Phosphodiesterase 10A in Schizophrenia: A PET Study Using [11C]IMA107
Abstract
Objective:
Phosphodiesterase 10A (PDE10A) is an enzyme present in striatal medium spiny neurons that degrades the intracellular second messengers triggered by dopamine signaling. The pharmaceutical industry has considerable interest in PDE10A inhibitors because they have been shown to have an antipsychotic-like effect in animal models. However, the status of PDE10A in schizophrenia is unknown. Using a newly developed and validated radioligand, [11C]IMA107, the authors report the first in vivo assessment of PDE10A brain expression in patients with schizophrenia.
Method:
The authors compared PDE10A availability in the brains of 12 patients with chronic schizophrenia and 12 matched healthy comparison subjects using [11C]IMA107 positron emission tomography (PET). Regional estimates of the binding potential (BPND) of [11C]IMA107 were generated from dynamic PET scans using the simplified reference tissue model with the cerebellum as the reference tissue for nonspecific binding.
Results:
There was no significant difference in [11C]IMA107 BPND between schizophrenia patients and comparison subjects in any of the brain regions studied (thalamus, caudate, putamen, nucleus accumbens, globus pallidus, and substantia nigra). There was also no significant correlation between [11C]IMA107 BPND and the severity of psychotic symptoms or antipsychotic dosage.
Conclusions:
Patients with schizophrenia have normal availability of PDE10A in brain regions thought to be involved in the pathophysiology of this disorder. The findings do not support the proposal of an altered PDE10A availability in schizophrenia. The implication of this finding for future drug development is discussed.
Schizophrenia is a chronic and disabling psychiatric illness, and despite extensive research, its molecular etiology remains unknown (1, 2). The current dopamine hypothesis postulates that excessive striatal dopamine transmission and reduced frontal dopamine stimulation underlie the pathophysiology of positive and negative symptoms, respectively (3, 4). All currently approved treatments (antipsychotic medications) are primarily antagonists at dopamine D2 receptors (5–7), and because they do little to enhance frontal dopamine transmission, they have limited effect against negative and cognitive symptoms. Therefore, the field is increasingly looking beyond the dopamine system to identify new targets and medications that improve both positive and negative symptoms (8). A promising strategy to modulate dopamine transmission without blocking D2 receptors is by acting at a postsynaptic receptor target, such as the phosphodiesterase (PDE) enzymes.
PDEs are a class of enzymes that degrade the intracellular second messengers cAMP and cGMP, which are triggered by receptor stimulation. The PDE10A enzyme is found in neurons expressing dopamine D1 and D2 receptors, thereby providing cellular and functional specificity for the dopamine system (9, 10). The inhibition of PDE10A in the medium spiny neurons of the striatum enhances functional output of the direct striatonigral pathway, functionally enhancing dopamine D1-related transmission, and it constrains the functional output of the indirect striatopallidal pathway by inhibiting D2 neurotransmission (11). Thus, in theory, PDE10A inhibitors may provide an ideal antipsychotic effect by reducing positive symptoms (via D2-antagonism-like effects) while simultaneously improving negative and cognitive symptoms (via enhancing D1-dependent neurotransmission) (see Figure S1 in the data supplement that accompanies the online edition of this article).
This notion is consistent with preclinical data demonstrating antipsychotic-like activity of PDE10A antagonists. Inhibition of PDE10A by papaverine and by more recent selective PDE10A inhibitors, such as TP-10 and MP-10, constrains psychostimulant-induced hyperactivity, constrains conditioned avoidance response, and shows positive results in several known animal models of positive symptoms in schizophrenia (10–14). Because PDE10A inhibitors augment D1-related signaling via the direct pathway, there has been considerable interest in their effect on cognitive and negative symptoms of schizophrenia, with PDE10A inhibitors showing a reversal of memory deficits induced by N-methyl-d-aspartate receptor antagonists such as phencyclidine, dizocilpine, and ketamine in rats and nonhuman primates (15–17).
This preclinical evidence has encouraged nearly 20 pharmaceutical companies to publish patent applications for PDE10A inhibitors, and five of these companies have reported clinical development of PDE10A inhibitors for the treatment of schizophrenia (18). Nonetheless, despite the biological relevance, preclinical evidence, and extensive investment from the drug companies, very little is known about PDE10A availability in patients with schizophrenia because of the absence of a suitable PET tracer for imaging of these receptors in the living human brain. Also, no study to our knowledge has investigated the availability of PDE10A in postmortem schizophrenia brain.
We recently developed a selective PET radiotracer ([11C]IMA107) and used it to image the PDE10A system in healthy comparison subjects, allowing robust quantification of PDE10A availability and the suitability of a simplified reference tissue model (19). In this study, we report the first investigation of PDE10A availability in patients with schizophrenia using [11C]IMA107.
Method
Participants
The study protocol was approved by the National Research Ethics Service, and permission to administer radioactive substances was granted by the U.K. Administration of Radioactive Substances Advisory Committee. All participants gave written informed consent to participate after receiving a full description of the study.
Patients with schizophrenia were recruited from the South London and Maudsley Foundation National Health Service Trust. Healthy comparison subjects were recruited from the same catchment area; they were assessed with the Psychosis Screening Questionnaire (20) and were excluded if they reported any psychotic symptom or a history of psychotic illness.
All subjects had a physical, psychiatric, and neurological examination. Drug screening tests were done on the days of the scans to identify the use of psychoactive drugs. Exclusion criteria included use of psychoactive drugs; a history of head trauma or injury with loss of consciousness longer than 1 hour; organic psychosis; learning disabilities; lack of English fluency; treatment with clozapine; consuming more than 500 mg/day of caffeine; and a history of substance abuse or dependence. None of the participants had a history of other neurological or psychiatric disorders other than schizophrenia in the patient group, and none were receiving treatment with substances with known actions on PDEs.
Clinical Assessment
All patients met DSM-IV criteria for schizophrenia, paranoid subtype, as determined by a review of their medical notes using the Operational Criteria Checklist for Psychotic Illness and Affective Illness (21).
None of the patients had experienced a symptomatic relapse, and all were on a stable dosage of an antipsychotic medication for at least 4 weeks prior to the screening visit. Three patients were taking first-generation antipsychotics (one each was taking flupentixol, pipotiazine, and fluphenazine), and the remaining nine patients were taking second-generation antipsychotics (four were taking risperidone, three were taking aripiprazole, and one each was taking amisulpride and paliperidone). Antipsychotic dosages were converted to chlorpromazine equivalents (22, 23). Clinical measures recorded at the PET scan included a physical examination, urine drug screen, medication history, and measurements of antipsychotic blood levels. Psychotic symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS) (24).
Image Acquisition
MRI and PET imaging were performed at Imanova in London. Participants were instructed to refrain from ingesting caffeine, tobacco, and alcohol for at least 12 hours before scanning. All participants were scanned on a Siemens Biograph Hi-Rez 6 PET-CT scanner (Erlangen, Germany) after the injection of an intravenous bolus [11C]IMA107 (mean radioactivity=268.25 MBq, SD=50.6; mean mass injected=3.1 μg, SD=1.74). Dynamic emission data were acquired continuously for 90 minutes following the injection of [11C]IMA107. The dynamic images were reconstructed with in-house software into 26 frames (8×15 seconds, 3×60 seconds, 5×120 seconds, 5×300 seconds, and 5×600 seconds), using a filtered back projection algorithm (direct inversion Fourier transform) with a matrix of 128 and a zoom of 2.6, producing images with an isotropic voxel size of 2×2×2 mm3, and a transaxial Gaussian filter of 5 mm.
MRI scans were acquired with a 32-channel head coil on a Siemens Magnetom Verio 3-T scanner and included a T1-weighted magnetization prepared rapid gradient echo sequence (time repetition=2300 ms, time echo=2.98 ms, flip angle=9°, time to inversion=900 ms, matrix=240×256) for coregistration with the PET images; fast GM T1 inversion recovery (time repetition=3000 ms, time echo=2.96 ms, flip angle=8°, time to inversion=409 ms, matrix=240×256); and fluid and white matter suppression (time repetition=5000 ms, time echo=2.94 ms, flip angle=5°, time to inversion=409/1100 ms, matrix=240×256) sequences for improving delineation of subcortical brain regions. All sequences used a 1-mm3 voxel size, anteroposterior phase encoding direction, and a symmetric echo.
[11C]IMA107 PET Data Processing
Movement minimization and correction.
Subjects were positioned supine with their transaxial planes parallel to the line intersecting the anterior-posterior commissure line. Head position was maintained with the help of individualized foam holders and monitored by video, with repositioning if movement was detected. Subjects were in a resting state with low light. Intrascan notes on participant movement were acquired during scanning.
Data analysis.
Data were analyzed using Imanova’s in-house MIAKAT software package (version 3.3.8). MIAKAT is implemented using MATLAB (version R2008b; The MathWorks, Inc., Natick, Mass.), and it makes use of the FMRIB Software Library (version 4.1.9; http://www.fmrib.ox.ac.uk/fsl/) (25, 26) functions for brain extraction and of SPM5 (http://www.fil.ion.ucl.ac.uk/spm) for image segmentation and registration. MIAKAT implements a robust and consistent analysis pipeline with built-in audit trail and prespecified quality-control points whereby the analyst is required to inspect results of intermediate stages (e.g., checking the success of automated brain extraction or assessing the quality of model fits).
Image processing and definition of regions of interest.
Each subject’s structural MRI image underwent brain extraction and gray matter segmentation, and it was coregistered to a standard reference space (MNI152) (27). The MNI152 template brain image and associated atlas (CIC atlas) (28) were nonlinearly warped to the subject’s MR image to enable automated definition of regions of interest.
The regions of interest defined in this manner were the dorsal caudate, dorsal putamen, nucleus accumbens, globus pallidus, thalamus, and cerebellum. In addition, the substantia nigra was manually delineated by reference to both fluid and white matter suppression and PET summed images using Analyze, version 4 (Mayo Foundation).
Dynamic PET images were registered to each subject’s MRI scan and corrected for motion using a frame-to-frame registration process with a normalized mutual information cost function. Regions of interest were applied to the dynamic PET data to derive regional time-activity curves.
Kinetic modeling.
We recently demonstrated the suitability of the cerebellum as a reference region for determining the regional estimation of [11C]IMA107 BPND (19). Therefore, regional time-activity curve data extracted from the PET images were fitted to the simplified reference tissue model (29). The simplified reference tissue model assumes a reference region (here, the cerebellum) that is similar to the target-rich regions, except that it is devoid of the target receptor. The basis function implementation of the simplified reference tissue model employed here (30) directly estimates the binding potential relative to the nondisplaceable component (BPND), which can be thought of as a measure of specific binding (31). The simplified reference tissue model was also used to generate parametric images, again using a basis function method implemented in MIAKAT.
Statistical Analysis
Statistical analysis was performed with SPSS, version 20 (IBM, Armonk, N.Y.). For all variables, variance homogeneity and normal distribution were tested with Kolmogorov-Smirnov tests, and we proceeded with parametric tests because our PET and clinical data were normally distributed. To determine whether there was an effect of group on BPND values and on sociodemographic and clinical data, analysis of variance and independent t tests were performed where appropriate. We interrogated correlations between PET and clinical data using Pearson’s r. All data are presented as means and standard deviations, and the alpha level was set for all comparisons at a corrected p threshold of 0.05.
Results
Twelve individuals with schizophrenia and 12 healthy comparison subjects were studied. The participants’ demographic and clinical characteristics are summarized in Table 1. All patients were on an antipsychotic medication at the time of the scan, and antipsychotic blood levels were within the normal range with reference to standardized laboratory values. Psychotic symptoms were evaluated using the PANSS (mean=61.5, SD=9.7). No between-group significant differences were observed in age, gender, ethnicity, weight, or radiation dose received. There were also no significant differences between groups in nicotine or caffeine intake.
| Measure | Schizophrenia Patients (N=12) | Healthy Comparison Subjects (N=12) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Male | 8 | 67 | 8 | 67 |
| Right-handed | 12 | 100 | 12 | 100 |
| Education | ||||
| High school diploma (GCSE) | 6 | 50 | 2 | 17 |
| High school diploma, advanced (A-level) | 3 | 25 | 4 | 33 |
| University | 3 | 25 | 6 | 50 |
| Antipsychotics used (% of atypicals) | 9 | 75 | ||
| Mean | SD | Mean | SD | |
| Age at scan (years) | 33.16 | 6.88 | 32.58 | 7.06 |
| Cotinine blood level (ng/ml) | 372.90 | 345.30 | 296.70 | 287.90 |
| Caffeine blood level (mg/L) | 3.10 | 0.60 | 3.08 | 0.23 |
| Positive and Negative Syndrome Scale score | 61.50 | 9.70 | ||
| Antipsychotic dosage (mg per day of chlorpromazine equivalents) | 259.70 | 169.90 | ||
| Radioactivity injected (MBq) | 275.30 | 43.50 | 261.20 | 57.90 |
| Specific activity (GBq/μmol) | 2.95 | 1.74 | 3.25 | 1.80 |
TABLE 1. Demographic and Clinical Characteristics of Schizophrenia Patients and Healthy Comparison Subjects in a PET Study of PDE10Aa
We found no significant differences in [11C]IMA107 BPND between the schizophrenia patients and healthy comparison subjects in any of the brain regions. Patients with schizophrenia showed no significant changes in [11C]IMA107 BPND in the caudate (−1%), putamen (6%), globus pallidus (2%), thalamus (8%), nucleus accumbens (−2%), and substantia nigra (−6%) compared with the healthy comparison subjects (Figure 1; Table 2).
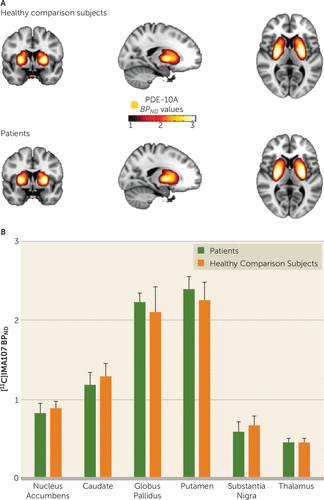
FIGURE 1. Differences in [11C]IMA107 BPND Between Schizophrenia Patients and Healthy Comparison Subjects in a PET Study of PDE10Aa
a Panel A shows mean [11C]IMA107 BPND parametric images derived from 12 healthy comparison subjects (top) and 12 chronic schizophrenia patients (bottom) in stereotaxic space overlaid onto the T1-weighted Montreal Neurological Institute template. The graph in panel B shows mean [11C]IMA107 BPND in anatomically defined brain regions between chronic schizophrenia patients and healthy comparison subjects. Error bars represent standard deviation.
| Schizophrenia Patients (N=12) | Healthy Comparison Subjects (N=12) | % Change in Patientsa | |||
|---|---|---|---|---|---|
| Region of Interest | Mean | SD | Mean | SD | |
| Thalamus | 0.47 | 0.09 | 0.43 | 0.09 | 8 |
| Substantia nigra | 0.63 | 0.16 | 0.67 | 0.18 | –6 |
| Caudate | 1.26 | 0.20 | 1.28 | 0.26 | –1 |
| Putamen | 2.39 | 0.35 | 2.26 | 0.35 | 6 |
| Nucleus accumbens | 0.87 | 0.18 | 0.88 | 0.15 | –2 |
| Globus pallidus | 2.20 | 0.30 | 2.17 | 0.40 | 2 |
TABLE 2. Comparison of [11C]IMA107 BPND Between Patients With Schizophrenia and Healthy Comparison Subjects in a PET Study of PDE10A
Correlations Between PDE-10A Availability and Clinical Characteristics
No significant correlation was found between [11C]IMA107 BPND and severity of psychotic symptoms, as assessed by the PANSS, in any region (Figure 2).
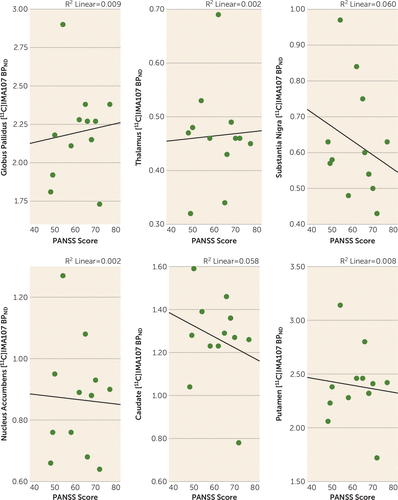
FIGURE 2. [11C]IMA107 BPND in Anatomically Defined Brain Regions and Severity of Psychotic Symptoms in a PET Study of PDE10Aa
a The scatterplots show correlations between [11C]IMA107 BPND in anatomically defined brain regions and Positive and Negative Syndrome Scale (PANSS) scores in chronic schizophrenia patients.
We also found no significant correlation between [11C]IMA107 BPND and exposure to antipsychotics, as measured in chlorpromazine equivalents, in any region (Figure 3).
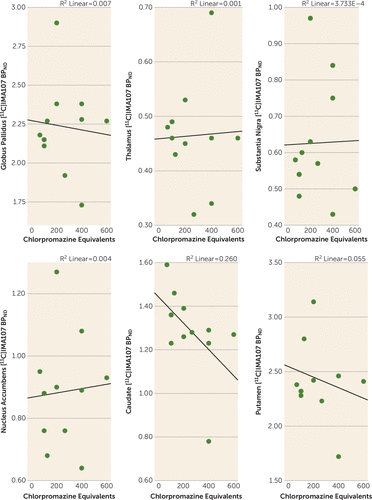
FIGURE 3. [11C]IMA107 BPND in Anatomically Defined Brain Regions and Exposure to Antipsychotics in a PET Study of PDE10Aa
a The scatterplots show correlations between [11C]IMA107 BPND in anatomically defined brain regions and medication dosage, converted to chlorpromazine equivalents, in chronic schizophrenia patients.
Discussion
To our knowledge, this is the first PET study to investigate the status of PDE10A in the brains of individuals with schizophrenia. We found no differences between patients and healthy comparison subjects and no association between PDE10A availability and clinical variables or medication exposure.
Before we discuss the implications of this finding, we should address the potential limitations of this study. [11C]IMA107 is a newly developed selective PET radiotracer. Nevertheless, it has demonstrated specific binding to the PDE10A enzyme, both in animal studies and in healthy volunteers (19). Furthermore, its high signal-to-noise ratio, reversible kinetics, and good brain penetration suggest that [11C]IMA107 has optimal properties to assess PDE10 availability (19). A potential limitation is the risk of a type II error because the relatively small sample size may not have provided adequate power to detect significant group differences. However, a post hoc power analysis shows that we had over 95% power to detect a 25% difference in PDE10A availability between groups, using the mean and variability of [11C]IMA107 BPND from our data. Although smaller differences between groups (i.e., less than 10%) cannot be rejected with our sample size, the clinical significance and implications of a smaller difference may be biologically questionable. Furthermore, some areas of the striatum, such as the caudate and the globus pallidus, showed a difference in PDE10A availability of less than 3% between groups, supporting our conclusion that availability is similar between the patients and the healthy comparison subjects. Finally, the prolonged exposure to antipsychotics in our patient sample could affect [11C]IMA107 binding. Because the PDE10A system acts downstream of the dopamine D2 receptors, it is possible that chronic D2 receptor blockage could alter PDE levels in the brain. Dlaboga et al. (32) reported that chronic treatment with haloperidol and clozapine increases striatal PDE10A protein expression in rats, suggesting that the up-regulation of PDE10A may also occur as a compensatory response to the effect of current treatments on cyclic nucleotide signaling. However, Dlaboga et al. did not measure enzyme levels using a binding marker; therefore, we examined this possibility (33) in a more recent animal PET study using [11C]MP-10, a selective and potent PDE10A inhibitor with good brain penetration and regional binding, combined with ex vivo tissue analysis for mRNA, protein levels, and enzyme activity. In that study, after 3 weeks of chronic haloperidol treatment in rodents, the PET imaging results showed no evidence of PDE10A enzyme elevation in the whole rodent striatum. Thus, as that study used the technique most comparable to our human PET data, it seems unlikely that antipsychotic use confounded the results. Investigating first-episode patients prior to the start of antipsychotic treatment could provide an unambiguous picture.
These results should also be discussed in the context of previous preclinical and human data. As a relatively newly characterized protein, PDE10A has not been part of any extensive human postmortem analysis of its potential role in schizophrenia. There are no preclinical studies showing a change in PDE10A availability in animal models of schizophrenia. The majority of the studies conducted so far have repeatedly demonstrated that PDE10A inhibitors produce behavioral effects predictive of antipsychotic activity, similar to D2 antagonists (10, 12, 13, 34–36). Thus, while our results cannot contradict the animal findings, they do not provide any support for PDE10A-based antipsychotics. These findings are then of interest in light of a recently completed trial of the first PDE10 inhibitor (MP-10) in schizophrenia (ClinicalTrials.gov identifier: NCT01175135). In this 4-week trial, MP-10 failed to differentiate from placebo in subjects with an acute exacerbation of schizophrenia. Our results complement these findings by indicating that there is no major alteration in PDE10A in schizophrenia.
However, it is important that our findings not be interpreted as a case against developing PDE10A drugs in schizophrenia. As we noted in the introduction, the study of intracellular signaling pathways makes a persuasive case for how PDE10A inhibitors, regardless of whether there is an intrinsic change in PDE10 in schizophrenia, could influence the overall signaling in a therapeutic direction. In fact, dopamine D2 blocking drugs have been effective antipsychotics with limited, if any, support for an intrinsic increase in the levels of dopamine D2 receptors (7). Similarly, selective serotonin reuptake inhibitors are effective treatments in depression without a primary pathology in these transporters. That said, those pursuing PDE10A in schizophrenia will have to do so knowing that it is at best a compensatory pathway, rather than a pathological mediator.
In conclusion, this work provides evidence that patients with schizophrenia have normal availability of PDE10A in brain regions thought to be involved in the pathophysiology of this disorder. The findings of this study provide critical information for the ongoing therapeutic developments for schizophrenia.
1 : Catching up on schizophrenia: natural history and neurobiology. Neuron 2000; 28:325–334Crossref, Medline, Google Scholar
2 : Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005; 10:40–68Crossref, Medline, Google Scholar
3 : The dopamine hypothesis of schizophrenia: version III: the final common pathway. Schizophr Bull 2009; 35:549–562Crossref, Medline, Google Scholar
4 : Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474–1486Link, Google Scholar
5 : Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27:1081–1090 Crossref, Medline, Google Scholar
6 : Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 2005; 10:79–104Crossref, Medline, Google Scholar
7 : The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 2012; 69:776–786Crossref, Medline, Google Scholar
8 : Building a better antipsychotic: receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics 2009; 6:78–85Crossref, Medline, Google Scholar
9 : Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 2006; 58:488–520Crossref, Medline, Google Scholar
10 : The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs 2008; 22:983–993Crossref, Medline, Google Scholar
11 : Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology 2006; 51:374–385Crossref, Medline, Google Scholar
12 : Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther 2008; 325:681–690Crossref, Medline, Google Scholar
13 : Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J Pharmacol Exp Ther 2009; 331:574–590Crossref, Medline, Google Scholar
14 : PDE10A inhibitors: novel therapeutic drugs for schizophrenia. Curr Pharm Des 2011; 17:137–150Crossref, Medline, Google Scholar
15 : Inhibition of phoshodiesterase type 2 or type 10 reverses object memory deficits induced by scopolamine or MK-801. Behav Brain Res 2013; 236:16–22Crossref, Medline, Google Scholar
16 : The novel phosphodiesterase 10A inhibitor THPP-1 has antipsychotic-like effects in rat and improves cognition in rat and rhesus monkey. Neuropharmacology 2013; 64:215–223Crossref, Medline, Google Scholar
17 : PDE10A inhibition reverses subchronic PCP-induced deficits in attentional set-shifting in rats. Eur J Neurosci 2005; 21:1070–1076Crossref, Medline, Google Scholar
18 : Phosphodiesterase 10A inhibitors: a 2009 - 2012 patent update. Expert Opin Ther Pat 2013; 23:31–45Crossref, Medline, Google Scholar
19 : Phosphodiesterase 10A PET radioligand development program: from pig to human. J Nucl Med 2014; 55:595–601Crossref, Medline, Google Scholar
20 : The psychosis screening questionnaire. Int J Methods Psychiatr Res 1995; 5:11–20Google Scholar
21 : A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48:764–770Crossref, Medline, Google Scholar
22 : Chlorpromazine equivalents: a consensus of opinion for both clinical and research implications. Psychiatr Bull 1997; 21:224–226Crossref, Google Scholar
23 : Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64:663–667Crossref, Medline, Google Scholar
24 : The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
25 : Fast robust automated brain extraction. Hum Brain Mapp 2002; 17:143–155Crossref, Medline, Google Scholar
26 : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(suppl 1):S208–S219Crossref, Medline, Google Scholar
27 : Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 2006; 9:58–66Medline, Google Scholar
28 : Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 2011; 54:264–277Crossref, Medline, Google Scholar
29 : Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4:153–158Crossref, Medline, Google Scholar
30 : Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997; 6:279–287Crossref, Medline, Google Scholar
31 : Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27:1533–1539Crossref, Medline, Google Scholar
32 : Chronic haloperidol and clozapine produce different patterns of effects on phosphodiesterase-1B, -4B, and -10A expression in rat striatum. Neuropharmacology 2008; 54:745–754Crossref, Medline, Google Scholar
33 : Effect of chronic antipsychotic treatment on striatal phosphodiesterase 10A levels: a [11C]MP-10 PET rodent imaging study with ex vivo confirmation. Transl Psychiatry 2014; 4:e376Crossref, Medline, Google Scholar
34 : Phosphodiesterase 10A inhibitor MP-10 effects in primates: comparison with risperidone and mechanistic implications. Neuropharmacology 2014; 77:257–267Crossref, Medline, Google Scholar
35 : Genetic deletion of PDE10A selectively impairs incentive salience attribution and decreases medium spiny neuron excitability. Behav Brain Res 2014; 268:48–54Crossref, Medline, Google Scholar
36 : Increased social interaction in mice deficient of the striatal medium spiny neuron-specific phosphodiesterase 10A2. J Neurochem 2008; 105:546–556Crossref, Medline, Google Scholar



