Microvascular Abnormality in Schizophrenia as Shown by Retinal Imaging
Abstract
Objective
Retinal and cerebral microvessels are structurally and functionally homologous, but unlike cerebral microvessels, retinal microvessels can be noninvasively measured in vivo by retinal imaging. The authors tested the hypothesis that individuals with schizophrenia exhibit microvascular abnormality and evaluated the utility of retinal imaging as a tool for schizophrenia research.
Method
Participants were members of the Dunedin Study, a population-representative cohort followed from birth with 95% retention. Study members underwent retinal imaging at age 38. The authors assessed retinal arteriolar and venular caliber for all members of the cohort, including individuals who developed schizophrenia.
Results
Study members who developed schizophrenia were distinguished by wider retinal venules, suggesting microvascular abnormality reflective of insufficient brain oxygen supply. Analyses that controlled for confounding health conditions suggested that wider retinal venules are not simply an artifact of co-occurring health problems in schizophrenia patients. Wider venules were also associated with a dimensional measure of adult psychosis symptoms and with psychosis symptoms reported in childhood.
Conclusions
The findings provide initial support for the hypothesis that individuals with schizophrenia show microvascular abnormality. Moreover, the results suggest that the same vascular mechanisms underlie subthreshold symptoms and clinical disorder and that these associations may begin early in life. These findings highlight the promise of retinal imaging as a tool for understanding the pathogenesis of schizophrenia.
Retinal imaging is a simple, noninvasive technology for assessing microvascular abnormalities in living individuals diagnosed with schizophrenia. Cerebrovascular abnormalities have been discussed as a pathological feature in schizophrenia, beginning with Meynert (1) (see Figure S1 in the data supplement that accompanies the online edition of this article). Advances in fundus photography (photographing the interior surface of the eye) and retinal image analysis now allow for the accurate quantitative assessment of the condition of small retinal blood vessels in large population-based samples (2).
Method
Participants
Participants were members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of the health and behavior of a complete birth cohort of consecutive births from April 1, 1972, to March 31, 1973, in Dunedin, New Zealand. The cohort of 1,037 children (91% of eligible births; 52% boys) was constituted at age 3 years. Cohort families represent the full range of socioeconomic status in the general population of New Zealand’s South Island and are primarily white. Follow-up assessments were conducted at ages 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and, most recently, 38 years, when 95% of the 1,007 living study members underwent assessment in the period 2010–2012.
The study protocol was approved by the institutional ethical review boards of the participating universities, and study members gave informed consent before participating.
Schizophrenia
Schizophrenia was assessed at ages 21, 26, 32, and 38. We previously described the schizophrenia cases up to age 32 (13–15), and here we update this information with data from age 38. Schizophrenia was assessed at each age with the Diagnostic Interview Schedule (DIS) (16, 17), using DSM criteria. To enhance the validity of our research diagnosis, we implemented special steps. First, we required the presence of hallucinations (not substance use-related) in addition to at least two other positive symptoms. This requirement is stricter than that of DSM-IV, which does not require hallucinations, although requiring them has been shown to reduce overdiagnosis (18). Second, because self-reports can be compromised by poor insight in schizophrenia, we required objective evidence of impairment resulting from psychosis, as reported by informants and as recorded in the study’s life-history calendars, which document continuous histories of employment and relationships. Third, in our research, the DIS is administered by experienced clinicians, not lay interviewers. These clinicians record detailed case notes. Staff also rate observable symptoms manifested in affect, grooming, and speech during the full day that participants spend at our research unit. Fourth, participants bring their medications, which are then classified by a pharmacist. Fifth, informants report study members’ positive and negative psychotic symptoms via postal questionnaires. Finally, study members’ parents were interviewed about their adult child’s psychotic symptoms and treatment as part of the Dunedin Family Health History Study (2003–2005). These data, accumulated in the Dunedin study at ages 21, 26, 32, and 38, were compiled into dossiers reviewed by four clinicians to achieve best-estimate diagnoses with 100% consensus. By age 38, 2% of the cohort (N=20) met criteria for schizophrenia and had, according to the multisource information collected in the dossiers, been hospitalized for schizophrenia (totaling 1,396 days of psychiatric hospitalization, according to official New Zealand administrative record searches) or received prescriptions for antipsychotic medications. An additional 1.7% (N=17) met all criteria, had hallucinations, and suffered significant life impairment but had not, to our knowledge, been treated specifically for psychotic illness. Together, these two groups constituted a total of 37 cases of diagnosed schizophrenia in the cohort. Of these 37 individuals, four died before the age-38 retinal vasculature assessment and two declined to participate. An additional four were excluded from the analysis because of pregnancy, a congenital health condition, or ungradable retinal images, leaving an effective group size of 27 (55% men) for this study.
The cohort’s 3.7% prevalence rate of schizophrenia is high and should be understood in the context of three methodological aspects of our study. First, our birth cohort, with a 95% participation rate, allows us to count psychotic individuals overlooked by previous surveys because individuals with psychotic disorders often decline to participate in surveys or die prematurely (19), and surveys often exclude homeless or institutionalized individuals with psychosis. Second, our cohort members are all from one city in the South Island of New Zealand. It is possible, given geographical variation in rates of schizophrenia (20–22), that the prevalence is somewhat elevated there. No data exist to compare prevalence rates of schizophrenia in New Zealand to rates in other countries, but the high prevalence of suicide in New Zealand could be consistent with an elevated prevalence of severe mental health conditions (23). Third, our research diagnoses did not make fine-grained distinctions among psychotic disorders (e.g., schizophrenia versus schizoaffective disorder). Thus, the cohort members diagnosed with schizophrenia here might not be considered by all clinicians to have schizophrenia. We note, however, that over half of those we diagnosed were confirmed by receipt of treatment. Moreover, etiological mechanisms appear to be similar across the continuum of psychosis (24).
Comparison Groups
Study members who did not meet criteria for schizophrenia were classified into the comparison groups detailed below. We selected these medical and psychiatric comparison groups because 1) these conditions occur more commonly in individuals diagnosed with schizophrenia than in the general population, in this cohort and in other research (25, 26), and 2) hypertension, diabetes, and smoking have been associated with vessel caliber in previous retinal imaging studies (2, 5).
Hypertension at age 38 (N=110).
Hypertension (27) was defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg (28). The prevalence of hypertension was 19% in the schizophrenia group and 12% in the rest of the cohort.
Prediabetes/diabetes at age 38 (N=154).
Prediabetes and diabetes were defined according to the American Diabetes Association: a hemoglobin A1c level in the range of 5.7%−6.5% for prediabetes, and ≥6.5% for diabetes (29). The prevalence was 29% in the schizophrenia group and 18% in the rest of the cohort.
Persistent tobacco dependence (N=210).
Cohort members who were diagnosed with DSM tobacco dependence on two or more occasions between ages 18 and 38 were classified into the persistent tobacco dependence group. The prevalence was 56% in the schizophrenia group and 23% in the rest of the cohort.
Persistent depression (N=188).
Cohort members who were diagnosed with DSM depression on two or more occasions between ages 18 and 38 were classified into the persistent depression group. The prevalence was 70% in the schizophrenia group and 21% in the rest of the cohort.
Healthy comparison subjects (N=412).
Healthy comparison subjects did not have any of the aforementioned health problems.
Psychosis Symptoms
Childhood psychosis symptoms were assessed at age 11 for 789 cohort members seen at the Dunedin Unit (those assessed at school were not seen by the child psychiatrist) using the Diagnostic Interview Schedule for Children (30). As previously described (13), children responded to four questions (see Table S1 in the online data supplement). Responses were summed, and children with scores of 0, 1, and ≥2 were grouped as having no, weak, or strong symptoms, respectively.
Adulthood psychosis symptoms were assessed with the DIS at ages 21, 26, 32, and 38. Cohort members reported on eight symptoms of hallucinations and delusions (see Table S1 in the data supplement), which were summed into a single scale at each age. These four scales were used in a confirmatory factor analysis to estimate a continuous latent variable representing dimensional liability to adult psychosis across ages 21–38. Because the four scales had positive skew, they were treated as ordinal, with values ranging from 0 to 8. Standardized factor loadings for the psychosis symptom scales at ages 21, 26, 32, and 38 were 0.60, 0.87, 0.81, and 0.83, respectively. The model fit for this single latent variable was excellent (χ2=2.13, p=0.345; root mean square error of approximation: 0.008, 95% CI=0.000–0.065; comparative fit index=1.00; Tucker-Lewis index=1.00).
Assessment of Retinal Vessel Caliber
As previously described (31), digital fundus photographs were taken at the Research Unit after 5 minutes of dark adaptation. The same camera (a Canon NMR-45 with a 20D SLR backing) was used for all photographs. For each participating cohort member, both eyes were photographed, and measurements for the two eyes were averaged. Retinal photographs were graded at the Singapore Eye Research Institute, National University of Singapore, using semiautomated computer software (Singapore “I” Vessel Assessment [SIVA], version 3.0). Trained graders, blind to participant characteristics, used the SIVA program to measure the retinal vessel diameters according to a standardized protocol with high intergrader reliability (32). Caliber (or diameter) denotes the size of the lumen, which is the internal space of the vessel. Measurements were made for arterioles and venules where they passed through a region located 0.50–2.00 disk diameters from the optic disk margin (Figure 1) (32). Vessel calibers were based on the six largest arterioles and venules passing though this region and were summarized as central retinal artery equivalent and central retinal vein equivalent using the revised Knudtson-Parr-Hubbard formula (32, 33). Of 938 study members for whom retinal images were available, only seven could not be graded because the images were either too dark or not centered on the optic disk. An additional nine study members were excluded from analyses because of pregnancy. This left 922 study members for whom retinal vessel data were available. Arteriolar and venular calibers were normally distributed within the cohort. The mean arteriolar caliber among the 922 study members was 137.33 measuring units (SD=10.86, median=137.30, range=105.66–179.47), and the mean venular caliber was 196.20 measuring units (SD=14.83, median=195.51, range=141.07–245.68).
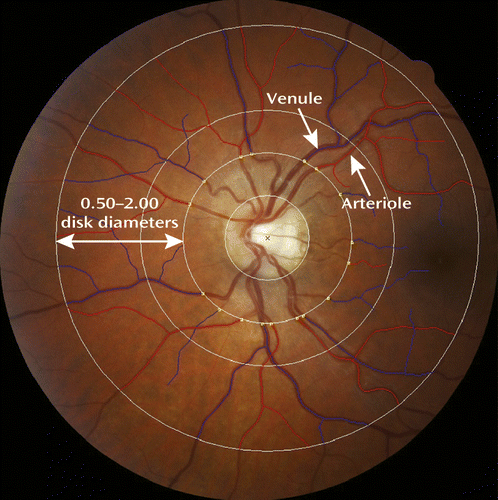
a Measurements were made for the six largest arterioles (red) and venules (blue) passing through the region located 0.50–2.00 disk diameters from the optic disk margin.
Statistical Analysis
Before all analyses, arteriolar and venular caliber were each adjusted for the effect of the other vessel, as recommended (2, 34), in order to isolate the unique effects for each vessel and adjust for any potential effects of refractive errors (35). Vessel calibers were then standardized on the population-representative cohort (mean=0.00, SD=1.00). Sex was included as a covariate in all analyses.
Our analyses proceeded as follows. First, to replicate previous studies documenting associations between vessel caliber and hypertension, diabetes, and smoking, we used analysis of variance to compare mean vessel calibers in each group with those in a group of healthy individuals (i.e., a group with none of the aforementioned conditions). Next, to test our hypothesis that individuals with schizophrenia are distinguished specifically by wider venules, we compared venular and arteriolar calibers in individuals with schizophrenia with those in individuals with hypertension, prediabetes/diabetes, persistent tobacco dependence, or persistent depression. As an additional check to ensure that an association between schizophrenia and wider venules could not be explained by the collective effects of these conditions, we conducted a multivariate regression, predicting venular caliber from schizophrenia while controlling for all of these conditions simultaneously. In this multivariate regression, we treated blood pressure (systolic and diastolic) and prediabetes/diabetes (i.e., hemoglobin A1c level) as continuous variables.
Second, to test our hypothesis that wider venular caliber is associated with psychosis symptoms in adulthood, we obtained the parametric correlation between latent dimensional liability to adult psychosis and venular caliber.
Third, to test our hypothesis that wider venular caliber is associated with psychosis symptoms in childhood, we obtained the polychoric correlation between childhood psychosis symptoms (a three-level ordinal variable), and venular caliber.
Results
Retinal Venular Caliber and Schizophrenia
Table 1 lists standardized mean venular and arteriolar calibers at age 38 for cohort members who developed schizophrenia (N=27) and for each of the comparison groups. Mean values reflect effect sizes for how different each group is from the cohort norm. Differences between pairs of groups can also be interpreted as effect sizes. Effect sizes of 0.20, 0.50, and 0.80 reflect small, medium, and large effects, respectively (36).
| Venule | Arteriole | ||||||
|---|---|---|---|---|---|---|---|
| Group | N | Mean | SD | 95% CI | Mean | SD | 95% CI |
| Schizophrenia | 27 | 0.59b | 1.16 | 0.14, 1.05 | –0.13 | 1.04 | –0.54, 0.28 |
| Healthy | 412 | –0.20c | 0.91 | –0.29, –0.12 | 0.15 | 0.93 | 0.06, 0.24 |
| Hypertension | 110 | 0.45b | 1.00 | 0.26, 0.64 | –0.75b,c | 1.06 | –0.95, –0.55 |
| Prediabetes/diabetes | 154 | 0.14b,c | 0.94 | –0.01, 0.29 | –0.08b | 1.00 | –0.24, 0.08 |
| Persistent tobacco dependence | 210 | 0.17b | 1.10 | 0.02, 0.32 | 0.02 | 1.04 | –0.12, 0.16 |
| Persistent depression | 188 | 0.13b,c | 1.11 | –0.03, 0.29 | –0.08b | 0.99 | –0.22, 0.06 |
The mean values indicate four noteworthy findings.
Schizophrenia was also associated with wider venular caliber in a multiple regression model, adjusted for arteriolar caliber and sex (β=0.61, SE=0.19; t=3.14, df=920, p=0.002), and this association remained statistically significant after we also controlled for systolic blood pressure, diastolic blood pressure, hemoglobin A1c level, persistent tobacco dependence, and persistent depression (β=0.49, SE=0.19; t=2.57, df=915, p=0.011). Wider venules among individuals diagnosed with schizophrenia could also not be explained by current use of antipsychotic medication; venular caliber for the subset of individuals diagnosed with schizophrenia who did not take antipsychotic medication during the year before retinal imaging (N=22) was even wider (mean=0.69, SD=1.15). Results were similar for those who had never (N=12, mean=0.63, SD=1.19) compared with those who had ever (N=15, mean=0.56, SD=1.17) received treatment specifically for psychotic illness.
Figure 2 shows the distribution of venular caliber (adjusted for arteriolar caliber and sex and standardized on the cohort) for each group. The figure shows that the distribution for venular caliber in the schizophrenia group was shifted to the right, reflecting wider venules. For example, 70% of the schizophrenia group members had venular calibers wider than the cohort mean (Z=0.00), compared with only 40% of the healthy group (χ2=9.91, p=0.002). This nonparametric test does not depend on extreme outlier observations. Thus, a significantly larger proportion of individuals with schizophrenia had wider-than-average venules (regardless of outliers).
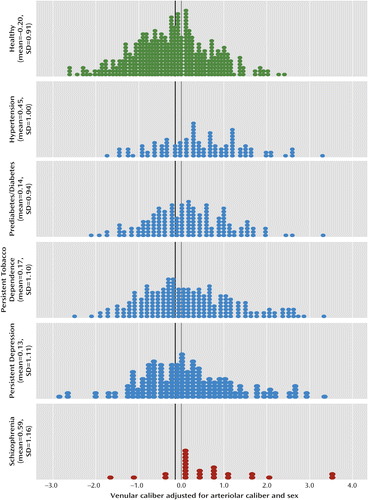
a Scores were adjusted for arteriolar caliber and sex and were standardized (mean=0.00, SD=1.00) on the population-representative cohort. The black vertical line represents the mean for the healthy group. Both parametric and nonparametric statistical analyses showed that individuals diagnosed with schizophrenia had significantly wider venules, a finding that does not depend on outliers.
Retinal Venular Caliber and Liability to Adult Psychosis
Approximately one-third of the cohort endorsed one or more psychotic symptoms in adulthood, which were aggregated into a latent liability score. The association between this latent liability to adult psychosis and venular caliber, adjusted for arteriolar caliber and sex, was 0.15 (p<0.001).
Retinal Venular Caliber and Childhood Psychosis Symptoms
Of the 13 cohort members who had significant psychotic symptoms at age 11, three were diagnosed with adult schizophrenia. The association between these childhood psychosis symptoms (age 11) and venular caliber (age 38), adjusted for arteriolar caliber and sex, was 0.13 (p=0.015) (Figure 3).
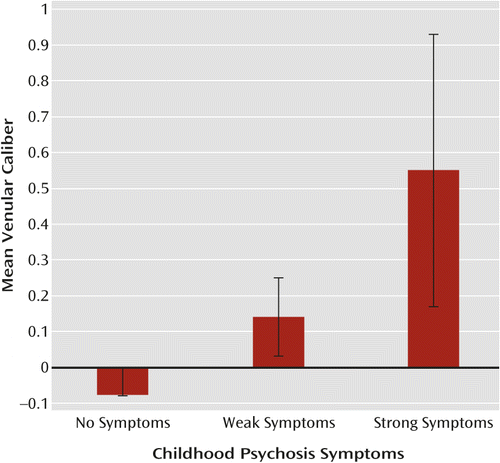
a Venular caliber values were adjusted for arteriolar caliber and sex and were standardized on the population-representative cohort. As previously described (13), children with scores of 0, 1, and ≥2 were grouped as having no symptoms (N=673; 50.2% were male), weak symptoms (N=103; 66.0% male), or strong symptoms (N=13; 61.5% male), respectively. At minimum, study members could enter the strong symptom group by obtaining a score of 1 (yes, likely) for two symptoms or by obtaining a score of 2 (yes, definitely) for one symptom. Children who exhibited strong psychosis symptoms had the widest venular calibers as adults. Error bars indicate standard error.
Discussion
In our population-based birth cohort, study members who developed schizophrenia exhibited retinal microvascular abnormality—specifically, wider venular caliber. Our analyses that controlled for confounding health conditions suggest the possibility that wider venules are a distinguishing feature of schizophrenia and not simply an artifact of co-occurring health problems in schizophrenia patients, as the widest venules were observed for the schizophrenia group. Moreover, wider venules were associated with a greater dimensional liability to experience psychosis symptoms in adulthood. This finding is consistent with theory and research suggesting that the same mechanisms underlie subthreshold symptoms and clinical disorder (24). Finally, wider venules were associated with childhood psychosis symptoms at age 11, which may suggest that pathological vascular mechanisms leading to the association between schizophrenia and wider venules operate from early life.
The specific pathophysiological mechanisms underlying wider retinal venular caliber are not entirely understood (2). Wider venules have been shown to be associated with inflammation (37–40), endothelial dysfunction (40, 41), and hypoxia/ischemia (12), for example. Notably, inflammation (42–44), endothelial dysfunction/dysregulation of the nitric oxide signaling pathway (45, 46), and hypoxia/ischemia (47) are all seen in schizophrenia. Genetic factors also influence retinal venular caliber (48–50). Interestingly, genetic linkage regions for venular caliber are implicated in endothelial function and vasculogenesis (48, 49). Thus, some individuals may have a genetic propensity to develop wider venules. It is unclear whether venular caliber plays a causal role in the development of schizophrenia or whether it might represent an associated epiphenomenon.
While our study is the first, to our knowledge, to investigate the microvasculature in living schizophrenia patients, our findings converge with a growing body of literature that implicates the vasculature in schizophrenia. First, individuals with schizophrenia are at increased risk of developing cardiovascular disease (25), and recent evidence suggests that the same genes influence both schizophrenia and risk for cardiovascular disease (51). Second, a large proportion of replicated candidate genes for schizophrenia are regulated by hypoxia or are expressed in the vasculature (47, 52). Third, altered cerebral blood flow and blood volume as well as mitochondrial dysfunction in schizophrenia have all been hypothesized to arise from vascular abnormalities (53–55). Fourth, individuals with schizophrenia show impaired vasodilation (56–58) and abnormalities of the nailfold capillary bed (59). Fifth, postmortem analysis of the brains of schizophrenia patients has revealed evidence of atypically simplified angioarchitecture and lack of normal arborization of vessels (60), ultrastructural capillary damage (61), and molecular alterations of the cerebral microvasculature (62). Overall, evidence of vascular involvement in schizophrenia is accumulating, and the long-standing hypothesis of vascular pathology in schizophrenia (see Figure S1 in the online data supplement) highlights the need for an innovative method to assess the microvasculature in living schizophrenia patients.
Results of our study should be interpreted in the context of limitations. First, the prevalence of schizophrenia in our cohort is high. Given the lack of clear boundaries between mental health and illness, it is possible that our cohort members diagnosed with schizophrenia would not be considered by all clinicians to have schizophrenia. However, over half of the members of our schizophrenia group had received treatment specifically for psychotic illness, and there were no differences in venular caliber between those who received treatment and those who did not. Second, our finding is based on a relatively small group of individuals who developed schizophrenia (N=27). Larger samples are needed to determine the replicability and robustness of the finding. We note, however, two aspects of our study that bolster our findings and are important for psychiatric research aimed toward identifying biological abnormalities (63): 1) we reported effect sizes for diagnosed schizophrenia in relation to both healthy and medically or psychiatrically unhealthy comparison groups, a practice that can substantially reduce or eliminate biased and invalid conclusions (64); and 2) we showed an association between wider venules and latent dimensional liability to adult psychosis in the population-representative cohort. Doing this circumvented some of the problems associated with studying categorical diagnoses (63).
Another limitation is that we assessed retinal vessel caliber at a single time point (age 38), and thus we cannot know whether wider venules might be detectable before the onset of schizophrenia. Retinal imaging of microvessels is a relatively new technology that was not available in the early years of our longitudinal study. However, our finding of an association between psychosis symptoms at age 11, before the onset of schizophrenia, and venular caliber at age 38 suggests the hypothesis that abnormal vascular processes may begin in childhood. In support of this possibility, research indicates that variation in the retinal vessel calibers of children is informative, at least with regard to blood pressure (65, 66).
Retinal imaging makes possible the prospective tracking of vascular changes as they relate to the onset, waxing, and waning of symptoms, and research using longitudinal, high-risk, and experimental designs could use this method to address a variety of important questions. For example, longitudinal and high-risk studies can determine whether retinal vessel caliber in juveniles predicts risk of developing psychosis or accompanies the progression of schizophrenia, as might be expected given the neurodevelopmental nature of schizophrenia (67). Research questions could also be extended to examine associations between retinal vessel caliber and neuropsychological impairment in schizophrenia, as we previously showed that wider venular caliber in adulthood was associated with poorer neuropsychological test performance in childhood and midlife (31). Experimental studies are ideally suited to addressing whether changes in retinal vessel caliber are associated with improvement or deterioration in symptoms. Along these lines, a recent study found that oxygen supplementation improved symptoms of schizophrenia, including neuropsychological functioning (68), and the addition of retinal imaging to this design could help elucidate the mechanisms by which treatment works.
Our results provide initial evidence of retinal vessel caliber abnormality (specifically wider venular caliber) in schizophrenia and psychosis, a finding that is consistent with the hypothesis of microvascular pathology in schizophrenia (69). The noninvasive nature of retinal imaging, its relative cost-effectiveness, and the availability of the technology in primary care, optometry, and ophthalmology centers all suggest the value of retinal imaging analysis as an exciting tool for schizophrenia research.
1 : Psychiatrie: Klinik der erkrankungen des vorderhirns. Vienna, Braumüller, 1884Google Scholar
2 : Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol 2009; 54:74–95Crossref, Medline, Google Scholar
3 : Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat 2005; 206:319–348Crossref, Medline, Google Scholar
4 : Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 2012; 60:1094–1103Crossref, Medline, Google Scholar
5 : Retinal vascular caliber measurements: clinical significance, current knowledge, and future perspectives. Ophthalmologica 2013; 229:125–136Crossref, Medline, Google Scholar
6 : Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol 2009; 170:1323–1332Crossref, Medline, Google Scholar
7 : Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology 2006; 66:1339–1343Crossref, Medline, Google Scholar
8 : Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain 2006; 129:182–188Crossref, Medline, Google Scholar
9 : Retinal vascular caliber and risk of dementia: the Rotterdam Study. Neurology 2011; 76:816–821Crossref, Medline, Google Scholar
10 : Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med 2006; 166:2388–2394Crossref, Medline, Google Scholar
11 ;
12 : Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters: the Rotterdam Study. Ophthalmology 2008; 115:887–892Crossref, Medline, Google Scholar
13 : Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry 2000; 57:1053–1058Crossref, Medline, Google Scholar
14 : Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 2002; 59:449–456Crossref, Medline, Google Scholar
15 : Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry 2010; 167:160–169Link, Google Scholar
16 : National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry 1981; 38:381–389Crossref, Medline, Google Scholar
17 : Diagnostic Interview Schedule for DSM-IV. St Louis, Mo, Washington University School of Medicine, 1995Google Scholar
18 : Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample: the National Comorbidity Survey. Arch Gen Psychiatry 1996; 53:1022–1031Crossref, Medline, Google Scholar
19 : Mortality in first-contact psychosis patients in the UK: a cohort study. Psychol Med 2012; 42:1649–1661Crossref, Medline, Google Scholar
20 : Evidence for geographical variations in the prevalence of schizophrenia in rural Ireland. Arch Gen Psychiatry 1991; 48:254–258Crossref, Medline, Google Scholar
21 : Prevalence and diagnosis of schizophrenia based on register, case record, and interview data in an isolated Finnish birth cohort born 1940–1969. Soc Psychiatry Psychiatr Epidemiol 2005; 40:808–816Crossref, Medline, Google Scholar
22 : Endemic psychosis in western Ireland. Am J Psychiatry 1984; 141:966–970Link, Google Scholar
23 : Suicide Rates in New Zealand: Exploring Associations With Social and Economic Factors. Wellington, New Zealand, Ministry of Health, 2005Google Scholar
24 : A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med 2009; 39:179–195Crossref, Medline, Google Scholar
25 : Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005; 150:1115–1121Crossref, Medline, Google Scholar
26 : Psychiatric comorbidities and schizophrenia. Schizophr Bull 2009; 35:383–402Crossref, Medline, Google Scholar
27 : Human blood pressure determination by sphygmomanometry. Circulation 1993; 88:2460–2470Crossref, Medline, Google Scholar
28 ;
29
30 : Diagnostic Interview Schedule for Children (DISC). Rockville, Md, National Institute of Mental Health, 1982Google Scholar
31 : Retinal vessel caliber and lifelong neuropsychological functioning: retinal imaging as an investigative tool for cognitive epidemiology. Psychol Sci 2013; 24:1198–1207Crossref, Medline, Google Scholar
32 : A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation 2010; 17:495–503Medline, Google Scholar
33 : Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003; 27:143–149Crossref, Medline, Google Scholar
34 : Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci 2007; 48:52–57Crossref, Medline, Google Scholar
35 : Does refractive error influence the association of blood pressure and retinal vessel diameters? The Blue Mountains Eye Study. Am J Ophthalmol 2004; 137:1050–1055Crossref, Medline, Google Scholar
36 : A power primer. Psychol Bull 1992; 112:155–159Crossref, Medline, Google Scholar
37 : Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol 2006; 124:87–94Crossref, Medline, Google Scholar
38 : Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004; 45:2129–2134Crossref, Medline, Google Scholar
39 : Relative importance of systemic determinants of retinal arteriolar and venular caliber: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol 2008; 126:1404–1410Crossref, Medline, Google Scholar
40 : Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi-Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci 2006; 47:2341–2350Crossref, Medline, Google Scholar
41 : Retinal vascular caliber and brachial flow-mediated dilation: the Multi-Ethnic Study of Atherosclerosis. Stroke 2010; 41:1343–1348Crossref, Medline, Google Scholar
42 : Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res 2007; 149:267–271Crossref, Medline, Google Scholar
43 : Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res 2010; 118:211–217Crossref, Medline, Google Scholar
44 : Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 2008; 63:801–808Crossref, Medline, Google Scholar
45 : Peripheral endothelial dysfunction in patients suffering from acute schizophrenia: a potential marker for cardiovascular morbidity? Schizophr Res 2011; 128:44–50Crossref, Medline, Google Scholar
46 : The many faces of nitric oxide in schizophrenia: a review. Schizophr Res 2005; 78:69–86Crossref, Medline, Google Scholar
47 : Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res 2006; 84:253–271Crossref, Medline, Google Scholar
48 : Genome-wide linkage study of retinal vessel diameters in the Beaver Dam Eye Study. Hypertension 2006; 47:797–802Crossref, Medline, Google Scholar
49 ;
50 : The relationship between retinal arteriolar and venular calibers is genetically mediated, and each is associated with risk of cardiovascular disease. Invest Ophthalmol Vis Sci 2011; 52:975–981Crossref, Medline, Google Scholar
51 ;
52 : An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry 2012; 17:1194–1205Crossref, Medline, Google Scholar
53 : Regional cerebral blood flow in schizophrenia and the local circuit neurons hypothesis. Schizophr Bull 1996; 22:163–182Crossref, Medline, Google Scholar
54 : Abnormalities of regional distribution of cerebral vasculature in schizophrenia detected by dynamic susceptibility contrast MRI. Am J Psychiatry 1995; 152:1801–1803Link, Google Scholar
55 : Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 2004; 9:684–697, 643Crossref, Medline, Google Scholar
56 : Niacin skin flush in schizophrenia: a preliminary report. Schizophr Res 1998; 29:269–274Crossref, Medline, Google Scholar
57 : A genome-wide quantitative linkage scan of niacin skin flush response in families with schizophrenia. Schizophr Bull 2013; 39:68–76Crossref, Medline, Google Scholar
58 : The niacin challenge test: clinical manifestation of altered transmembrane signal transduction in schizophrenia? Biol Psychiatry 1997; 41:507–513Crossref, Medline, Google Scholar
59 : Relationship between nailfold plexus visibility and clinical, neuropsychological, and brain structural measures in schizophrenia. Biol Psychiatry 1999; 46:102–109Crossref, Medline, Google Scholar
60 : [Neuronal structure abnormality in the orbito-frontal cortex of schizophrenics.] J Hirnforsch 1991; 32:149–158 (German)Medline, Google Scholar
61 : Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry 2010; 11:567–578Crossref, Medline, Google Scholar
62 : The cerebral microvasculature in schizophrenia: a laser capture microdissection study. PLoS ONE 2008; 3:e3964Crossref, Medline, Google Scholar
63 : Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012 17:1174–1179Crossref, Medline, Google Scholar
64 : The use of well controls: an unhealthy practice in psychiatric research. Psychol Med 2011; 41:1127–1131Crossref, Medline, Google Scholar
65 : Influence of blood pressure on retinal vascular caliber in young children. Ophthalmology 2011; 118:1459–1465Crossref, Medline, Google Scholar
66 : Blood pressure is associated with retinal vessel signs in preadolescent children. J Hypertens 2010; 28:1406–1412Crossref, Medline, Google Scholar
67 : Rethinking schizophrenia. Nature 2010; 468:187–193Crossref, Medline, Google Scholar
68 : Normobaric hyperoxia treatment of schizophrenia. J Clin Psychopharmacol 2012; 32:525–530Crossref, Medline, Google Scholar
69 : Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet 2005; 6:7Crossref, Medline, Google Scholar



