child Behavior Checklist rating at 3.5 years | ClinicalTrials.gov, NCT03028857; 2017 |
| J. Zhu, China | Effects of genomic and metabolomic variations of choline on risk of preterm birth and clinical outcomes in preterms | Prospective, observational; effects of plasma choline level during gestation | Preterm birth incidence | ClinicalTrials.gov, NCT02841813; 2016 |
TABLE 4. Prenatal Nutrient Studies Registered as in Progress
To obtain such evidence for any nutrient will require new research agendas that emphasize prenatal clinical trials of interventions, early biomarkers of their effectiveness developed in translational models, and then longer-term follow-up through childhood developmental stages into adulthood. Deficiencies in some nutrients, folic acid and vitamin D, have been found to persist into the first psychotic episode (98). Trial designs based upon randomized clinical trials with relatively short-term follow-up for medication in adults are not applicable because of the unique features of prenatal trials. Randomization to placebo requires consideration that fetal development is a unique stage; later reassignment to an active treatment cannot fully remediate defects in brain development that occur before birth. Preconception initiation of a nutrient often is more effective than initiation during gestation, but informed women who plan their children before conception may not consent to be randomized. Instead, they can choose to initiate the nutrients themselves. Follow-up is lengthy, essentially spanning two generations of patients, mother and child, and thus two generations of the researchers themselves. Intermediate effects on childhood behavior will be valuable findings because waiting decades for final data on illness in adulthood means that the generations born in that interval will not receive any guidance on treatment. Finally, large numbers of subjects are necessary because of the many additional risk factors that impinge between conception and illness. These considerations suggest that such trials may need to be conducted over platforms like social media rather than in traditional biomedical settings.
In the absence of definitive evidence, parents currently planning pregnancy now have difficult decisions about nutrient supplements. The mother is unlikely to receive fully effective levels of the currently studied nutrients from diet alone. Adverse effects of supplements are few at the doses studied, but it would be premature to conclude that they are nonexistent. Conversely, there is only one opportunity in each child’s life for intervention to enhance fetal brain development and protect the child against developmental risks that arise in this period (99).
1 : The Dutch famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol 1998; 33:373–379Crossref, Medline, Google Scholar
2 : Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167:261–280Link, Google Scholar
3 : Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population-based study and meta-analysis. JAMA Psychiatry 2015; 72:635–641Crossref, Medline, Google Scholar
4 : Developmental regulation of human cortex transcription and its clinical relevance at single base resolution. Nat Neurosci 2015; 18:154–161Crossref, Medline, Google Scholar
5 : Prenatal expression patterns of genes associated with neuropsychiatric disorders. Am J Psychiatry 2014; 171:758–767Link, Google Scholar
6 : Schizophrenia in the offspring of antenatally depressed mothers in the northern Finland 1966 birth cohort: relationship to family history of psychosis. Am J Psychiatry 2010; 167:70–77Link, Google Scholar
7 : Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am J Psychiatry 2016; 173:705–713Link, Google Scholar
8 : Prenatal nicotine exposure and risk of schizophrenia among offspring in a national birth cohort. Am J Psychiatry 2016; 173:799–806Link, Google Scholar
9 : Maternal tobacco, cannabis and alcohol use during pregnancy and risk of adolescent psychotic symptoms in offspring. Br J Psychiatry 2009; 195:294–300Crossref, Medline, Google Scholar
10 : Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry 2009; 166:1025–1030Link, Google Scholar
11 : Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441–451Crossref, Medline, Google Scholar
12 : Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112Crossref, Medline, Google Scholar
13 : Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA 2017; 317:1544–1552Crossref, Medline, Google Scholar
14 : Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ 2007; 334:464Crossref, Medline, Google Scholar
15 : Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991; 338:131–137Crossref, Medline, Google Scholar
16 : Longitudinal observations of biological deviations in a schizophrenic infant. Am J Psychiatry 1959; 116:25–31Link, Google Scholar
17 : The New York High-Risk Project: a followup report. Schizophr Bull 1987; 13:451–461Crossref, Medline, Google Scholar
18 : Childhood emotional expressions, educational attainment, and age at onset of illness in schizophrenia. J Abnorm Psychol 1994; 103:784–790Crossref, Medline, Google Scholar
19 : Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry 2017; 174:319–328Link, Google Scholar
20 : Disorganized attachment in infancy: a review of the phenomenon and its implications for clinicians and policy-makers. Attach Hum Dev 2017; 19:534–558Crossref, Medline, Google Scholar
21 : Behavioral neurodevelopment abnormalities and schizophrenic disorder: a retrospective evaluation with the Childhood Behavior Checklist (CBCL). Schizophr Res 2000; 44:121–128Crossref, Medline, Google Scholar
22 : Some premorbid characteristics related to breakdown in children with schizophrenic mothers. J Psychiatr Res 1968; 6(Supp 1):267–291Crossref, Google Scholar
23 : Fish oil–derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med 2016; 375:2530–2539Crossref, Medline, Google Scholar
24 : Seven-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA 2017; 317:1173–1175Crossref, Medline, Google Scholar
25 : Folate and long-chain polyunsaturated fatty acid supplementation during pregnancy has long-term effects on the attention system of 8.5-y-old offspring: a randomized controlled trial. Am J Clin Nutr 2016; 103:115–127Crossref, Medline, Google Scholar
26 : Maternal folic acid supplement use in early pregnancy and child behavioural problems: the Generation R study. Br J Nutr 2010; 103:445–452Crossref, Medline, Google Scholar
27 : Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R study. Am J Clin Nutr 2012; 95:1413–1421Crossref, Medline, Google Scholar
28 : Maternal folic acid supplementation during pregnancy and early childhood asthma. Epidemiology 2015; 26:934–941Crossref, Medline, Google Scholar
29 : Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry 2013; 170:290–298Link, Google Scholar
30 : Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. Am J Psychiatry 2016; 173:509–516Link, Google Scholar
31 : Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One 2012; 7:e43448Crossref, Medline, Google Scholar
32 : A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1). FASEB J 2013; 27:1245–1253Crossref, Medline, Google Scholar
33 : Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the Coronary After Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc 2016; 5:e003970Crossref, Medline, Google Scholar
34 : Maternal serum docosahexaenoic acid and schizophrenia spectrum disorders in adult offspring. Schizophr Res 2011; 128:30–36Crossref, Medline, Google Scholar
35 : Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 2010; 304:1675–1683Crossref, Medline, Google Scholar
36 : Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 2010; 67:889–894Crossref, Medline, Google Scholar
37 : Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res 2004; 67:237–245Crossref, Medline, Google Scholar
38 : Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open 2017; 3:85–90Crossref, Medline, Google Scholar
39 : Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics 2012; 130:e913–e920Crossref, Medline, Google Scholar
40 : Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res 2003; 63:73–78Crossref, Medline, Google Scholar
41 : Early methyl donor deficiency may induce persistent brain defects by reducing Stat3 signaling targeted by miR-124. Cell Death Dis 2013; 4:e755Crossref, Medline, Google Scholar
42 : Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC, National Academy Press, 1998Google Scholar
43 : Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 2006; 314:1610–1613Crossref, Medline, Google Scholar
44 : Long-term improvements in sensory inhibition with gestational choline supplementation linked to α7 nicotinic receptors through studies in Chrna7 null mutation mice. Brain Res 2014; 1552:26–33Crossref, Medline, Google Scholar
45 : Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc Natl Acad Sci USA 1996; 93:12559–12563Crossref, Medline, Google Scholar
46 : Omega-3 fatty acids and decidual cell prostaglandin production in response to the inflammatory cytokine IL-1beta. Am J Obstet Gynecol 2006; 195:1693–1699Crossref, Medline, Google Scholar
47 : Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci Lett 2010; 486:220–223Crossref, Medline, Google Scholar
48 : 1α,25-dihydroxyvitamin D3 mechanism of action: modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim Biophys Acta 2012; 1823:1708–1719Crossref, Medline, Google Scholar
49 : Manual for the ASEBA Preschool Forms and Profiles: An Integrated System of Multi-Informant Assessment. Burlington, Vt, ASEBA, 1991Google Scholar
50 : Folate concentrations during pregnancy and autistic traits in the offspring: the Generation R study. Eur J Public Health 2015; 25:431–433Crossref, Medline, Google Scholar
51 : Maternal dietary patterns during pregnancy and child internalising and externalising problems: the Generation R study. Clin Nutr 2014; 33:115–121Crossref, Medline, Google Scholar
52 : Maternal fatty acid status during pregnancy and child autistic traits: the Generation R study. Am J Epidemiol 2016; 183:792–799Crossref, Medline, Google Scholar
53 : Randomized controlled trial of maternal omega-3 long-chain PUFA supplementation during pregnancy and early childhood development of attention, working memory, and inhibitory control. Am J Clin Nutr 2014; 99:851–859Crossref, Medline, Google Scholar
54 : Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003; 111:e39–e44Crossref, Medline, Google Scholar
55 : Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev 2004; 75:1254–1267Crossref, Medline, Google Scholar
56 : Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2008; 93:F45–F50Crossref, Medline, Google Scholar
57 : Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. J Nutr 2011; 141:1216–1223Crossref, Medline, Google Scholar
58 : Four-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA 2014; 311:1802–1804Crossref, Medline, Google Scholar
59 : The influence of supplemental docosahexaenoic and arachidonic acids during pregnancy and lactation on neurodevelopment at eighteen months. Prostaglandins Leukot Essent Fatty Acids 2011; 84:139–146Crossref, Medline, Google Scholar
60 : Can early intake of dietary omega-3 predict childhood externalizing behaviour? Acta Paediatr 2009; 98:1805–1808Crossref, Medline, Google Scholar
61 : Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol 2008; 167:1171–1181Crossref, Medline, Google Scholar
62 : The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2013; 97:531–544Crossref, Medline, Google Scholar
63 : Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA 2009; 301:175–182Crossref, Medline, Google Scholar
64 : DHA supplementation during the perinatal period and neurodevelopment: do some babies benefit more than others? Prostaglandins Leukot Essent Fatty Acids 2013; 88:87–90Crossref, Medline, Google Scholar
65 : Fish oil supplementation improves pregnancy outcomes and size of the newborn: a meta-analysis of 21 randomized controlled trials. J Matern Fetal Neonatal Med 2016; 29:2017–2027Crossref, Medline, Google Scholar
66 : Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 2002; 288:728–737Crossref, Medline, Google Scholar
67 Hierholzer RS: American Medical Association House of Delegates: Report of Reference Committee E. Resolution 517. Choline Supplementation in Prenatal Vitamins. 2017; https://www.ama-assn.org/sites/default/files/media-browser/public/hod/a17-refcomm-e-addendum-updated.pdfGoogle Scholar
68 : Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther 1983; 225:320–324Medline, Google Scholar
69 : Pregnant Canadian women achieve recommended intakes of one-carbon nutrients through prenatal supplementation but the supplement composition, including choline, requires reconsideration. J Nutr 2015; 145:1824–1834Crossref, Medline, Google Scholar
70 : Unexpected depletion in plasma choline and phosphatidylcholine concentrations in a pregnant woman with bipolar affective disorder being treated with lithium, haloperidol, and benztropine: a case report. J Med Case Rep 2008; 2:55Crossref, Medline, Google Scholar
71 : Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2012; 96:1465–1472Crossref, Medline, Google Scholar
72 : Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behav Dev 1996; 19:159–170Crossref, Google Scholar
73 : Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull 2011; 37:1200–1208Crossref, Medline, Google Scholar
74 : Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587–592Crossref, Medline, Google Scholar
75 : High maternal serum choline at 16 weeks gestation protects against infection-associated impairments in infant auditory P50 sensory gating. Neuropsychopharm 2016; 41:S343Google Scholar
76 : Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J 2012; 26:3563–3574Crossref, Medline, Google Scholar
77 : Low maternal retinol as a risk factor for schizophrenia in adult offspring. Schizophr Res 2012; 137:159–165Crossref, Medline, Google Scholar
78 : Gestational vitamin D deficiency and autism-related traits: the Generation R study. Mol Psychiatry 2018; 23:240–246Crossref, Medline, Google Scholar
79 : Perinatal vitamin A status in relation to neurodevelopmental outcome at two years of age. Int J Vitam Nutr Res 2009; 79:238–249Crossref, Medline, Google Scholar
80 : Maternal vitamin D deficiency and the risk of autism spectrum disorders: population-based study. BJPsych Open 2016; 2:170–172Crossref, Medline, Google Scholar
81 : Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring. J Psychosom Res 2016; 89:98–101Crossref, Medline, Google Scholar
82 : Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 2012; 129:485–493Crossref, Medline, Google Scholar
83 : Maternal vitamin D status and infant outcomes in rural Vietnam: a prospective cohort study. PLoS One 2014; 9:e99005Crossref, Medline, Google Scholar
84 : Gestational vitamin 25(OH)D status as a risk factor for receptive language development: a 24-month, longitudinal, observational study. Nutrients 2015; 7:9918–9930Crossref, Medline, Google Scholar
85 : Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol 2014; 28:434–444Crossref, Medline, Google Scholar
86 : Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011; 127:e180–e187Crossref, Medline, Google Scholar
87 : Post-hoc comparison of vitamin D status at three timepoints during pregnancy demonstrates lower risk of preterm birth with higher vitamin D closer to delivery. J Steroid Biochem Mol Biol 2015; 148:256–260Crossref, Medline, Google Scholar
88 : Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC, National Academy Press, 2011Google Scholar
89 : Teratogenicity of high vitamin A intake. N Engl J Med 1995; 333:1369–1373Crossref, Medline, Google Scholar
90 : Metabolomics of human amniotic fluid and maternal plasma during normal pregnancy. PLoS One 2016; 11:e0152740Crossref, Medline, Google Scholar
91 : Evidence for negative selection of gene variants that increase dependence on dietary choline in a Gambian cohort. FASEB J 2015; 29:3426–3435Crossref, Medline, Google Scholar
92 : A study of the PEMT gene in schizophrenia. Neurosci Lett 2007; 424:203–206Crossref, Medline, Google Scholar
93 : Increased vitamin D intake differentiated according to skin color is needed to meet requirements in young Swedish children during winter: a double-blind randomized clinical trial. Am J Clin Nutr 2017; 106:105–112Crossref, Medline, Google Scholar
94 : Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am J Psychiatry 2010; 167:1083–1091Link, Google Scholar
95 : Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317:183–189Crossref, Medline, Google Scholar
96 : A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012; 366:54–63Crossref, Medline, Google Scholar
97 Bell CC: AMA’s stance on choline, prenatal vitamins could bring ‘staggering’ results. ObGynNews 2017; http://www.mdedge.com/obgynnews/article/144765/adhd/amas-stance-choline-prenatal-vitamins-could-bring-staggering-resultsGoogle Scholar
98 : Nutritional deficiencies and clinical correlates in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull (Epub ahead of print, Nov 30, 2017)Google Scholar
99 : Fish oil supplementation in pregnancy. N Engl J Med 2016; 375:2599–2601Crossref, Medline, Google Scholar
100 : Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006; 26:229–250Crossref, Medline, Google Scholar
101 : The acetylcholine innervation of cerebral cortex: new data on its normal development and its fate in the hAPP(SW,IND) mouse model of Alzheimer’s disease. J Neural Transm (Vienna) 2005; 112:149–162Crossref, Medline, Google Scholar
102 : Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci 1998; 18:1187–1195Crossref, Medline, Google Scholar
103 : Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 1997; 8:2831–2835Crossref, Medline, Google Scholar
104 : Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J Neurosci 2012; 32:7651–7661Crossref, Medline, Google Scholar
105 : Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 2011; 31:11088–11095Crossref, Medline, Google Scholar
106 : Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience 1990; 39:25–32Crossref, Medline, Google Scholar
107 : Vitamin D in fetal brain development. Semin Cell Dev Biol 2011; 22:629–636Crossref, Medline, Google Scholar
108 : The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav Immun 2015; 46:192–202Crossref, Medline, Google Scholar
109 : Development of the human fetal hippocampal formation during early second trimester. Neuroimage 2015; 119:33–43Crossref, Medline, Google Scholar
110 : Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388Crossref, Medline, Google Scholar
111 : Nicotinic acetylcholine receptors in rat and human placenta. Placenta 2005; 26:735–746Crossref, Medline, Google Scholar
112 : The nicotinic acetylcholine receptor α7 subunit is an essential negative regulator of bone mass. Sci Rep 2017; 7:45597Crossref, Medline, Google Scholar
113 : The role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophrenia. Schizophr Res 2008; 99:48–55Crossref, Medline, Google Scholar
114 : Prenatal alcohol exposure and prenatal stress differentially alter glucocorticoid signaling in the placenta and fetal brain. Neuroscience 2017; 342:167–179Crossref, Medline, Google Scholar
115 : Placental gene expression mediates the interaction between obstetrical history and genetic risk for schizophrenia. bioRxiv 2017. Available at doi:
116 : Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564; correction, 2009; 360:648Crossref, Medline, Google Scholar
117 : It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 2008; 84:329–342Crossref, Medline, Google Scholar
118 : Bupropion sustained release for pregnant smokers: a randomized, placebo-controlled trial. Am J Obstet Gynecol 2017; 216:420.e1–420.e9Crossref, Google Scholar
119 : Pregnancy outcomes after maternal varenicline use; analysis of surveillance data collected by the European Network of Teratology Information Services. Reprod Toxicol 2017; 67:26–34Crossref, Medline, Google Scholar
120 : First-trimester exposure to bupropion and risk of cardiac malformations. Pharmacoepidemiol Drug Saf 2014; 23:1066–1075Crossref, Medline, Google Scholar
121 : Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 2009; 166:557–566Link, Google Scholar
122 : Depression and serotonin reuptake inhibitor treatment as risk factors for preterm birth. Epidemiology 2012; 23:677–685Crossref, Medline, Google Scholar
123 : Does fetal exposure to SSRIs or maternal depression impact infant growth? Am J Psychiatry 2013; 170:485–493; correction, 2013; 170:1218Link, Google Scholar
124 : Antidepressant use in pregnant and postpartum women. Annu Rev Clin Psychol 2014; 10:369–392Crossref, Medline, Google Scholar
125 : Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. Am J Psychiatry 2012; 169:616–624Link, Google Scholar



