Lamotrigine Dosing for Pregnant Patients With Bipolar Disorder
Abstract
Objective
Little information is available on the need for dosage changes for lamotrigine in pregnant women with bipolar disorder. The authors present new data on serial serum levels of lamotrigine in pregnant patients on lamotrigine monotherapy. They also review the epilepsy literature on use of lamotrigine during pregnancy.
Method
Lamotrigine serum samples were obtained from eight mother-infant pairs at different time points during pregnancy and the postpartum period.
Results
All of the women were taking lamotrigine throughout pregnancy. Serum-level-to-dose ratios were lower during pregnancy than the postpartum period. Lamotrigine was taken once daily in doses ranging from 100 mg to 300 mg. Three patients had an increase of 50 mg to their daily dose across pregnancy. The change in serum lamotrigine levels in the postpartum period ranged from a 30% decrease to a 640% increase compared with the first level obtained during pregnancy. Level-to-dose ratios obtained within 4 weeks after delivery reflected a mean level 402% greater than the baseline level during gestation. Compared with the third trimester, lamotrigine serum concentration increased an average of 154% within 5 weeks after delivery. The most dramatic increase in lamotrigine serum level early after delivery occurred at 1.5 weeks. The mean infant cord level was 66% of the maternal serum level at delivery. The mean breast-fed infant serum level was 32.5% of the maternal serum levels.
Conclusions
The pattern of lamotrigine changes during pregnancy in these women with bipolar disorder was consistent with that described in the epilepsy literature.
The typical onset of bipolar disorder in the early 20s means that many women manage this chronic illness during their childbearing years. Pregnancy was once believed to be protective against bipolar disorder (1); however, recent studies suggest that pregnancy is a vulnerable period for symptom recurrence. In one study, discontinuation of mood stabilizers was estimated to increase the risk of recurrence during pregnancy by 85% (2). In a study of lithium discontinuation in pregnant and nonpregnant women, recurrence rates were similar over a 40-week period and increased threefold during the postpartum period compared with nonpregnant women (3), which demonstrates the propensity for relapse in the immediate postpartum period (4).
Lamotrigine is an anticonvulsant that has been approved by the U.S. Food and Drug Administration (FDA) for the maintenance treatment of bipolar disorder. Compared with other anticonvulsants, it has a favorable reproductive risk profile (5–8), and it is a preferred option for women of childbearing age. However, minimal information is available on the utility of therapeutic dose monitoring for the management of pregnant women with bipolar disorder, or even to inform dosing during pregnancy. In this article, we present data on the serum levels of lamotrigine in eight gravid women who participated in an observational study. We review the epilepsy literature on lamotrigine use during pregnancy and summarize its application to women with bipolar disorder.
Pharmacokinetics
Pharmacokinetics in Pregnancy
In most pregnant women, lamotrigine clearance increases (9–14). Lamotrigine is metabolized primarily through the liver by glucuronidation (15, 16), unlike other anticonvulsants, which are eliminated primarily through the cytochrome P450 system or cleared by the kidneys. Uridine diphosphate-glucuronosyltransferase (UGT) 1A4 (UGT1A4) catalyzes 90% of lamotrigine conjugation (17). The major lamotrigine metabolite, 2-N-glucuronide, is excreted through the kidneys (16). Estradiol up-regulates the expression of UGT1A4 (18), which increases lamotrigine clearance associated with rising estrogen levels during pregnancy (18). Further evidence of estradiol's effect on lamotrigine clearance has been provided by data from patients receiving combined oral contraceptives, demonstrating that estradiol, not progestogens, is associated with the reduction of lamotrigine serum levels by >50% (19). Ohman et al. (20) studied 15 women with epilepsy treated with lamotrigine monotherapy or combination treatment with lamotrigine and a noninteractive anticonvulsant during 17 pregnancies and compared them with 20 nonpregnant women with epilepsy on lamotrigine monotherapy. The ratio of 2-N-glucuronide to lamotrigine increased up to 175% in the third trimester compared with the ratio 1 month after delivery, which indicates increased clearance of lamotrigine during late pregnancy. One month after delivery, the mean ratios of 2-N-glucuronide to lamotrigine of the postpartum participants were not significantly different from those of the nonpregnant comparison women, which indicates that lamotrigine clearance returned to baseline.
Fotopoulou et al. (17) prospectively followed nine pregnant women with epilepsy and reported that lamotrigine clearance increased in all trimesters, up to a striking 248% above the prepregnancy level in the third trimester. These findings were consistent with several other studies (9–14, 21). Case reports have identified a linear decline in lamotrigine plasma concentrations (9); however, several studies have documented a wide interindividual variation in the pattern of declining lamotrigine concentrations (11–14, 21, 22). This variation is influenced by several factors, including ethnicity, cigarette smoking, disease states, concomitant medications, and genetic polymorphisms that affect glucuronidation (23).
Lamotrigine clearance increases rapidly within the first 2 months of pregnancy (12, 13, 21). A study of 10 women demonstrated a decrease in lamotrigine serum levels across the first 10 weeks of pregnancy, with a mean serum concentration 82% (SD=14%) of prepregnancy baseline level (12). Similarly, Pennell et al. (13) analyzed total lamotrigine levels before conception and monthly during pregnancy in 14 women. The best-fit curve of lamotrigine clearance ascended during the first 10 weeks of pregnancy and reached peak clearance at 32 weeks. Decreases in lamotrigine serum concentration across pregnancy increase the risk for symptom recurrence.
“Ms. L,” a 22-year-old married Caucasian woman with bipolar I disorder and a 24-week pregnancy, presented to a perinatal specialty psychiatry program. She was referred by her community psychiatrist for consultation on optimal management of lamotrigine during pregnancy. Ms. L was euthymic on a once-daily dose of 200 mg of lamotrigine. She was stable for the year preceding her pregnancy. Under her psychiatrist’s care, she continued lamotrigine throughout pregnancy because she “enjoyed feeling stable.” Her medication history included no response to aripiprazole and toxicity while taking lithium.
The research program provided Ms. L and her psychiatrist continued consultation through the pregnancy and the postpartum period. Lamotrigine serum levels and mood assessments were obtained regularly.
At 30 weeks’ gestation, Ms. L reported a 3-week period of diurnal variation in mood and sadness, crying spells, helplessness, and worthlessness for hours every morning. Her daily lamotrigine dose was increased from 200 mg to 225 mg. Her serum-level-to-dose ratio increased from 0.86 to 0.90. By week 36, she reported that her depression had dissipated. At 39 weeks, Ms. L vaginally delivered a baby girl. Two days after delivery, the newborn had difficulty breast-feeding and was admitted to the hospital for jaundice and dehydration, which resolved with intravenous fluids and bilirubin light therapy. Ms. L continued to breast-feed fully. At 12 days, the infant had mild hypotonia but was otherwise normal on examination. At 4 weeks, the infant’s lamotrigine concentration was 46% of the maternal serum concentration. The baby had no further complications after resuming exposure to lamotrigine through breast milk. The infant’s 12-month pediatric examination was normal.
At 11 weeks postpartum, Ms. L was euthymic. Her daily lamotrigine dose was decreased to 200 mg without any change in mood symptoms. At 3, 6.5, and 12 months, Ms. L remained euthymic. At 6.5 months, the infant’s score on the Bayley Scales of Infant Development was normal, and her length, weight, and head circumference were at the 64th, 46th, and 76th percentile, respectively.
Postpartum Pharmacokinetics
Lamotrigine serum levels return to prepregnancy values within 3 to 4 weeks after delivery (10, 12, 17). Because pregnant women are not often enrolled in research protocols before conception, most researchers have not obtained true prepregnancy baseline lamotrigine levels. Instead, researchers have compared postpartum levels to levels obtained during pregnancy. As a result, the time it takes for lamotrigine levels return to prepregnancy values has not been systematically studied.
In women with epilepsy, the lamotrigine dosage is increased during pregnancy to prevent seizure recurrence as a result of declining serum concentrations. However, the rapid postpartum rise in lamotrigine concentration can result in toxicity, manifested as diplopia, ataxia, nausea, and dizziness, and can occur as early as 3 days after delivery (12). Pennell et al. (22) found that a structured postpartum taper (decreasing the lamotrigine dosage in steady increments on the third, seventh, and 10th days postpartum) reduced the risk of postpartum toxicity.
Sabers and Tomson (24) recommended comparing the therapeutic prepregnancy serum lamotrigine level (baseline) to levels obtained every 4–5 weeks during pregnancy and adjusting the lamotrigine dosage to maintain the prepregnancy level. If the lamotrigine level is less than the baseline level, the dosage should be increased 20%−25%. After delivery, the lamotrigine serum level should be monitored every 1–2 weeks. If the serum level is unchanged from baseline, lamotrigine should be continued at the same dosage. If the serum level is higher than at baseline, the dosage should be reduced 20%−25%. Having more than four dosage increases during pregnancy increases the risk for toxic levels when lamotrigine clearance returns to baseline after delivery, and a 20%−25% dose reduction the day after delivery is recommended (24).
Transplacental Transfer to the Fetus
Placental transfer of lamotrigine has been documented in 83 mother-infant pairs through case reports, case series, and cohort studies (9, 10, 12, 17, 25–28). Sixty-eight of these mother-infant pairs were treated with lamotrigine monotherapy and 15 were treated with lamotrigine combined with other anticonvulsants. Serum concentrations of lamotrigine for mother and infant (measured in the umbilical cord) at delivery are essentially equivalent. In the largest study to explore transplacental transfer, Kacirova et al. (25) analyzed maternal and umbilical cord lamotrigine concentrations at delivery in 55 patients. The ratio of infant umbilical cord to maternal serum ranged from 0.4 to 1.38, with a mean of 0.89; although the mean is close to 1, the range of ratios reflected a greater than twofold difference. Similar to the interindividual variation in lamotrigine metabolism, the expression and activity of UGTs in the placenta may affect lamotrigine transfer to the fetus (29).
Lamotrigine and Breast-Feeding
Milk-to-plasma ratios have been reported in 40 lactating women taking lamotrigine in case reports (9, 28, 30, 31), case series (10, 17, 32–34), and a prospective study (26). On average, the milk-to-plasma ratio is 60% (9, 10, 17, 28), with wide interindividual variation (17, 26). Newport et al. (26), in a prospective study of 30 women with epilepsy (63.3%) or bipolar disorder (36.7%) and their breast-fed infants during lamotrigine monotherapy, in which 25 participants provided 210 breast milk samples, found that the mean milk-to-plasma ratio was 41.3% (range, 5.7%−147.1%).
Infant plasma concentrations have been obtained in 40 infants whose mothers were taking lamotrigine while breast-feeding (9, 10, 12, 17, 26, 28, 31, 32). All but one had a detectable lamotrigine serum level (32). Serum lamotrigine levels in the infants ranged from 6%−50% of maternal serum levels (9, 10, 12, 17, 26, 28, 32). Maternal dosage did not correlate with the percentage of lamotrigine measured in the infant serum. Similar to maternal lamotrigine concentrations, infant lamotrigine concentration varies by individual determinants, such as whether and how much the mother supplements breast milk with formula and the baby’s age and genetic characteristics. Additionally, infants are inefficient at metabolizing lamotrigine. Mature forms of UGT, which allow the infant to metabolize lamotrigine, take up to 3 months to appear, and it may take 3 years for adult levels to be reached (32). The wide variability of lamotrigine levels found in breast milk makes it difficult to predict which infants are at risk of increased lamotrigine exposure.
The only reported adverse event of lamotrigine exposure through breast milk (31) was in a 16-day-old infant who experienced a brief apneic episode while sleeping and again 3 hours later while breast-feeding. The infant’s serum lamotrigine concentration 12.5 hours after birth was 7.71 µg/mL. At the time of the infant’s apneic event, on day 16, the lamotrigine serum level was 4.87 µg/mL, slightly higher than previous reports of levels in infants exposed through breast milk (>4 µg/mL) (9, 17, 26, 28). Nine days postpartum, the mother developed symptoms of toxicity, although her lamotrigine level was not reported. She continued breast-feeding and on day 16, when the infant became apneic, her level was also not reported. On day 17, the mother’s lamotrigine level was 14.9 µg/mL. During pregnancy, the mother’s daily dose had been gradually titrated from 450 mg to 875 mg by the fifth month of gestation to control seizures. Because of a seizure shortly after delivery (the serum lamotrigine level was not reported), the mother’s daily lamotrigine dose was decreased by only 25 mg the second week after delivery. The mother’s high serum lamotrigine levels likely contributed to the high levels in her newborn. This case underscores the need to reduce the lamotrigine dosage to prevent toxicity in mothers after birth.
Method
We enrolled women with bipolar I disorder in an observational study at the University of Pittsburgh. All women chose, under the care of their community psychiatrists, to take lamotrigine during pregnancy, in the postpartum period, or both. All women provided written informed consent, and seven of them consented for their infants’ participation.
Participants had to be at least 18 years old, be pregnant, speak English, meet DSM-IV criteria for bipolar disorder (any subtype), and be treated with lamotrigine. Women were excluded if they had actively abused substances within 6 months of intake, had a positive urine drug screen at intake (except for cannabis), or were taking medication in the FDA pregnancy categories D or X that were not antimanic drugs. Patients were recruited at or before week 20 of gestation. Steady-state serum trough lamotrigine levels were obtained at, or as close as possible to, weeks 20, 30, and 36 of gestation and postpartum weeks 2, 12, and 30. Paired umbilical cord and maternal serum samples were obtained at delivery. The ratios of lamotrigine serum level to daily dose (level-to-dose ratios) were calculated using the following formula: lamotrigine level (µg/mL) × 100/prescribed daily lamotrigine dose (mg) (12) to compensate for dose adjustments. Total lamotrigine serum level was used instead of free lamotrigine serum level because of the limited protein binding of lamotrigine.
Assessments
Patients were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (35) to confirm the diagnosis of bipolar disorder. All patients completed the Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement (SIGH-ADS) (36) and the Mania Rating Scale (derived from the Schedule for Affective Disorders and Schizophrenia) (37) at weeks 20, 30, and 36 of gestation and postpartum weeks 2, 12, 30, and 52.
Infant Outcomes
Infant total serum lamotrigine levels were obtained 2–4 weeks after delivery. Infant development was assessed with the Bayley Scales of Infant Development, including the Mental Development Index and the Psychomotor Development Index scores (38). Gestational duration, head circumference, length, and weight were obtained to calculate Centers for Disease Control and Prevention (CDC) growth percentiles.
Lamotrigine Serum Analysis
Blood samples were obtained by venipuncture into 5-mL BD Vacutainer serum tubes without additives (Franklin Lakes, N.J.). All samples were analyzed by liquid-liquid extraction with ethyl acetate, evaporation, reconstitution, and C-18 high-performance liquid chromatography separation with ultraviolet detection. The lamotrigine levels were run in batches as part of the research protocol and were not used to guide patient dosing. The interassay variation coefficients for lamotrigine ranged from 5.0% to 10.5% for the controls.
Results
Sample Characteristics
Maternal demographic data, data on concomitant medications, and infant data are summarized in Table 1. All seven infants whose mothers provided consent were full term (mean=38.5 weeks’ gestation), healthy, and without congenital malformations. Bayley Scales of Infant Development scores were within normal limits. At birth, all infants were within the normal range of CDC growth percentiles (5th to 95th), with a range from 5th to 62nd. No rashes were reported in the infants exposed to lamotrigine. The only infant with a complication was the daughter of patient 7, our case example.
| Patient Number | Age (Years) | Education | Marital Status | Gestational Age at Delivery (Weeks) | Birth Weight | Bayley Scales of Infant Development | Concomitant Medications | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (g) | Percentile | Infant Sex | MDI Score | Month | PDI Score | Month | ||||||
| 1 | 31 | Some college | Married | 37 | 3,572 | 54 | Male | 87 | 12 | 105 | 12 | Paliperidone, bupropion, quetiapine, prenatal vitamins, folic acid |
| 2 | 30 | Some college | Single | 37 | 3,543 | 52 | Male | 99 | 3 | 88 | 3 | Aripiprazole, carbamazepine, omega-3, prenatal vitamins |
| 3 | 25 | Some college | Divorced | 39 | n/a | n/a | n/a | n/a | n/a | Prenatal vitamins, magnesium supplement | ||
| 4 | 33 | Some college | Married | 40 | 2,835 | 33 | Female | 91 | 12 | 95 | 12 | Risperidone, alprazolam |
| 5 | 30 | College graduate | Single | 37 | 2,495 | 5 | Female | 103 | 12 | 114 | 12 | Carbamazepine, topiramate, lithium, paroxetine, sertraline, clonazepam, low-dosage estrogen birth control pill |
| 6 | 31 | High school graduate (GED) | Single | 39 | 3,685 | 62 | Female | 89 | 12 | 72 | 12 | Atomoxetine, aripiprazole, bupropion, risperidone, omega-3 |
| 7 | 22 | College graduate | Married | 39 | 2,863 | 35 | Female | 99 | 12 | 101 | 12 | Diphenhydramine, prenatal vitamins |
| 8 | 31 | College graduate | Married | 40 | 3,203 | 34 | Male | 100 | 6.5 | 100 | 6.5 | Levothyroxine, bupropion, ziprasidone, cetirizine, prenatal vitamins, folic acid |
Two of the eight women were on lamotrigine monotherapy (patients 3 and 7), and the remaining women took antidepressants (selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors), antipsychotics, or benzodiazepines (alprazolam, clonazepam, or lorazepam). Patient 4 took lorazepam between postpartum weeks 8 and 40. Two mothers were taking carbamazepine and discontinued it in the first trimester. Lamotrigine daily doses ranged from 100 mg to 300 mg. Dosage adjustments during pregnancy or the postpartum period were made by the patient’s psychiatrist in response to manic, hypomanic, or depressive symptoms. Three patients had an average increase of 50 mg (range, 25–100 mg) across pregnancy.
Lamotrigine Level-to-Dose Ratios
Maternal lamotrigine level-to-dose ratios were calculated to correct for dosage changes during the course of the study. Serum levels were obtained for six participants during pregnancy or at delivery. For one patient (patient 8), a serum level was also obtained at 11 weeks’ gestation. The level-to-dose ratios were lower during pregnancy and at delivery than during the postpartum period (Figure 1). The changes in level-to-dose ratios varied between and within individuals across gestation. Ratios were lower during pregnancy than during the postpartum period for five patients (patients 1, 3, 5, 7, and 8). For two patients (patients 1 and 5), ratios reached their lowest value in the third trimester. For patient 8, the lamotrigine concentration increased in the third trimester despite a consistent lamotrigine dosage. Two patients (patients 3 and 7) had consistent increases in level-to-dose ratios during pregnancy, although the levels were less than during the postpartum period.
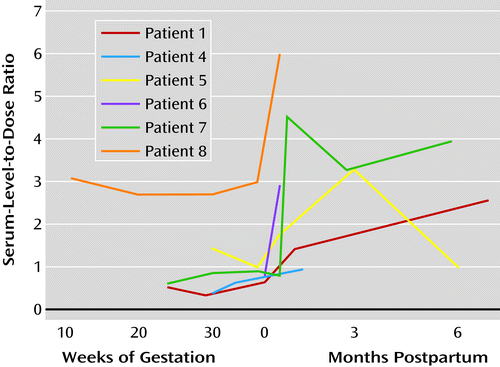
a Ratio was calculated using the following formula: lamotrigine level (µg/mL) × 100/prescribed daily lamotrigine dose (mg).
Patients whose daily dose of lamotrigine was increased had symptom worsening in the second trimester. Their doses were changed at the end of second trimester (>24 weeks for patient 1) and during the early third trimester (29–36 weeks for patients 1, 5, and 7). Patient 1 had dose increases of 50 mg and 25 mg in the second and third trimesters, respectively. Patient 5 had a dose increase of 100 mg. Patient 7 had a dose increase of 25 mg, which was later decreased by 25 mg between postpartum weeks 3 and 11. Although patient 7’s lamotrigine level was 10.26 µg/mL at postpartum week 3 compared with 2.10 µg/mL at 36 weeks’ gestation, she did not report toxic symptoms.
Postpartum lamotrigine serum levels were available for six women. The change in lamotrigine concentrations after delivery ranged from a 30% decrease to a 640% increase compared with the first level (baseline) obtained during pregnancy (Figure 1). Level-to-dose ratios obtained within 4 weeks after delivery reflected a mean level 402% greater than the baseline level obtained during gestation. Compared with the early (30 weeks) and late (≥36 weeks) third trimester, lamotrigine serum levels increased an average of 172% (range, 24%−428%) and 137%, respectively, within 5 weeks after delivery. The most dramatic increase occurred 2 weeks after delivery.
Maternal and Cord Lamotrigine Levels
Four mother-infant pairs provided maternal serum levels and umbilical cord levels at delivery. Maternal lamotrigine daily doses ranged from 25 mg to 275 mg and were at steady state at the time of delivery. The mean infant cord lamotrigine level was 66% (range, 38%−152%) of the maternal serum level at delivery.
Breast-Feeding and Infant Lamotrigine Concentrations
Serum lamotrigine levels were obtained during breast-feeding from four mother-infant pairs between 1.5 and 5 weeks after delivery. Maternal lamotrigine daily doses ranged from 100 mg to 300 mg. All women fully breast-fed their infants. The mean infant lamotrigine serum level was 32.5% (range, 18%−46%) of the maternal serum levels.
Treatment Considerations
Pregnant women taking lamotrigine for bipolar disorder are at risk of symptom recurrence because of declining serum levels of lamotrigine and at risk of toxicity after delivery because of the rapid reversal of pregnancy-induced hypermetabolism. Based on our synthesis of observations from this case series and the epilepsy literature, we propose the following considerations for management:
| 1. | Before conception, titrate lamotrigine to the optimal therapeutic dosage and obtain a total lamotrigine serum level. If the patient presents during pregnancy, obtain the serum level as early as possible. The serum lamotrigine concentration will begin to decrease within the first trimester and will reach its lowest concentration mid-third trimester. Lamotrigine concentration decline is variable. It is likely that the second-trimester levels will be less than those in the first trimester, and the third-trimester levels will be less than those in the second trimester. | ||||
| 2. | Educate patients on the potential for depressive or manic symptom recurrence and the potential need to increase the lamotrigine dosage because of decreasing serum concentrations during pregnancy. In addition, use the Young Mania Rating Scale (39) and the Montgomery-Åsberg Depression Rating Scale (40) or self-report tools, including the Altman Self-Rating Mania Scale (41) and the Inventory of Depressive Symptomatology–Self-Report (42, 43), to quantitatively monitor the return of manic or depressive symptoms during pregnancy and adjust dosage accordingly. | ||||
| 3. | Check the serum lamotrigine level every 4 weeks (44). Increase the dosage by 20%−25% to maintain the target level or to reduce symptoms (44). | ||||
| 4. | Coordinate the patient’s management with the treating obstetrician. Serum lamotrigine levels could be obtained at obstetric appointments for convenience. Dosage management is ideally a collaborative decision between the patient, the psychiatrist, and the obstetrician. | ||||
| 5. | If a patient’s dosage is increased four or more times during pregnancy, decrease the dosage immediately after delivery by 20%−25% to prevent lamotrigine toxicity from the rapid increase in concentration that may occur within the first 2 weeks after delivery and as early as 3 days after delivery. Otherwise, check the lamotrigine serum level every 1–2 weeks and reduce the dosage by 20%−25% until the serum level returns to the prepregnancy level. | ||||
| 6. | If a prepregnancy serum lamotrigine level is not available and the dosage was increased during pregnancy, gradually taper the dose over the first 2 weeks after delivery to the preconception therapeutic dose. Begin the taper within 3 days after delivery to prevent toxicity, and check lamotrigine serum levels every 1–2 weeks. Also, monitor the patient for symptoms of toxicity, including ataxia, blurry or double vision, nausea, and dizziness. | ||||
| 7. | After delivery and during lactation, routine pediatric monitoring is reasonable. Given the case report of apnea in an infant exposed to lamotrigine through breast milk from a mother with postpartum lamotrigine toxicity, close monitoring is warranted. | ||||
Discussion
The lower serum concentrations of lamotrigine observed during pregnancy compared with the postpartum period in this case series are consistent with the epilepsy literature. Three mothers in this study required an increase in lamotrigine dosage during pregnancy. This suggests that pregnant patients with bipolar disorder, like women with epilepsy, experience an increase in symptoms with declining concentrations of lamotrigine during pregnancy. The decreasing concentrations of lamotrigine during pregnancy may precipitate a recurrence of bipolar disorder. Although no therapeutic level for lamotrigine has been established, studies in women with epilepsy have shown that maintaining individual preconception therapeutic serum levels by increasing the dosage of lamotrigine during pregnancy decreases the likelihood of seizure recurrence (44). In this study, no correlation was observed between declining lamotrigine concentration and scores on the SIGH-ADS or the Mania Rating Scale. The lack of correlation likely resulted from the practical problem of capturing an increase in symptoms before the patient’s dosage was increased by her community psychiatrist. Additional studies to correlate declining serum levels with clinical symptoms in real time are needed.
Further study is required to determine the extent to which estrogen in pregnancy decreases serum lamotrigine concentration. We suspect that the effects of estrogen on glucuronidation were associated with the 30% reduction in lamotrigine concentration we observed in patient 5, who started a low-dosage estrogen birth control pill 13 weeks after delivery.
Infants exposed to lamotrigine in utero and through breast milk had umbilical cord and serum lamotrigine levels within the published range. Consistent with infants of women with epilepsy, the mean lamotrigine concentration was lower in the serum of nursing infants than in the cord blood. As with previous studies, this case series underscores the considerable interindividual variability in lamotrigine metabolism in mothers and infants.
This case series adds to the sparse data in the psychiatric literature on lamotrigine dosing in pregnant patients with bipolar disorder, and the consistency of our findings with the neurology literature informs our management recommendations. However, our ability to draw firm conclusions from this study is limited because of the small sample of patients. A formal pharmacokinetic study is needed to confirm the variability in lamotrigine clearance among pregnant women and to determine whether pregnant patients with bipolar disorder would benefit from dosage adjustments to prevent episode recurrence.
1 : Protective effect of pregnancy in women with lithium-responsive bipolar disorder. J Affect Disord 2000; 61:31–39Crossref, Medline, Google Scholar
2 : Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry 2007; 164:1817–1824Link, Google Scholar
3 : Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000; 157:179–184Link, Google Scholar
4 : Reproductive cycle-associated mood symptoms in women with major depression and bipolar disorder. J Affect Disord 2007; 99:221–229Crossref, Medline, Google Scholar
5 ;
6 : Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res 2008; 81:1–13Crossref, Medline, Google Scholar
7 : Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol 2008; 64:200–211Crossref, Medline, Google Scholar
8 : Valproate is associated with new-onset oligoamenorrhea with hyperandrogenism in women with bipolar disorder. Biol Psychiatry 2006; 59:1078–1086Crossref, Medline, Google Scholar
9 : Lamotrigine in pregnancy and lactation: a case report. Epilepsia 1997; 38:1039–1041Crossref, Medline, Google Scholar
10 : Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia 2000; 41:709–713Crossref, Medline, Google Scholar
11 : Lamotrigine clearance during pregnancy. Neurology 2002; 59:251–255Crossref, Medline, Google Scholar
12 : Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology 2004; 63:571–573Crossref, Medline, Google Scholar
13 : The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology 2004; 62:292–295Crossref, Medline, Google Scholar
14 : Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res 2005; 65:185–188Crossref, Medline, Google Scholar
15 : Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther 1987; 42:535–541Crossref, Medline, Google Scholar
16 : Expressed human UGT1.4 protein catalyzes the formation of quaternary ammonium-linked glucuronides. Drug Metab Dispos 1995; 23:299–302Medline, Google Scholar
17 : Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation, and the neonatal period. Epilepsy Res 2009; 85:60–64Crossref, Medline, Google Scholar
18 : Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17β-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos 2009; 37:1841–1847Crossref, Medline, Google Scholar
19 : Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia 2005; 46:1414–1417Crossref, Medline, Google Scholar
20 : Plasma concentrations of lamotrigine and its 2-N-glucuronide metabolite during pregnancy in women with epilepsy. Epilepsia 2008; 49:1075–1080Crossref, Medline, Google Scholar
21 : Changes in lamotrigine pharmacokinetics during pregnancy and the puerperium. Ther Drug Monit 2008; 30:544–547Medline, Google Scholar
22 : Pharmacology of antiepileptic drugs during pregnancy and lactation. Epilepsy Behav 2007; 11:263–269Crossref, Medline, Google Scholar
23 : Drug glucuronidation in clinical psychopharmacology. J Clin Psychopharmacol 2001; 21:500–515Crossref, Medline, Google Scholar
24 : Managing antiepileptic drugs during pregnancy and lactation. Curr Opin Neurol 2009; 22:157–161Crossref, Medline, Google Scholar
25 : Serum levels of lamotrigine during delivery in mothers and their infants. Epilepsy Res 2010; 91:161–165Crossref, Medline, Google Scholar
26 : Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics 2008; 122:e223–e231Crossref, Medline, Google Scholar
27 : Transplacental passage of lamotrigine in a human placental perfusion system in vitro and in maternal and cord blood in vivo. Eur J Clin Pharmacol 2003; 58:677–682Crossref, Medline, Google Scholar
28 : Concentrations of lamotrigine in a mother on lamotrigine treatment and her newborn child. Eur J Clin Pharmacol 1997; 51:481–484Crossref, Medline, Google Scholar
29 : UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem Pharmacol 2002; 63:409–419Crossref, Medline, Google Scholar
30 : Prophylactic treatment of bipolar disorder in pregnancy and breastfeeding: focus on emerging mood stabilizers. Bipolar Disord 2006; 8:207–220Crossref, Medline, Google Scholar
31 : Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother 2009; 43:1893–1897Crossref, Medline, Google Scholar
32 : Concerns regarding lamotrigine and breast-feeding. Epilepsy Behav 2004; 5:102–105Crossref, Medline, Google Scholar
33 : Transfer of lamotrigine into breast milk. Ann Pharmacother 2006; 40:1470–1471Crossref, Medline, Google Scholar
34 : Neonatal outcomes with the use of lamotrigine for bipolar disorder in pregnancy and breastfeeding: a case series and review of the literature. Psychopharmacol Bull 2009; 42:91–98Medline, Google Scholar
35 : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
36 : Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement (SIGH-ADS). New York, New York State Psychiatric Institute, 2003Google Scholar
37 : Schedule for Affective Disorders and Schizophrenia–Change Version, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
38 : Scales of Infant Development. New York, Psychological Corp, 1969Google Scholar
39 : A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
40 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
41 : The Altman Self-Rating Mania Scale. Biol Psychiatry 1997; 42:948–955Crossref, Medline, Google Scholar
42 : The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res 1986; 18:65–87Crossref, Medline, Google Scholar
43 : The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and Self-Report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res 2006; 9:45–59Crossref, Google Scholar
44 : Algorithm for lamotrigine dose adjustment before, during, and after pregnancy. Acta Neurol Scand 2012; 126:e1–e4Crossref, Medline, Google Scholar



