Prediction of Relapse After Discontinuation of Antipsychotic Treatment in Alzheimer’s Disease: The Role of Hallucinations
Abstract
Objective:
In Alzheimer’s disease, antipsychotic medications are often used for a period, with relief of symptoms, and then discontinued, after which relapse may occur. The authors sought to determine which neuropsychiatric symptoms predict relapse.
Method:
In the Antipsychotic Discontinuation in Alzheimer’s Disease trial, 180 patients with Alzheimer’s disease and symptoms of agitation or psychosis were treated with risperidone for 16 weeks, after which patients who responded (N=110) were randomly assigned to continue risperidone for 32 weeks, to continue risperidone for 16 weeks followed by switch to placebo for 16 weeks, or to receive placebo for 32 weeks. As reported previously, discontinuation of risperidone was associated with a two- to fourfold increased risk of relapse over 16–32 weeks. In planned post hoc analyses, the authors examined associations between the 12 symptom domains in the Neuropsychiatric Inventory (NPI) and relapse in the first 16-week phase after randomization.
Results:
Compared with patients with mild hallucinations or no hallucinations, patients with severe hallucinations as a presenting symptom at baseline had a higher likelihood of relapse (hazard ratio=2.96, 95% CI=1.52, 5.76). This effect was present for the subgroup with auditory hallucinations, but not the subgroup with visual hallucinations. Among patients with baseline hallucinations, 13 of 17 (76.5%) who discontinued risperidone relapsed, compared with 10 of 26 (38.5%) who continued risperidone (p<0.02). This group difference remained significant for severe (77.8%) compared with mild (36%) hallucinations. NPI domain scores after the initial open-treatment phase were not associated with relapse.
Conclusions:
Patients with severe baseline hallucinations were more likely to relapse after randomization, and the presence of baseline hallucinations was associated with a higher risk of relapse after discontinuation of risperidone compared with continued risperidone treatment. For patients with hallucinations, particularly auditory hallucinations, antipsychotic discontinuation should be approached cautiously because of high relapse risk.
Currently in the United States there are approximately 5 million individuals age 65 or older with Alzheimer’s disease, and by the year 2050 the number will reach 13.8 million (1). During the course of illness, the majority of patients with Alzheimer’s disease suffer from behavioral disturbances (2, 3), with a lifetime risk of nearly 100% for the occurrence of these symptoms (4).
The U.S. Food and Drug Administration (FDA) has not approved antipsychotics for the treatment of agitation or psychosis in dementia or Alzheimer’s disease, but some countries, such as Germany, have approved risperidone for this type of indication. Antipsychotics are nevertheless used off-label to treat agitation and psychosis in the United States, usually because of clinical need. Concern about antipsychotic toxicity has led to federal regulations that require discontinuation after a few months of antipsychotic treatment for patients in nursing homes (5). Data are lacking on which types of neuropsychiatric symptoms increase the risk of relapse after discontinuation of antipsychotic treatment. In order for clinicians to make informed judgments about which patients can safely discontinue their antipsychotic medication and which patients need to continue treatment, it is important to identify which symptoms are associated with an increased likelihood of relapse after antipsychotic discontinuation. In our multicenter Antipsychotic Discontinuation in Alzheimer’s Disease (ADAD) trial, patients who responded to risperidone over 16 weeks of open-label treatment showed a two- to fourfold higher risk of relapse 16 to 32 weeks after discontinuation of risperidone compared with continuation on risperidone (6). In planned post hoc analyses from this trial, we examined the associations between neuropsychiatric symptoms, both at study baseline and immediately after open risperidone treatment, and computed the likelihood of relapse after subsequent randomization to continuation risperidone treatment versus discontinuation to placebo.
Method
ADAD Study Design
The rationale, design, and methods of the ADAD trial have been published previously (6). Briefly, to participate in the trial, outpatients or residents of assisted-living facilities or nursing homes had to meet the following inclusion criteria: DSM-IV criteria for dementia; National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for probable Alzheimer’s disease (7); a Mini-Mental State Examination (MMSE) (8) score in the range of 5–26 for outpatients and 2–26 for nursing home residents; and a Neuropsychiatric Inventory (NPI) (9) score of 4 or more on the delusions or hallucinations subscale (psychosis score) or the agitation-aggression subscale (agitation score). (Scores on all NPI subscales range from 0 to 12, with higher scores indicating more pronounced symptoms.) Patients were excluded if they had a history of stroke, transient ischemic attack, or uncontrolled atrial fibrillation or if they met DSM-IV criteria for any primary psychotic disorder predating the onset of Alzheimer’s disease. At baseline, subjects were assessed for the presence or absence of the following cardiovascular risk factors: hypertension, hyperlipidemia, diabetes, and tobacco use. Subjects or their representatives provided written informed consent after receiving a complete description of the study.
In phase A of the trial (weeks 0 to 16), patients received open treatment with risperidone, with the dosage adjusted by the study physician as clinically indicated. At week 16, patients were identified as responders to treatment if they had at least a 30% reduction in the core NPI score (sum of agitation-aggression, hallucinations, and delusions) and a score of 1 (very much improved) or 2 (much improved) on the change scale of the Clinical Global Impression of Change Scale (CGIC) assessing behavioral symptoms. At week 16, patients who responded to treatment in phase A were then randomly assigned to continue risperidone for 32 weeks, to continue risperidone for 16 weeks and then to receive placebo for 16 weeks, or to receive placebo for 32 weeks. In phase B, relapse was defined as an increase of at least 30% in the core NPI score or a 5-point increase in the core NPI score from the end of phase A open treatment and a score of 6 (much worse) or 7 (very much worse) on the CGIC at any study visit. Patients who met criteria for relapse immediately exited the protocol and received open treatment based on doctor’s choice.
The Present Substudy
This substudy included all patients who responded to risperidone in phase A and underwent randomized treatment assignment at week 16. Of the 112 patients who responded to treatment in phase A, 110 underwent randomization at week 16 to enter phase B (Figure 1). Symptoms at initial presentation, prior to open-label treatment, are referred to as baseline symptoms. Results from baseline until the first 16 weeks after randomization were examined. The final 16 weeks (week 32 to week 48) were not analyzed for prediction of relapse because of the relatively small sample sizes.
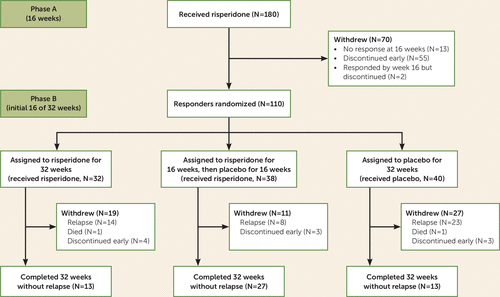
FIGURE 1. Patient Flow Through 16 Weeks of Open Treatment and the Initial 16 Weeks After Randomizationa
a In phase B (combining all groups), in addition to patient relapse, subjects exited the study because of death (N=2), early discontinuation (N=10), unacceptable side effects (N=3), or other reasons (N=7). The final 16 weeks in the trial were not analyzed because of small sample sizes. The full CONSORT diagram has been published previously (6).
Statistical Analysis
Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, N.C.). Only patients who underwent randomization were included, and analyses were conducted for all patients according to the intent-to-treat principle. NPI domains were evaluated at week 0 (baseline) and at week 16 (the time point of randomization) as potential predictors of relapse during the first 16 weeks after randomization (weeks 16–32 in the trial).
Each individual NPI domain was dichotomized as present or absent at week 0 and week 16 and compared for differences between relapsed and nonrelapsed groups using Fisher’s exact test.
To examine clinically relevant relationships of the NPI domains in predicting relapse, the NPI domain scores were classified into three categories: 0 for not present, 1–6 for mild to moderate symptoms, and 7–12 for severe symptoms. This was done for all NPI domains at week 0 and at week 16.
Individual NPI domain scores at week 0 and week 16 were analyzed using SAS’s PROC PHREG with the discrete method to address ties (10). Stratified Cox proportional hazard regression analyses were conducted to evaluate the associations between NPI domain scores at week 0 and at week 16 and relapse between weeks 16 and 32. All analyses were conducted with the two stratification variables employed in the randomization: presence or absence of psychosis and residence in an assisted-living facility or nursing home versus living at home (6). The Cox proportional hazard regression analysis was performed initially with dichotomized NPI domain scores and then for NPI domain scores classified into three groups, based on severity, at week 0 and at week 16. The twelve NPI domains were examined separately with treatment group and their interactions. The chi-square test was used to analyze the effect of treatment group on relapse. The proportional hazards assumption was evaluated by testing a nonzero slope in linear regression models of the Schoenfeld residuals as a function of event time. All models satisfied the assumption. Kaplan-Meier estimates of the risk of relapse were prepared for visual comparison.
Results
As previously reported (6), in phase A open-label treatment with risperidone for 16 weeks (N=180), NPI scores declined significantly for all 12 domains except appetite. A total of 112 patients were responders, 110 of whom underwent randomization. Of these 110 patients, 32 were assigned to receive risperidone for 32 weeks, 38 to receive risperidone for 16 weeks and placebo for the following 16 weeks (for these two groups combined, N=70 on risperidone), and 40 to receive placebo for 32 weeks. In this subsample, the mean age was 79.97 years (SD=7.83), 60% were female, and 50.9% were residing in a nursing home or assisted living facility. The breakdown by race was 71.82% white, 19.09% African American, 8.18% Hispanic, and 0.91% other. After randomization, patients who discontinued risperidone to receive placebo were more likely to relapse (24/40, 60%) than patients who continued on risperidone (23/70, 33%) (p<0.001) (6). Gender, race, and nursing home/outpatient status were not associated with relapse.
Baseline NPI Domain Scores and Prediction of Relapse
In the total randomized sample, in the stratified Cox models there was no significant association between any of the 12 baseline dichotomized NPI domain scores and time to relapse. Using the classification of absent, mild/moderate, and severe symptoms, the only NPI domain that was significantly associated with relapse at week 32 was hallucinations (p=0.005) (Table 1). In the Cox regression analysis, severe hallucinations were significantly associated with an increased risk of relapse when compared with the group with no hallucinations (hazard ratio=2.70, 95% CI=1.19, 6.12, p<0.02) (Figure 2). Auditory hallucinations showed a significant effect in predicting relapse (hazard ratio=2.36, 95% CI=1.18, 4.71, p<0.02). Visual hallucinations were not predictive of relapse. Fourteen of 18 patients with severe hallucinations had severe delusions, and these two variables were associated with each other (likelihood ratio χ2=9.69, p<0.05). Adding delusions into the model did not alter the significance of severe hallucinations in predicting relapse.
| NPI Domain | Symptom Present | Symptoms Among Those Who Relapsed at Week 32 (%) | p (Fisher’s Exact Test) | |||
|---|---|---|---|---|---|---|
| N | % | None | Mild | Severe | ||
| At baseline | ||||||
| Hallucinations | 43 | 39.1 | 35.8 | 36.0 | 77.8 | 0.005 |
| Delusions | 95 | 86.4 | 33.3 | 35.1 | 50.0 | 0.28 |
| Agitation/aggression | 102 | 92.7 | 75.0 | 35.4 | 44.4 | 0.10 |
| Depression/dysphoria | 48 | 43.6 | 38.7 | 46.3 | 57.1 | 0.50 |
| Anxiety | 56 | 50.9 | 50.0 | 32.5 | 43.8 | 0.23 |
| Elation/euphoria | 8 | 7.3 | 44.1 | 33.3 | 0.0 | 0.60 |
| Apathy/indifference | 44 | 40.0 | 43.9 | 34.6 | 50.0 | 0.59 |
| Disinhibition | 53 | 48.2 | 42.1 | 44.7 | 40.0 | 0.96 |
| Irritability/lability | 82 | 74.5 | 35.7 | 42.6 | 48.6 | 0.59 |
| Aberrant motor | 45 | 40.9 | 41.5 | 47.6 | 41.7 | 0.93 |
| Sleep | 47 | 42.7 | 41.3 | 42.9 | 47.4 | 0.90 |
| Appetite | 29 | 26.4 | 43.2 | 30.0 | 66.7 | 0.19 |
| At randomization (week 16) | ||||||
| Hallucinations | 15 | 13.6 | 42.1 | 46.7 | 0.0 | 0.78 |
| Delusions | 36 | 32.7 | 45.9 | 39.4 | 0.0 | 0.32 |
| Agitation/aggression | 50 | 45.5 | 45.0 | 40.4 | 33.3 | 0.87 |
| Depression/dysphoria | 20 | 18.2 | 40.0 | 50 | 100.0 | 0.20 |
| Anxiety | 33 | 30.0 | 40.3 | 46.9 | 100.0 | 0.39 |
| Elation/euphoria | 1 | 0.9 | 43.1 | 0.0 | 0.0 | 1.00 |
| Apathy/indifference | 42 | 38.2 | 42.6 | 48.3 | 30.8 | 0.58 |
| Disinhibition | 15 | 13.6 | 38.9 | 71.4 | 0.0 | 0.04 |
| Irritability/lability | 40 | 36.4 | 48.6 | 32.5 | 0.0 | 0.11 |
| Aberrant motor | 33 | 30.0 | 44.2 | 37.5 | 44.4 | 0.90 |
| Sleep | 20 | 18.2 | 47.8 | 17.6 | 33.3 | 0.04 |
| Appetite | 18 | 16.4 | 41.3 | 41.7 | 66.7 | 0.61 |
TABLE 1. Neuropsychiatric Inventory (NPI) Domain Scores at Baseline and After 16 Weeks of Open-Label Risperidone Treatment in the Combined Sample of 110 Responders Who Were Randomly Assigned to a Subsequent 16 Weeks of Continuation Risperidone (N=70) or Discontinuation to Placebo (N=40)
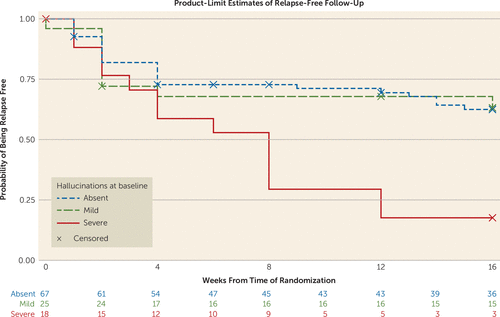
FIGURE 2. Kaplan-Meier Curves, With Numbers of Patients Who Did Not Relapse, by Severity Score for Hallucinations at Baseline
The additional covariates of MMSE score, number of cardiovascular risk factors, number of medications, and number of medications classified according to the Beers criteria, which cover medications with potential toxicity in older adults (11), were included in the main analysis. None of these measures changed the significant effect of hallucinations in predicting relapse. Twenty-three patients were taking pain medications at baseline, and they were significantly less likely to relapse (p<0.02), but including the number of pain medications as a covariate did not alter the significant association between baseline hallucinations and risk of relapse. Forty-eight patients were on other psychotropic medications at baseline, and class of psychotropic medication was not associated with risk of relapse.
In additional analyses, a three-way contingency table was analyzed with the baseline hallucination domain and the variables of treatment and relapse during weeks 16 to 32. Among patients with baseline hallucinations, 13 of 17 (76.5%) who switched to placebo relapsed, compared with 10 of 26 (38.5%) who continued risperidone treatment (relative risk=1.99, 95% CI=1.14, 3.46, p<0.03). This difference was also observed for severe (77.8%) compared with mild (36%) hallucinations (relative risk=2.88, 95% CI=1.16, 7.18, p<0.03). Within the subgroup of patients with severe baseline hallucinations, 10 of 11 (90.9%) who switched to placebo relapsed, compared with four of seven (57.1%) who continued risperidone treatment (relative risk=1.59, 95% CI=0.82, 3.10, p=0.17). In this analysis, the p value did not reach statistical significance because of the small size of the subgroup with mild baseline hallucinations: three of six (50%) who switched to placebo relapsed, compared with six of 19 (31.6%) on risperidone (relative risk=1.58, 95% CI=0.56, 4.47, p=0.39).
In stratified Cox models that examined the interaction between baseline symptom severity for each NPI domain and treatment group after randomization (risperidone continuation versus discontinuation) in the prediction of time to relapse, only irritability/lability was significant (χ2=7.16, p<0.03). Among patients with severe irritability/lability at baseline, those who discontinued risperidone had a higher risk of relapse than those who continued risperidone treatment (hazard ratio=7.78, 95% CI=2.32, 26.07, p<0.01), while those with no irritability/lability or mild irritability/lability did not show a significantly increased risk of relapse (Table 2). Few patients demonstrated severe lability/irritability at baseline (Table 2).
| NPI Domain and Symptom Severity | Relapses by Week 32 (N/Total) | pa | |
|---|---|---|---|
| Risperidone Continued | Risperidone Discontinued | ||
| At baseline | |||
| Hallucination | |||
| Absent | 13/44 | 11/23 | 0.35 |
| Mild | 6/19 | 3/6 | |
| Severe | 4/7 | 10/11 | |
| Delusions | |||
| Absent | 5/11 | 0/4 | 0.44 |
| Mild | 6/28 | 7/9 | |
| Severe | 12/31 | 17/27 | |
| Agitation/aggression | |||
| Absent | 2/3 | 4/5 | 0.15 |
| Mild | 11/33 | 6/15 | |
| Severe | 10/34 | 14/20 | |
| Depression/dysphoria | |||
| Absent | 13/41 | 11/21 | 0.14 |
| Mild | 8/25 | 11/16 | |
| Severe | 2/4 | 2/3 | |
| Anxiety | |||
| Absent | 7/25 | 20/29 | 0.17 |
| Mild | 10/32 | 3/8 | |
| Severe | 6/13 | 1/3 | |
| Elation/euphoriab | |||
| Absent | 22/64 | 23/38 | 0.88 |
| Present | 1/6 | 1/2 | |
| Apathy/indifference | |||
| Absent | 15/44 | 14/22 | 0.07 |
| Mild | 1/13 | 8/13 | |
| Severe | 7/13 | 2/5 | |
| Disinhibition | |||
| Absent | 14/38 | 10/19 | 0.38 |
| Mild | 6/23 | 11/15 | |
| Severe | 3/9 | 3/6 | |
| Irritability/lability | |||
| Absent | 6/17 | 4/11 | 0.03 |
| Mild | 13/32 | 7/15 | |
| Severe | 4/21 | 13/14 | |
| Aberrant motor | |||
| Absent | 12/39 | 15/26 | 0.65 |
| Mild | 6/15 | 4/6 | |
| Severe | 5/16 | 5/8 | |
| Sleep | |||
| Absent | 15/49 | 20/32 | 0.27 |
| Mild | 3/14 | 3/6 | |
| Severe | 5/7 | 1/2 | |
| Appetite | |||
| Absent | 12/39 | 14/24 | 0.33 |
| Mild | 8/19 | 4/9 | |
| Severe | 3/12 | 6/7 | |
| At randomization (week 16) | |||
| Hallucination | |||
| Absent | 19/60 | 21/35 | 0.31 |
| Mild | 4/10 | 3/5 | |
| Delusionsb | |||
| Absent | 15/50 | 19/24 | 0.02 |
| Present | 8/20 | 5/16 | |
| Agitation/aggression | |||
| Absent | 12/36 | 15/24 | 0.82 |
| Mild | 11/32 | 8/15 | |
| Severe | 0/2 | 1/1 | |
| Depression/dysphoria | |||
| Absent | 17/57 | 19/33 | 0.97 |
| Mild | 4/11 | 5/7 | |
| Severe | 2/2 | 0/0 | |
| Anxiety | |||
| Absent | 12/47 | 19/30 | 0.10 |
| Mild | 10/22 | 5/10 | |
| Severe | 1/1 | 0/0 | |
| Elation/euphoria | |||
| Absent | 23/69 | 24/40 | NA |
| Severe | 0/1 | 0/0 | |
| Apathy/indifference | |||
| Absent | 11/39 | 18/29 | 0.36 |
| Mild | 9/20 | 5/9 | |
| Severe | 3/11 | 1/2 | |
| Disinhibitionb | |||
| Absent | 18/62 | 19/33 | 0.29 |
| Present | 5/8 | 5/7 | |
| Irritability/lability | |||
| Absent | 17/44 | 17/26 | 0.89 |
| Mild | 6/26 | 7/14 | |
| Aberrant motor | |||
| Absent | 14/46 | 20/31 | 0.68 |
| Mild | 6/18 | 3/6 | |
| Severe | 3/6 | 1/3 | |
| Sleep | |||
| Absent | 18/59 | 20/33 | 0.56 |
| Mild | 3/8 | 2/4 | |
| Severe | 2/3 | 2/3 | |
| Appetite | |||
| Absent | 21/56 | 22/34 | 0.40 |
| Mild | 1/11 | 2/6 | |
| Severe | 1/3 | 0/0 | |
TABLE 2. Frequency of Relapse by Neuropsychiatric Inventory (NPI) Domain Symptom Severity at Baseline and 16 Weeks, and Risperidone Continuation Versus Discontinuation in the Randomized Phase (weeks 16 to 32)
Prerandomization Week 16 NPI Domain Scores and Prediction of Relapse
Each individual NPI domain was dichotomized as present or absent at week 16 (the time point of randomization) and was compared for differences between relapsed and nonrelapsed groups in the first 16 weeks after randomization (weeks 16 to 32), using Fisher’s exact test. The NPI domain score at week 16 for absence of sleep problems (Fisher’s exact test, p=0.04; 4/20 with sleep problems relapsed compared with 43/90 without sleep problems) was significantly associated with relapse (Table 1). In stratified Cox regression analyses that examined time to relapse, absence of sleep problems at week 16 was associated with an increased risk of relapse (hazard ratio=0.28, 95% CI=0.10, 0.79, p<0.02). For sleep problems at week 16, analyses with the clinically relevant classification of no symptoms, mild symptoms, and severe symptoms were not conducted because of small sample sizes in the subgroups that relapsed. Of four patients with sleep problems at week 16 who relapsed, three with mild sleep problems and one with severe sleep problems relapsed.
At week 16 (after open treatment and before randomization), patients had improved markedly in symptoms of psychosis and agitation; for example, 15/110 patients had hallucinations, compared with 43/110 at baseline, and 50/110 patients had agitation, compared with 102/110 at baseline. The relapse rate in the 53 patients with baseline agitation who improved from baseline was 41.51%, while the relapse rate in the 49 patients who continued to have agitation at week 16 was 38.78%.
In the stratified Cox models, there was no significant interaction between any of the 12 prerandomization dichotomized NPI domain scores at week 16 and randomized group assignment (risperidone continuation or discontinuation) in the prediction of time to relapse, with the exception of delusions (χ2=5.65, p<0.02) (Table 2). Patients who did not have delusions at randomization had a higher risk of relapse on discontinuation of risperidone than did patients who continued risperidone (hazard ratio=3.77, 95% CI=1.77, 7.84, p<0.001), while among patients with delusions at randomization, there was no significant difference in relapse risk between those who continued risperidone treatment and those who discontinued (hazard ratio=0.70, 95% CI=0.22, 2.23, p=0.55). The majority of patients who had delusions at baseline (61/95, 64%) improved to the extent of not having delusions at randomization, and the number of relapses in this subgroup (29/61, 47.54%) did not differ significantly (p=0.35) from that of the rest of the sample (18/49, 37.73%).
Discussion
The majority of patients with hallucinations as a presenting symptom at baseline relapsed during the first 16 weeks after randomization. Severe hallucinations were associated with a threefold higher risk of relapse compared with mild or no hallucinations. Patients with severe hallucinations at baseline were more likely than the overall randomized sample to relapse, and the risk of relapse was higher for patients assigned to placebo compared with those assigned to continue risperidone treatment. Patients with auditory hallucinations at baseline, but not those with visual hallucinations at baseline, were more likely to relapse, indicating that if patients with severe auditory hallucinations respond to treatment with antipsychotics, discontinuation should be approached with caution, and close monitoring after discontinuation is advisable. It is unclear why auditory hallucinations and not visual hallucinations predicted relapse. One speculation is that illusions that can occur in patients with Alzheimer’s disease were rated as visual hallucinations, and since illusions may not improve with antipsychotic treatment, the medication effect on visual hallucinations may have been diluted. Also, it is possible that some patients with Alzheimer’s disease and visual hallucinations had Lewy body dementia, a condition associated with low antipsychotic tolerability.
At week 16, remission of delusions was associated with a higher risk of relapse, suggesting that the persistence of delusions after treatment response is not an indicator of an increased risk of relapse. However, the number of patients with delusions at week 16 was small (Table 2). Absence of sleep problems at week 16 was associated with a lower likelihood of relapse between weeks 16 and 32, but the effect was marginally significant, and its clinical relevance is unclear because of the small number of patients with sleep problems who relapsed. Overall, the results suggest that the initial severity of symptoms, particularly the severity of hallucinations, rather than the residual severity of symptoms after treatment response, is important to consider when evaluating the potential risk of relapse after antipsychotic discontinuation.
There have been few previous efforts to evaluate individual symptoms in patients with Alzheimer’s disease to predict treatment response to antipsychotics or to predict relapse after antipsychotic discontinuation. In the Dementia Antipsychotic Withdrawal Trial–Alzheimer’s Disease (DART-AD) trial, in which discontinuation occurred after 3 months of antipsychotic treatment, patients with higher NPI scores (>14) worsened behaviorally and had worse outcomes when switched to placebo (12). Among patients whose total NPI score was >14 at baseline, those in the placebo arm manifested more neuropsychiatric symptoms than did those in the antipsychotic treatment arm by 12 months of follow-up (13). As previously reported in the ADAD trial, total NPI score did not predict future relapse (6), and the present post hoc analyses indicate that presence of hallucinations at baseline specifically, rather than the total NPI score, was predictive of relapse.
There are several hypothesized mechanisms for the occurrence of hallucinations in Alzheimer’s disease, including receptor changes, denervation, dysregulation of image perception/production, and a combination of attention and processing disturbances (14). One study used data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) with brain MRI and fluorodeoxyglucose positron emission tomography (FDG-PET) and found that Alzheimer’s patients with hallucinations had greater brain atrophy and more hypometabolism than did those without hallucinations (14). The areas of structural change included the left superior frontal gyrus and the lingual gyri, with increased hallucination intensity associated with greater atrophy in the right precentral gyrus, right superior temporal gyrus, and left precuneus. Increased hallucination intensity was also associated with extensive hypometabolism on FDG-PET in the right frontal lobe, orbitofrontal region, and insula and in the left midcingulate gyrus. Alzheimer’s patients with hallucinations had overlapping regions of focal atrophy and metabolic changes in the right anterior insula and inferior frontal gyrus (14). These findings suggest that Alzheimer’s patients with hallucinations have a wide neuronal network of dysfunction in brain regions involved in sensory processing, judgment, and attention (14). We did not conduct functional brain imaging during the ADAD trial, and the magnitude and complexity of involvement of different brain regions in the prediction of relapse may need further investigation.
There were some limitations to this study. This was the largest prospective randomized controlled trial of antipsychotic discontinuation in Alzheimer’s disease to date, but the size of the study sample decreased over time because patients who relapsed immediately exited the study, according to the study protocol. This is a difficult group of patients to enroll and treat in a research protocol, and other antipsychotic withdrawal trials with smaller samples have had higher dropout rates (15). The study inclusion criteria led to nearly all patients having agitation/aggression at baseline, and as a result, the comparison group of patients without agitation/aggression was small. Therefore, the present/absent classification of NPI domains, particularly for agitation/aggression, became less useful in the prediction of relapse after antipsychotic withdrawal, and examination of severity of symptoms became more important. In the open-label treatment phase, the average dosage of risperidone was 0.97 mg/day, which is similar to the average dosage of 1 mg/day used in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, in which dosing was also based on doctor’s choice (16). Nonetheless, we cannot rule out the possibility that dosing was lower or higher than optimal for each patient. We did not utilize a statistical correction for multiple comparisons, but the main finding of severe hallucinations at baseline predicting an increased risk of relapse would have survived a Bonferroni correction (Table 2).
Prediction of relapse after treatment response is clinically important because concerns about antipsychotic side effects and increased mortality have led to increased scrutiny of antipsychotic use and federal regulations designed to further decrease their use (5). Antipsychotic discontinuation should be considered in many patients in order to decrease morbidity and mortality due to side effects, but it is important to recognize that many patients respond well to antipsychotics without problematic side effects, and maintaining these patients on antipsychotics may be advantageous (6). In line with the goals of precision medicine, it is essential for clinicians to identify the patients who are likely to demonstrate an advantageous benefit-to-risk ratio with antipsychotic treatment. Our findings in this study indicate that the subgroup of patients with hallucinations is at a high risk of relapse after discontinuation of antipsychotic treatment. In patients with dementia and severe psychotic symptoms, particularly auditory hallucinations, subsequent discontinuation after treatment response, if attempted, should be accompanied by close monitoring for symptom recurrence. Antipsychotic medication may need to be promptly reinstated if relapse occurs.
1 : Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783Crossref, Medline, Google Scholar
2 : The clinical problem of neuropsychiatric signs and symptoms in dementia. Continuum (Minneap Minn) 2013; 19(2 Dementia):382–396Medline, Google Scholar
3 : The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry 1997; 54:257–263Crossref, Medline, Google Scholar
4 : ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008; 33:957–970Crossref, Medline, Google Scholar
5 Centers for Medicare and Medicaid Services: CMS announces partnership to improve dementia care in nursing homes. May 30, 2012. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2012-Press-releases-items/2012-05-30.htmlGoogle Scholar
6 : Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med 2012; 367:1497–1507Crossref, Medline, Google Scholar
7 : Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
8 : “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
9 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
10 : The efficiency of Cox’s likelihood function for censored data. J Am Stat Assoc 1977; 72:557–565Crossref, Google Scholar
11 : American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246Crossref, Medline, Google Scholar
12 : A 3-month, randomized, placebo-controlled, neuroleptic discontinuation study in 100 people with dementia: the Neuropsychiatric Inventory median cutoff is a predictor of clinical outcome. J Clin Psychiatry 2004; 65:114–119Crossref, Medline, Google Scholar
13 : A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med 2008; 5:e76Crossref, Medline, Google Scholar
14 : Right anterior insula: core region of hallucinations in cognitive neurodegenerative diseases. PLoS One 2014; 9:e114774Crossref, Medline, Google Scholar
15 : Withdrawal of haloperidol, thioridazine, and lorazepam in the nursing home: a controlled, double-blind study. Arch Intern Med 1999; 159:1733–1740Crossref, Medline, Google Scholar
16 : Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538Crossref, Medline, Google Scholar



