A Randomized, Double-Blind, Placebo-Controlled Trial of Citicoline for Cocaine Dependence in Bipolar I Disorder
Abstract
Objective:
Although drug dependence is common in patients with bipolar disorder, minimal data are available on the treatment of drug dependence in this patient population. The authors previously reported a decreased risk of relapse to cocaine use in a pilot study of citicoline in patients with bipolar disorder and cocaine dependence. The primary aim of the present study was to determine whether citicoline reduces cocaine use in outpatients with bipolar I disorder and current cocaine dependence and active cocaine use.
Method:
A total of 130 outpatients with bipolar I disorder (depressed or mixed mood state) and cocaine dependence received citicoline or placebo add-on therapy for 12 weeks. Results of thrice-weekly urine drug screens were analyzed using a generalized linear mixed model that was fitted to the binary outcome of cocaine-positive screens at each measurement occasion for 12 weeks. Mood was assessed with the Inventory of Depressive Symptomatology–Self Report, the Hamilton Depression Rating Scale, and the Young Mania Rating Scale.
Results:
In the intent-to-treat sample (N=61 in both groups), significant treatment group and group-by-time effects were observed, whether or not missing urine screens were imputed as cocaine positive. The group effect was greatest early in the study and tended to decline with time. No between-group differences in mood symptoms or side effects were observed.
Conclusions:
Citicoline was well tolerated for treatment of cocaine dependence in patients with bipolar disorder. Cocaine use was significantly reduced with citicoline initially, although treatment effects diminished over time, suggesting the need for augmentation strategies to optimize long-term benefit.
Bipolar disorder is a severe and often chronic psychiatric illness affecting 1.3%–3.5% of the population (1–3). Substance use disorders are common in persons with bipolar disorder. Regier et al. (1) found a lifetime prevalence of 61% for substance abuse or dependence in patients with bipolar I disorder and 48% in patients with bipolar II disorder. This prevalence was substantially greater than the 6% prevalence reported in the general population (odds ratio=8), and more than twice that of persons with major depressive disorder. Cocaine dependence is particularly common among persons with bipolar disorder, with lifetime rates ranging from 15% to 39% (4). An analysis of 330 hospitalized patients with bipolar disorder found that cocaine was second only to alcohol among substances of abuse, with a current abuse or dependence rate of 7% and a lifetime rate of 20% (5).
The strong negative impact of substance use on bipolar disorder is well documented. Studies report increased hospitalization rates and lower rates of recovery during hospitalization (6–10), greater rates of aggression and violence (11, 12), and greater rates of medication nonadherence (13–15) in patients with bipolar disorder who have substance use disorders. In addition, a diagnosis of bipolar disorder is associated with a poor response to substance abuse treatment (12). Thus, patients with bipolar disorder and substance dependence are a large and challenging population and an important public health concern. However, little research has been conducted on the treatment of this dual-diagnosis population.
Few placebo-controlled studies have focused on the treatment of cocaine use in bipolar disorder. Brady et al. (16) reported greater reductions in cocaine-positive urine tests and depressive symptom severity with carbamazepine than with placebo in cocaine-dependent patients with mood disorders (N=57, mostly with major depressive disorder, but some with bipolar disorders), while a group of patients without mood disorders (N=82) did not show greater reductions in cocaine use than with placebo. These findings suggest that pharmacotherapy can be more effective in dual-diagnosis patients than in those with only cocaine dependence.
Our group has conducted several clinical trials in patients with bipolar disorder and cocaine dependence. In a small pilot study (17), we randomly assigned 12 outpatients with bipolar disorder and cocaine dependence to 12 weeks of either quetiapine or placebo. No significant between-group differences were observed in cocaine use or craving, but the quetiapine group had significantly fewer heavy drinking days than the placebo group, and depression scores showed a large effect size favoring quetiapine. In another study, we (18) randomly assigned 120 outpatients with bipolar disorder and cocaine dependence to lamotrigine or placebo for 10 weeks. The lamotrigine group reported significantly fewer dollars spent on cocaine than the placebo group, with a trend in the same direction for days of cocaine use. We recently reported on a placebo-controlled pilot study of citicoline in 44 outpatients with bipolar disorder and cocaine dependence (19). The citicoline group was significantly less likely to have a cocaine-positive urine sample at study exit than the placebo group (odds ratio=6.41). The completion rate for citicoline was more than twice that for placebo, and citicoline was associated with significantly fewer side effects than placebo.
These results are in contrast to the somewhat mixed findings on citicoline in the small studies in patients with cocaine dependence but without a mood disorder. Renshaw et al. (20) administered citicoline (1000 mg/day) or placebo for 2 weeks to 14 participants with cocaine dependence. The citicoline group tended to have greater reduction in cocaine craving than the placebo group. For example, after viewing a cocaine-related video, the citicoline-treated group showed less posttreatment “urge for cocaine” than the placebo-treated group (p=0.01). However, in a study of 29 patients with cocaine dependence randomly assigned to receive 8 weeks of citicoline (1000 mg/day) or placebo, Licata et al. (21) found that cocaine-positive urine tests were not significantly different between the citicoline and placebo groups, although a significant reduction in alcohol use was observed in the citicoline group.
Citicoline is sold as a prescription drug in Japan and Europe and over the counter as a dietary supplement in the United States (22). Citicoline has a mild side effect profile, and a Cochrane review observed that citicoline “tended to be associated with fewer adverse effects than placebo” in elderly patients with cognitive disorders (23). It is also relatively inexpensive and has no known drug-drug interactions (22). In animal models, citicoline has been found to increase incorporation of phospholipids into membranes, enhance the synthesis of structural phospholipids, and increase cerebral metabolism (24). In the rat brain, citicoline has been reported to increase norepinephrine levels in the cerebral cortex and hypothalamus, increase dopamine levels in the corpus striatum, increase serotonin levels in the cerebral cortex, striatum, and hypothalamus (25), and increase acetylcholine levels in the hippocampus and neocortex (26). Citicoline has been found to be neuroprotective in animal models of ischemia (27).
In this study, we compared citicoline to placebo in 130 patients with bipolar I disorder and cocaine dependence with active cocaine use. The primary aim was to determine whether citicoline was more effective than placebo in reducing cocaine use, as assessed by urine drug screen results. Secondary aims included evaluating the impact of citicoline on depressive and manic symptoms and study retention. Citicoline was selected for this study because of our promising pilot data in this population (19) and the literature suggesting that cholinergic systems are critical to cocaine dependence (28). In addition, citicoline’s favorable side effect profile and lack of known drug-drug interactions make it a potentially useful treatment option in dual-diagnosis patients.
Method
A 12-week, randomized, double-blind, parallel-group, placebo-controlled trial of citicoline was conducted in 130 outpatients with bipolar I disorder and cocaine dependence. The first participant was enrolled on May 1, 2008, and the final assessment was conducted on March 14, 2012; the trial was stopped when the predetermined enrollment goal was achieved. Potential participants were identified through physician referral and through flyers and brochures at clinics that treat the population needed for this study; assessments were conducted at the University of Texas Southwestern Medical Center. After receiving a complete description of the study, participants provided written informed consent, in accordance with the university’s institutional review board. The Structured Clinical Interview for DSM-IV, Clinician Version (SCID) (29), was used to establish the diagnoses of bipolar I disorder and cocaine dependence. Eligible participants were also assessed with the Addiction Severity Index (30), the Cocaine Craving Questionnaire (31), the 30-item Inventory of Depressive Symptomatology–Self Report (IDS-SR) (32), the 17-item Hamilton Depression Rating Scale (HAM-D) (33), the Young Mania Rating Scale (YMRS) (34), the Psychobiology of Recovery in Depression III–Somatic Symptom Scale (35), and a urine drug screen. At each weekly assessment, the HAM-D, IDS-SR, YMRS, and Somatic Symptom Scale were administered. Urine samples were obtained three times a week.
Changes in concomitant medications were managed through the use of a treatment algorithm developed for the study. The algorithm suggested considering a concomitant medication change if the participant’s HAM-D or YMRS score increased by >10 points from the previous weekly assessment. Adherence with study medication was assessed with the Medication Event Monitoring System (metered medication bottle caps) and pill counts. In addition, all participants received manual-based cognitive-behavioral therapy (CBT) (two sessions a week for 4 weeks followed by weekly sessions, for a total of 16 sessions) specifically designed for persons with bipolar disorder and substance abuse (36). The therapy was provided by a therapist with experience in CBT (either A.N., who received training in Houston by J.M.S., or an advanced clinical psychology graduate student under A.N.’s supervision). Citicoline (or identical placebo) was initiated at 500 mg/day and increased to 1000 mg/day at week 2, 1500 mg/day at week 4, and 2000 mg/day at week 6. Study subjects were paid for their participation. In addition, to minimize missing data, participants were given bonus vouchers for food and nonalcoholic beverages or for use in certain stores on an escalating payment scale for attending appointments and providing urine samples (payment was unrelated to urine screen results). The payments were reset to baseline if an appointment was missed (37).
Participants were adult outpatients with bipolar I disorder (depressed or mixed mood state, based on DSM-IV criteria using the SCID), current cocaine dependence with self-reported cocaine use within 7 days before baseline, a cocaine-positive urine screen at baseline, a baseline HAM-D score <35 and a baseline YMRS score <35 (to exclude those with severe mood symptoms), and current treatment with a mood stabilizer (lithium, divalproex/valproic acid, lamotrigine, carbamazepine, oxcarbazepine [a derivative of carbamazepine that may have mood-stabilizing properties] [38], quetiapine, risperidone, olanzapine, aripiprazole, or ziprasidone) at a stable dosage for at least 14 days. The study excluded vulnerable populations (e.g., inmates, pregnant women), patients who were medically unstable, patients who had initiated psychotropic medications or psychotherapy in the past 14 days or were receiving intensive outpatient treatment for substance abuse, individuals whose current symptoms included psychotic features (delusions, hallucinations, or disorganized thought processes) as defined using DSM-IV criteria, individuals at high risk of suicide (defined as any suicide attempt in the past 6 months, current suicidal ideation with plan and intent, or a score ≥2 on the suicide item of the HAM-D), and individuals whose drug of choice was not cocaine. Urine samples were obtained on Monday, Wednesday, and Friday when possible. Quantitative urine drug screens were performed by Redwood Toxicology Laboratory (Santa Rosa, Calif.), with initial screening done using an enzyme immunoassay procedure for benzoylecgonine (cutoff, 300 ng/mL) and confirmation of positive samples done with gas chromatography and mass spectrometry.
Statistical Analysis
Randomization (1:1 allocation) was performed by a statistician using a random number sequence. All study personnel who had contact with participants (e.g., raters, physicians) were blind to treatment assignment, as were the participants themselves. A staff member who had no participant contact (T.H.) maintained a password-protected randomization list. Demographic and baseline characteristics were compared between groups using independent-sample t tests or chi-square tests. The primary outcome measure was the presence or absence of a cocaine-positive urine screen. Urine drug screen data were analyzed using a random regression for binary outcome (presence or absence of cocaine) at each visit, using SAS Proc GLIMMIX. All participants who completed the baseline assessment and at least one additional assessment were included in the primary analysis (the intent-to-treat sample). The random regression analysis was conducted with the treatment group (citicoline and placebo) as the between-subject factor, time as the within-subject factor, and a group-by-time interaction. In the predetermined primary analysis of urine screens, missing data were imputed as cocaine positive. We also conducted a predetermined secondary analysis of the data that did not make assumptions about missing data. The thrice-weekly urine drug screen data were collapsed into a weekly score that was coded as positive if at least one sample during the week was cocaine positive. In addition, a post hoc analysis of the percentage in each group with no postbaseline cocaine-positive urine screens was conducted using a chi-square analysis. Number needed to treat was also calculated (39, 40).
HAM-D, IDS-SR, and YMRS scores (secondary outcome measures) were compared between the citicoline and placebo groups with an intent-to-treat sample and using a random regression analysis for continuous data. Retention was assessed between groups using a Kaplan-Meier survival curve. An a priori alpha of 0.05 was used for all analyses.
The sample size estimate was based on comparing the slopes for the two groups on the primary outcome measure and urine drug screens in an earlier pilot study (19). Group sample sizes of 65 in each group achieved 80% power to detect a standardized difference between the slopes of 0.44 (medium effect size), assuming that the standardized regression slope is −0.10 for the placebo group and −0.54 for the treatment group using a two-sided test with a significance threshold of 0.05. The study was not powered for secondary analyses.
Results
The baseline demographic and clinical characteristics of the two treatment groups (intent-to-treat sample) are summarized in Table 1. There were no statistically significant differences between groups except that the mean duration of cocaine use was longer in the placebo group. Baseline concomitant medications were similar in the two groups. Lamotrigine (included in the category “anticonvulsants” in Table 1), the only medication that to our knowledge has shown some efficacy in reducing cocaine use in patients with bipolar disorder in a placebo-controlled trial (18), was taken by six participants (9.8%) in the citicoline group and five (8.2%) in the placebo group.
| Characteristic | Citicoline (N=61) | Placebo (N=61) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 41.1 | 9.1 | 43.6 | 8.3 |
| Education (years) | 12.5 | 2.4 | 13.2 | 1.9 |
| Time since onset of bipolar disorder (years) | 20.02 | 9.5 | 20.12 | 9.6 |
| Duration of cocaine use (years)a | 16.1 | 7.6 | 21.3 | 9.9 |
| % Days of cocaine use, past 2 weeks | 36.0 | 30.0 | 36.0 | 30.0 |
| N | % | N | % | |
| Female | 16 | 26.2 | 24 | 39.3 |
| Race/ethnicity | ||||
| Caucasian | 17 | 27.9 | 28 | 45.9 |
| African American | 41 | 67.2 | 30 | 49.2 |
| Hispanic | 2 | 3.3 | 3 | 4.9 |
| Other | 1 | 1.6 | 0 | 0.0 |
| Current mood state | ||||
| Depressed | 58 | 95.1 | 61 | 100.0 |
| Mixed | 3 | 4.9 | 0 | 0.0 |
| Route of cocaine use | ||||
| Smoking (crack cocaine) | 31 | 50.8 | 38 | 62.3 |
| Otherb | 15 | 24.6 | 14 | 23.0 |
| Unknown | 15 | 24.6 | 9 | 14.8 |
| Other current substance use disordersc | ||||
| Alcohol | 36 | 59.0 | 38 | 62.3 |
| Opioids | 2 | 3.3 | 2 | 3.3 |
| Sedatives, hypnotics, or anxiolytics | 3 | 4.9 | 2 | 3.3 |
| Cannabis | 33 | 54.1 | 23 | 37.7 |
| Hallucinogens | 2 | 3.3 | 0 | 0.0 |
| Amphetamines | 3 | 4.9 | 6 | 9.8 |
| Otherd | 6 | 9.8 | 4 | 6.6 |
| Classes of concomitant medications | ||||
| Lithium | 26 | 42.6 | 23 | 37.7 |
| Anticonvulsants | 29 | 47.5 | 29 | 47.5 |
| Antidepressants | 25 | 41.0 | 27 | 44.3 |
| Antipsychotics | 25 | 41.0 | 33 | 54.1 |
| Anxiolytics | 12 | 19.7 | 9 | 14.8 |
| Hypnotics | 19 | 31.1 | 14 | 23.0 |
| Stimulants | 0 | 0.0 | 1 | 1.6 |
| Mean | SD | Mean | SD | |
| Number of concomitant medications | 2.6 | 1.4 | 2.3 | 1.3 |
| Hamilton Depression Rating Scale score | 17.9 | 5.6 | 18.0 | 6.3 |
| Young Mania Rating Scale score | 10.2 | 5.9 | 10.1 | 6.1 |
| Inventory of Depressive Symptomatology–Self Report score | 33.8 | 23.6 | 29.4 | 27.1 |
| Addiction Severity Index subscale scorese | ||||
| Medical | 0.3 | 0.3 | 0.4 | 0.4 |
| Employment | 0.8 | 0.2 | 0.8 | 0.2 |
| Alcohol | 0.1 | 0.2 | 0.2 | 0.2 |
| Drug | 0.2 | 0.1 | 0.2 | 0.1 |
| Legal | 0.1 | 0.2 | 0.1 | 0.2 |
| Family | 0.2 | 0.2 | 0.3 | 0.3 |
| Psychiatric | 0.5 | 0.2 | 0.5 | 0.2 |
TABLE 1. Baseline Demographic and Clinical Characteristics of Participants With Bipolar Disorder and Cocaine Dependence Who Received Citicoline or Placebo (Intent-to-Treat Sample)
Table 2 presents results of a generalized linear mixed model fitted to the binary outcome of cocaine-positive urine drug screens, with missing values imputed as cocaine positive. During the time they were in the study, 59.0% of the citicoline group and 49.2% of the placebo group had at least one urine drug screen for every study week; urine screens were missing for more than half of the study weeks for 16.4% of the citicoline group and 19.7% of the placebo group. Significant treatment group (F=5.2, df=1, 1351, p=0.022) and group-by-time effects (F=5.9, df=1, 1351, p=0.015) were observed. As shown in Figure 1, the between-group difference in cocaine-positive urine screens was greatest early in the study and tended to decline with time. The number needed to treat was 6.9 at week 1, 11.1 at week 6, and 43.8 at week 12. Similar results were obtained for group (F=4.5, df=1, 1057, p=0.035) and group-by-time effects (F=11.2, df=1, 1057, p<0.001) in the secondary analysis that did not impute missing values. A total of 19.7% (12/61) of the placebo group and 23.0% (14/61) of the citicoline group (n.s.) were total cocaine abstainers. No significant group or group-by-time effects were observed on the Cocaine Craving Questionnaire, the HAM-D, the IDS-SR, or the YMRS.
| Outcome Measure | F | df | p |
|---|---|---|---|
| Urine drug screen positive for cocaine (with missing data imputed as positive) | |||
| Treatment group | 5.2 | 1, 1351 | 0.022 |
| Treatment group by time | 5.9 | 1, 1351 | 0.015 |
| Urine drug screen positive for cocaine (with no imputed data) | |||
| Treatment group | 4.5 | 1, 1057 | 0.035 |
| Treatment group by time | 11.2 | 1, 1057 | <0.001 |
| Cocaine Craving Questionnaire | |||
| Treatment group | 2.4 | 1, 108 | 0.127 |
| Treatment group by time | 1.3 | 1, 101 | 0.249 |
| Hamilton Depression Rating Scale | |||
| Treatment group | 0.0 | 1, 106 | 0.830 |
| Treatment group by time | 0.8 | 1, 103 | 0.380 |
| Inventory of Depressive Symptomatology–Self Report | |||
| Treatment group | 1.5 | 1, 111 | 0.216 |
| Treatment group by time | 0.2 | 1, 90 | 0.632 |
| Young Mania Rating Scale | |||
| Treatment group | 0.0 | 1, 105 | 0.976 |
| Treatment group by time | 0.1 | 1, 97 | 0.768 |
TABLE 2. Results of Between-Group Analysis for Participants With Bipolar Disorder and Cocaine Dependence Who Received Citicoline (N=61) or Placebo (N=61)
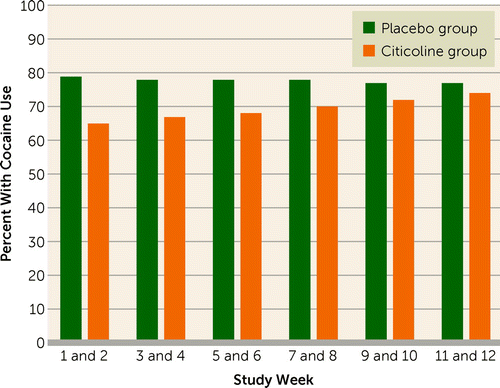
FIGURE 1. Percent of Participants With Bipolar Disorder and Cocaine Dependence Receiving Citicoline (N=61) or Placebo (N=61) Who Had Cocaine-Positive Urine Samples, Averaged Over 2-Week Intervalsa
a Based on an imputed data model in which missing urine samples for any given week were assumed to be positive.
Citicoline was well tolerated. No between-group differences were observed on the Somatic Symptom Scale. A total of 13 serious adverse events were recorded during the study, five in the citicoline group and eight in the placebo group. Adverse events in the citicoline group were an emergency room visit related to a foot injury sustained before study enrollment, a sexual assault, hospitalization related to a motor vehicle accident, and two separate hospitalizations for flu-like symptoms in one participant. In the placebo group, adverse events were a hospitalization due to a motor vehicle accident, active suicidal ideation in two participants, a pulmonary embolism, leucopenia and thrombocytopenia, chest pain secondary to cocaine use, an ankle injury caused by a golf ball, and a hospitalization for nausea, emesis, and vertigo.
Figure 2 presents the Kaplan-Meier survival curve. Results of a log-rank test indicated no significant between-group difference in study survival. Completion rates were 71% for the citicoline group and 57% for the placebo group. Study drug adherence, defined as the total number of times the medication bottle was opened (as monitored with the Medication Event Monitoring System cap) divided by the number of times it should have been opened, was 82.3% for the citicoline group and 79.2% for the placebo group (n.s.). Some concomitant medication changes occurred in both groups, but the between-group differences were not statistically significant. These concomitant medication changes consisted of dosage increases (18.0% of the citicoline group and 13.1% of the placebo group), dosage decreases (19.7% of the citicoline group and 8.2% of the placebo group), discontinuation of medication (23.0% of the citicoline group and 26.2% of the placebo group), and addition of a medication (24.6% of the citicoline group and 16.4% of the placebo group). There were no changes in 57.4% of the citicoline group and 55.7% of the placebo group.
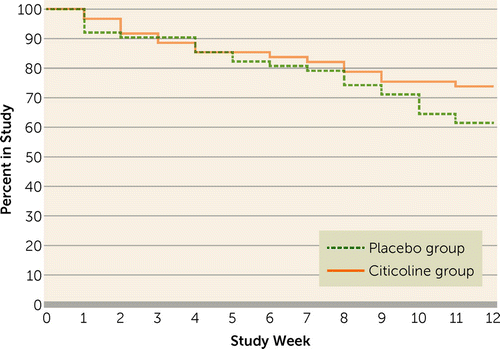
FIGURE 2. Study Survival for Participants With Bipolar Disorder and Cocaine Dependence Receiving Citicoline (N=61) or Placebo (N=61)
Discussion
The study found a significant difference in cocaine-positive urine screens between groups of participants with bipolar I disorder receiving citicoline or placebo, whether or not missing urine samples were imputed as cocaine positive. The findings are consistent with our previous pilot study of citicoline in patients with bipolar disorder and cocaine dependence (19). It is noteworthy that the two studies had similar findings using different designs. The previous study was largely a relapse prevention study of persons with recent self-reported abstinence from cocaine, and it demonstrated that participants receiving citicoline, whose screens were generally cocaine negative at baseline, were less likely than those receiving placebo to have cocaine-positive urine screens during the study. In the present study, in which participants had active cocaine use and a cocaine-positive urine screen at baseline, we also observed a reduction in cocaine use with citicoline as compared with placebo. Thus, citicoline appears both to decrease active cocaine use and to decrease relapse to active use in patients with bipolar disorder. We did not observe a significant between-group difference in Cocaine Craving Questionnaire scores, which suggests that the effects of citicoline on cocaine use are not mediated through a reduction in craving. To our knowledge, citicoline is the only medication that has shown positive findings in reducing cocaine use, based on urine drug screens, in patients with bipolar disorder and cocaine dependence. A study of lamotrigine in this population (18) showed positive findings on self-reported cocaine use, but the urine drug screen findings did not reach statistical significance.
The effects of citicoline in reducing cocaine use appeared to occur quickly and tended to decline during the study. Furthermore, in our previous study we observed a robust reduction in relapse to cocaine use in patients with bipolar disorder and cocaine dependence who received citicoline (19). These findings suggest that citicoline might be most effectively used in an acute treatment to reduce cocaine use in inpatient settings while other treatments are initiated rather than as a long-term monotherapy. Alternatively, in light of the slow upward dosage escalation in the study, it is possible that the lower initial dosage of 500 mg/day was more effective than higher dosages. Consistent with this idea are somewhat counterintuitive phosphorous MR spectroscopy data suggesting that a 500 mg/day dosage of citicoline is associated with a greater change in membrane phospholipids than the 2000 mg/day dosage (41). However, a linear dose-dependent relationship was observed in stroke patients, with better outcomes associated with higher dosages of citicoline (2000–4000 mg/day) (42). Another possibility is that tolerance develops to the effects of citicoline in reducing cocaine use, and an additional dosage titration may be needed. Finally, the decrease in effectiveness of citicoline over time could be due to differential attrition, with citicoline-treated patients remaining in the study when using cocaine while those on placebo discontinue treatment. We observed a large reduction in cocaine-positive urine screens near the end of our earlier citicoline pilot study because of dropout among participants in the placebo group (19).
The study did not find significant group differences in manic or depressive symptom measures, which is consistent with the results from our pilot study of citicoline in bipolar disorder and cocaine dependence (19). However, we observed a significant reduction in depressive symptoms, but not drug use, in a study of citicoline in patients with bipolar disorder or major depressive disorder and methamphetamine dependence (43). The mean depressive symptom severity, based on the IDS-SR, was somewhat lower in the present study than in the methamphetamine study, which may explain in part the different findings. In the present study, no significant differences in YMRS scores were observed between groups. Because the study participants were almost all in the depressed, not mixed, mood state, mean YMRS scores were low at baseline and provided little ability to observe changes in manic symptom severity. Because citicoline appeared to decrease cocaine use without having a positive effect on mood, the observed reduction in cocaine use does not appear to be secondary to mood-stabilizing effects of citicoline. Therefore, the efficacy of citicoline for reducing cocaine use may extend beyond the dual-diagnosis population studied.
Citicoline was safe and well tolerated in this population. Side effects and treatment retention did not differ significantly between the citicoline and placebo groups. In our previous pilot study of citicoline in bipolar disorder and cocaine dependence, we observed significantly fewer side effects and greater treatment retention with citicoline than placebo (19). A Cochrane review of citicoline studies in vascular dementia also reported that citicoline tended to be associated with fewer side effects than placebo (23). While the present study did not replicate these findings, it suggests a favorable safety profile for citicoline in patients with active mood symptoms and cocaine use. The favorable safety profile and absence of known drug-drug interactions may make citicoline a useful choice in bipolar disorder and cocaine-dependent patients who are taking a variety of concomitant medications.
In our earlier pilot study of citicoline in bipolar disorder and cocaine dependence (19) and our trial of citicoline in patients with mood disorders and methamphetamine dependence (43), we observed more than twice the completion rates with citicoline as compared with placebo. While completion rates in the present study favored citicoline (71% compared with 57%), study survival did not differ significantly between groups. This may be due to greater treatment retention in both groups in the present study as compared with the previous studies. The CBT used in this study has been shown to improve retention but not to affect substance use in patients with bipolar disorder (36). In addition, we also used a contingency management strategy in which payments increased for each urine drug screen provided (irrespective of whether it was positive or negative) and reset to baseline if a visit was missed. Similar voucher-based systems have been shown to improve treatment retention in patients with substance dependence (37).
A strength of the study is the randomized, placebo-controlled, blinded design. The sample size is, to our knowledge, the largest for a trial in patients with bipolar disorder and cocaine dependence. However, some limitations should be noted. Because the patients had bipolar disorder, they were taking concomitant medications at baseline, although these medications were similar in the two treatment groups. To minimize the potential impact of concomitant medications, we required stable dosages for a minimum of 14 days at baseline. Some medication changes occurred during the study, although these were again similar in the two groups. We did not formally assess the integrity of the blind at the end of the study by asking participants, raters, and clinicians to guess which treatment the participant was receiving. Citicoline’s highly favorable side effect profile (19, 23), however, would suggest that it would be difficult to distinguish it from placebo. Previous research has not been conducted to determine whether citicoline could have an effect on cocaine urine drug screen sensitivity. Although this possibility cannot be entirely ruled out, citicoline is not structurally similar to cocaine or other substances of abuse and has no known drug-drug or other interactions (22). The use of CBT as a psychosocial platform potentially had advantages and disadvantages. The specific CBT used was designed for patients with bipolar disorder and substance abuse and has been shown to increase treatment retention while not decreasing substance use (36). Thus, the potential benefit of lower attrition was thought to outweigh the possibility that the ability to detect between-group differences might be reduced as a result of a reduction in cocaine use in both groups.
In summary, citicoline shows promise as a treatment for cocaine use in patients with bipolar disorder, although the effects were reduced over time. Adequately powered trials exploring citicoline in combination with other medications that appear to decrease cocaine use, as well as in other dual-diagnosis populations, are needed. Given the lack of effect on mood symptoms, citicoline may also be effective in patients without mood disorders. Thus, trials in patients with cocaine use alone seem warranted to determine the range of efficacy of citicoline and the generalizability of the present findings.
1 : Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990; 264:2511–2518Crossref, Medline, Google Scholar
2 : The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry 1996; 66:17–31Crossref, Medline, Google Scholar
3 : Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 2007; 64:543–552Crossref, Medline, Google Scholar
4 : Drug abuse and bipolar disorder: comorbidity or misdiagnosis? J Affect Disord 2001; 65:105–115Crossref, Medline, Google Scholar
5 : Prevalence and distinct correlates of anxiety, substance, and combined comorbidity in a multi-site public sector sample with bipolar disorder. J Affect Disord 2005; 85:301–315Crossref, Medline, Google Scholar
6 : Substance abuse and bipolar affective disorder. J Nerv Ment Dis 1994; 182:349–352Crossref, Medline, Google Scholar
7 : Sources of lithium resistance in mixed mania. Psychopharmacol Bull 1986; 22:613–620Medline, Google Scholar
8 : Outcome in mania: a 4-year prospective follow-up of 75 patients utilizing survival analysis. Arch Gen Psychiatry 1990; 47:1106–1111Crossref, Medline, Google Scholar
9 : Outcome of bipolar disorder on long-term treatment with lithium. Br J Psychiatry 1991; 159:123–129Crossref, Medline, Google Scholar
10 : Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry 1995; 152:856–861Link, Google Scholar
11 : Substance misuse and risk of aggression and offending among the severely mentally ill. Br J Psychiatry 1998; 172:345–350Crossref, Medline, Google Scholar
12 : Using the general behavior inventory to screen for mood disorders among patients with psychoactive substance dependence. Am J Addict 1994; 3:296–305Crossref, Google Scholar
13 : Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry 2010; 71:296–303Crossref, Medline, Google Scholar
14 : Predictors of nonadherence among individuals with bipolar disorder receiving treatment in a community mental health clinic. Compr Psychiatry 2009; 50:100–107Crossref, Medline, Google Scholar
15 : Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol 2008; 23:95–105Crossref, Medline, Google Scholar
16 : Carbamazepine in the treatment of cocaine dependence: subtyping by affective disorder. Exp Clin Psychopharmacol 2002; 10:276–285Crossref, Medline, Google Scholar
17 : A pilot study of quetiapine in patients with bipolar disorder and cocaine dependence. J Dual Diagn 2010; 6:16–24Crossref, Google Scholar
18 : A randomized, double-blind, placebo-controlled trial of lamotrigine therapy in bipolar disorder, depressed or mixed phase, and cocaine dependence. Neuropsychopharmacology 2012; 37:2347–2354Crossref, Medline, Google Scholar
19 : A randomized, placebo-controlled trial of citicoline add-on therapy in outpatients with bipolar disorder and cocaine dependence. J Clin Psychopharmacol 2007; 27:498–502Crossref, Medline, Google Scholar
20 : Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report. Psychopharmacology (Berl) 1999; 142:132–138Crossref, Medline, Google Scholar
21 : Effects of daily treatment with citicoline: a double-blind, placebo-controlled study in cocaine-dependent volunteers. J Addict Med 2011; 5:57–64Crossref, Medline, Google Scholar
22 : Citicoline in addictive disorders: a review of the literature. Am J Drug Alcohol Abuse 2014; 40:262–268Crossref, Medline, Google Scholar
23 : Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev 2005; 2:CD000269Medline, Google Scholar
24 : Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol 2006; 28(suppl B):1–56Medline, Google Scholar
25 : Changes in brain biogenic monoamines induced by the nootropic drugs adafenoxate and meclofenoxate and by citicholine (experiments on rats). Gen Pharmacol 1990; 21:71–75Crossref, Medline, Google Scholar
26 : Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma 1997; 14:161–169Crossref, Medline, Google Scholar
27 : Ischemic brain injury caused by interrupted versus uninterrupted occlusion in hypotensive rats with subarachnoid hemorrhage: neuroprotective effects of citicoline. Arch Physiol Biochem 2001; 109:161–167Crossref, Medline, Google Scholar
28 : The role of acetylcholine in cocaine addiction. Neuropsychopharmacology 2008; 33:1779–1797Crossref, Medline, Google Scholar
29 : Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
30 : An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis 1980; 168:26–33Crossref, Medline, Google Scholar
31 : The development of a cocaine craving questionnaire. Drug Alcohol Depend 1993; 34:19–28Crossref, Medline, Google Scholar
32 : The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med 2004; 34:73–82Crossref, Medline, Google Scholar
33 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
34 : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
35 : A placebo-controlled, randomized clinical trial comparing sertraline and imipramine for the treatment of dysthymia. Arch Gen Psychiatry 1996; 53:777–784Crossref, Medline, Google Scholar
36 : Cognitive behavioral treatment of bipolar disorder and substance abuse: a preliminary randomized study. Addict Disord Their Treat 2002; 1:17–24Crossref, Google Scholar
37 : Voucher-based incentives: a substance abuse treatment innovation. Addict Behav 2002; 27:887–910Crossref, Medline, Google Scholar
38 : Oxcarbazepine treatment of bipolar disorder. J Clin Psychiatry 2003; 64:943–945Crossref, Medline, Google Scholar
39 : An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 1988; 318:1728–1733Crossref, Medline, Google Scholar
40 : Reporting number needed to treat and absolute risk reduction in randomized controlled trials. JAMA 2002; 287:2813–2814Crossref, Medline, Google Scholar
41 : Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed 2008; 21:1066–1075Crossref, Medline, Google Scholar
42 : Efficacy and safety of oral citicoline in acute ischemic stroke: drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol 2009; 31:171–176Crossref, Medline, Google Scholar
43 : A randomized, double-blind, placebo-controlled trial of citicoline for bipolar and unipolar depression and methamphetamine dependence. J Affect Disord 2012; 143:257–260Crossref, Medline, Google Scholar



