Diffusion Tensor Imaging White Matter Endophenotypes in Patients With Schizophrenia or Psychotic Bipolar Disorder and Their Relatives
Abstract
Objective
Both schizophrenia and bipolar disorder are hypothesized to involve disordered brain connectivity. Prior studies show low white matter integrity, measured with diffusion tensor imaging, for both disorders. The authors studied disease specificity and endophenotypic status of these abnormalities by examining patients and their unaffected relatives.
Method
The 513 participants included probands with schizophrenia, probands with psychotic bipolar disorder, their first-degree relatives, and healthy comparison subjects. Fractional anisotropy measures of white matter integrity were collected at two sites as a part of the Bipolar-Schizophrenia Network on Intermediate Phenotypes project. Relatives with cluster A or B personality characteristics were further examined.
Results
Both the probands with schizophrenia and those with psychotic bipolar disorder showed lower fractional anisotropy than the comparison subjects in multiple white matter regions; differences were more marked in schizophrenia. No significant differences existed between proband groups, but in some brain regions scores on a measure of the dimensional continuum between schizophrenia and bipolar disorder, the Schizo-Bipolar Scale, showed correlations with fractional anisotropy. Many regions affected in schizophrenia probands showed similar but smaller effects in relatives, with a continuous fractional anisotropy decrease from healthy subjects to relatives to cluster A/B relatives to probands. The pattern for psychotic bipolar disorder was similar but involved fewer brain regions. Effects in bipolar relatives were limited to younger subjects. Fractional anisotropy decreased with age in all groups; this decrease was exaggerated in schizophrenia but not psychotic bipolar disorder.
Conclusions
Fractional anisotropy was highly heritable, supporting its value as a potential endophenotype.
Since Kraepelin’s initial distinction (1), schizophrenia and bipolar disorder have been viewed as separate clinical entities with distinct clinical courses and outcomes. However, significant overlaps exist in symptoms (2), alterations in cognition (3), brain structure (4), brain functioning, and disease risk genes, especially between schizophrenia and psychotic bipolar disorder (4). Both disorders are heritable; large meta-analytic linkage studies based on clinical schizophrenia and bipolar phenotypes have shown several overlapping genetic risk loci (5). Schizophrenia and affective psychoses co-occur within kindreds, suggesting shared familial risk and consistent with shared genes that confer risk for both illnesses, most convincingly for schizophrenia and psychotic bipolar disorder. Thus, some illness risk genes and associated brain processes are shared by schizophrenia and psychotic bipolar disorder, while others are likely disease-specific. Such deficits also occur in unaffected relatives of probands, in whom illness risk genes are likely overrepresented (6) in comparison to the population at large. Such research in schizophrenia has identified several functional and anatomical indicators of disease risk, known as endophenotypes. Endophenotypes are heritable, quantifiable, trait-related, illness-associated biological features that cosegregate with disease in families, are overrepresented in the unaffected relatives compared with the general population (7), and are conceived of as situated between genes and phenomenology.
The dysconnectivity model of schizophrenia (8) posits that several brain circuits interact abnormally to generate the schizophrenia phenotype; this proposal is supported by reported functional connectivity deficits (9). While empirical support for dysconnectivity is strongest at the functional level, it is plausible that connection problems originate in disrupted white matter connections. Integrity of those connections is usually measured by using estimates of brain tissue water diffusion obtained with diffusion tensor imaging (DTI), mainly fractional anisotropy. In schizophrenia, differences (10) in white matter structure and integrity have been observed both as a global measure and in specific structures. The relationship between altered structural and functional measures has been demonstrated previously (11). Analogous abnormalities may be present among unaffected relatives of persons with schizophrenia (9), suggesting that brain dysconnectivity may be a schizophrenia endophenotype (12). Similar but less consistent deficits are reported for bipolar disorder (13).
Confirming other findings (9), we previously reported functional network differences assessed with resting-state functional magnetic resonance imaging (fMRI) (14). In a large multicenter study, we have now investigated whether white matter connectivity is similarly impaired in probands with schizophrenia, probands with psychotic bipolar disorder, and their first-degree relatives, to address the endophenotypic status of any such deviations. Given the known associations between cluster A and B personality traits and risk for schizophrenia (15) and psychotic bipolar disorder (16), we also examined abnormalities in relatives with cluster A or B personality traits, the relationship between fractional anisotropy and a measure of the continuum between schizophrenia and affective disorder (2), and the heritability of observed abnormalities. As the validity of the diagnostic status of schizoaffective disorder is controversial (17), we decided not to analyze probands with schizoaffective disorder and their relatives as separate groups. These 35 probands were classified as having either bipolar disorder (schizoaffective disorder, manic) or schizophrenia (schizoaffective disorder, depressed), as suggested elsewhere (2, 5).
Method
Participants
Data were collected from 513 subjects participating at two sites of the Bipolar-Schizophrenia Network on Intermediate Phenotypes study (B-SNIP): 104 healthy comparison subjects, 109 probands with schizophrenia, 35 probands with schizoaffective disorder (16 depressed, 19 manic), 63 probands with psychotic bipolar disorder, 95 relatives of the schizophrenia probands, 43 relatives of the probands with schizoaffective disorder (24 relatives of depressed probands, 19 relatives of manic probands), and 64 relatives of the probands with psychotic bipolar disorder. The groups compared in the study, after regrouping of the probands with schizoaffective disorder, are shown in Table 1. The participants were recruited through word of mouth, advertisements, and community support groups. All subjects provided written informed consent statements approved by review boards of Hartford Hospital/Yale University and the University of Maryland/Johns Hopkins University. The participants were independent of study groups analyzed in previous publications on DTI studies.
| Schizophrenia | Psychotic Bipolar Disorder | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Healthy Comparison Subjects (N=104) | Probands (N=125) | Relatives (N=119) | Relatives With Cluster A/B Traitsa (N=17) | Probands (N=82) | Relatives (N=83) | Relatives With Cluster A/B Traitsa (N=18) | Total (N=513) |
| Age (years) | ||||||||
| Mean | 38.9 | 33.8b | 42.5b | 42.5 | 36.4 | 40.6 | 40.6 | 38.4 |
| SD | 1.3 | 1.0 | 1.5 | 3.8 | 1.4 | 2.5 | 4.1 | 0.6 |
| Gender | ||||||||
| Female | 61 | 44b | 79 | 9 | 53 | 55 | 11 | 292 |
| Male | 43 | 81b | 40 | 8 | 29 | 28 | 7 | 221 |
| Race | ||||||||
| White | 74 | 72 | 70 | 9 | 65 | 71 | 13 | 352 |
| Black | 24 | 45 | 27 | 7 | 15 | 7 | 3 | 118 |
| Ethnicity | ||||||||
| Non-Hispanic | 100 | 115 | 110 | 14 | 75 | 74 | 16 | 474 |
| Hispanic | 4 | 10 | 9 | 3 | 6 | 9 | 2 | 38 |
| Site | ||||||||
| Hartford | 65 | 60 | 70 | 9 | 54 | 58 | 13 | 307 |
| Baltimore | 39 | 65 | 49 | 8 | 28 | 25 | 5 | 206 |
| Medication | ||||||||
| First-generation antipsychotic | 0 | 18 | 0 | 0 | 8 | 0 | 0 | 26 |
| Second-generation antipsychotic | 0 | 109 | 0 | 0 | 57 | 0 | 0 | 166 |
| Antidepressant | 3 | 56 | 16 | 2 | 38 | 17 | 4 | 130 |
| Anticonvulsant mood stabilizer | 0 | 32 | 3 | 1 | 45 | 5 | 0 | 85 |
| Lithium | 0 | 11 | 0 | 0 | 22 | 2 | 0 | 35 |
| Stimulant | 0 | 1 | 0 | 0 | 7 | 2 | 0 | 11 |
| Sedative-hypnotic | 3 | 21 | 3 | 0 | 21 | 6 | 2 | 65 |
| Anticholinergic | 0 | 23 | 0 | 0 | 9 | 0 | 0 | 32 |
| None | 99 | 9 | 71 | 14 | 8 | 51 | 7 | 238 |
Consensus diagnoses were established by trained clinical raters and senior diagnosticians using clinical data and the Structured Clinical Interview for DSM-IV (SCID). The probands were clinically stable and had been taking stable doses of medications for at least 4 weeks. All of the bipolar subjects were psychotic, as determined by the presence of hallucinations or delusions during prior affective episodes (14) or current psychosis at the time of scanning. Current psychosis was judged according to the relatively permissive criterion of a score of 3 or higher on one or more of the following scales of the Positive and Negative Syndrome Scale (PANSS) (18): delusions, conceptual disorganization, hallucinations, and suspiciousness/persecutory delusions. Other positive symptoms (grandiosity, excitement) did not qualify as psychosis. The relatives were further classified by the presence or absence of symptoms of DSM-IV-TR cluster A and cluster B personality disorders with the Structured Interview for DSM-IV Personality (19). Relatives with cluster A or B symptoms were included even if they possessed one fewer symptom than is required to meet the full criteria for the axis II disorder. The comparison subjects and the probands’ relatives were free of current axis I disorders, as assessed by the SCID (those who had axis I disorders were excluded). All subjects were free of known neurological illness and were not substance abusers, as judged by negative urine toxicology screens. Though we strove to achieve balanced subject groups, matching was not exact. The subject population overall contained more African Americans at the Baltimore site and more Hispanics at the Hartford site, consistent with the 2010 U.S. Census. More relatives were female, likely because of family volunteer bias.
Imaging and DTI Processing
DTI data were obtained on 3-T scanners (Siemens, Erlangen, Germany). The scanners at Hartford (Allegra, head only) and Baltimore (Trio) used highly similar but not identical scanning sequences; both used single-shot spin-echo planar imaging (EPI) with a twice-refocused balance echo sequence to reduce eddy current distortions. Hartford used TR/TE=6,300/85 ms, field of view=220 m, b=1,000 s/mm2 along 32 directions, 45 contiguous slices, three imaging series, and a voxel size of 1.7×1.7×3 mm. Baltimore used TR/TE=6,700/92 ms, field of view=230 m, b=1,000 s/mm2 along 30 directions, 48 contiguous slices, two imaging series, and a voxel size of 1.8×1.8×3 mm.
All data processing was executed at Hartford by investigators who were blind to diagnosis and used the same methods and quality thresholds. Data were checked for noise, motion, and artifacts (seven subjects at Hartford and 34 at Baltimore were excluded). FSL software (www.fmrib.ox.ac.uk/fsl) was used for standard analysis including motion correction and eddy current correction, with coregistration to the B0 image with gradient directions corrected. Fractional anisotropy was used to assess white matter integrity. To enhance reproducibility and overlap of white matter fibers, we calculated a common white matter skeleton and used tract-based spatial statistics (20).
Data Analysis
Effects of scanner, age, sex, race, and ethnicity were included in the general linear model and thus removed from main effects. Analysis was performed both at the voxel level and by region of interest.
Regions of interest were delineated on the white matter skeleton obtained by using all 50 predefined regions from the International Consortium of Brain Mapping (21) white matter atlas. Those regions were then overlaid with the white matter skeleton. The atlas covers only deep white matter structures that are most reproducible on standard coregistration. Tract-based spatial statistics allow for reliable definition of more fiber tracts. To investigate those tracts, we manually defined 26 additional regions using anatomical information only.
Voxel-based whole-skeleton analysis was performed on the skeletonized data by using threshold-free cluster enhancement (22) to correct for multiple comparisons. Since the voxel-level data showed group differences in variance, permutation-based approaches were used instead of the standard general linear model. The region-of-interest data showed uniform variance at p<0.05 for all regions.
In a direct comparison of probands’ relatives and either probands or healthy comparison subjects, using regions of interest or threshold-free cluster enhancement whole-map analysis, we detected no significant differences at a p<0.01 corrected level. Statistical power in this initial analysis was hampered by the large number of voxels in the whole-brain analysis and/or by large anatomical regions, diluting focal regional differences. We therefore analyzed the relatives’ data within smaller regions, defined as those showing the most significant differences in the probands. We selected the 29 anatomically defined regions showing significant statistical differences when we compared both proband groups to the comparison subjects in both voxel-based and region-of-interest analyses. Within each such region, we defined smaller, focused regions of interest contained within the clusters identified with threshold-free cluster enhancement, around the maxima of contrast between all probands and the healthy comparison subjects. Of these 29 subregions; all showed differences in schizophrenia, and 14 also showed differences for psychotic bipolar disorder. Table 2 shows Montreal Neurological Institute (MNI) coordinates for the centers of the primary anatomical regions and for the focal subregions. Coordinates of the subregions in all of the defined regions are presented in Table S1 in the data supplement accompanying the online version of this article.
| Secondary Analysisc (C=comparison group, P=probands, R=relatives, A/B=relatives with cluster A or B traitsd) | |||||||
|---|---|---|---|---|---|---|---|
| Region of Interesta, Proband Diagnosis, and Prior Studiesb | MNI Coordinates of Mass Center | Group Effect | MNI Coordinates | C > R | R > P | Non-A/B > A/B | Heritability (h2)e |
| Genu of corpus callosum | –0, 29, 12 | 0.36 | |||||
| Schizophrenia; see 4, 23, 24 | Probandsf | 17, 32, 20 | X | X | X | ||
| Bipolar disorder; see 25, 26 | Probandsf | −8, 26, 16 | X | X | |||
| Body of corpus callosum | –0, –4, 30 | 0.30 | |||||
| Schizophrenia; see 4, 23, 24 | Probandsf | 15, 13, 31 | X | ||||
| Bipolar disorder; see 27 | Probands | 15, 13, 31 | X | ||||
| Splenium of corpus callosum | –0, –43, 19 | 0.71g | |||||
| Schizophrenia | Probandsf | –25, –51, 20 | X | ||||
| Bipolar disorder | |||||||
| Anterior limb of internal capsule, left | –18, 10, 9 | 0.38 | |||||
| Schizophrenia | Probands | –22, 12, 15 | |||||
| Bipolar disorder | |||||||
| Anterior limb of internal capsule, right | 17, 10, 9 | 0.76g | |||||
| Schizophrenia; see 28 | Probandsf | 20, 12, 13 | |||||
| Bipolar disorder; see 23, 25 | Probands | 22, 17, 15 | |||||
| Anterior corona radiata, left | –23, 30, 12 | — | |||||
| Schizophrenia; see 24, 29 | Probandsf, relatives | –20, 36, 25 | X | X | |||
| Bipolar disorder | Probands | −19, 35, 21 | |||||
| Anterior corona radiata, right | 21, 30, 13 | 0.31 | |||||
| Schizophrenia; see 24 | Probandsf | 24, 29, 14 | X | X | |||
| Bipolar disorder | |||||||
| Posterior corona radiata, right | 24, –37, 31 | 0.32 | |||||
| Schizophrenia | Probands | 19, –37, 35 | X | X | |||
| Bipolar disorder; see 26 | Probands | 21, −35, 33 | |||||
| Posterior thalamic radiation, left | –34, –56, 7 | — | |||||
| Schizophrenia; see 23, 29 | Probands | –32, –46, 16 | X | ||||
| Bipolar disorder; see 30 | Probands | −28, −58, 18 | |||||
| Sagittal stratum, right | 40, –28, –12 | 0.53g | |||||
| Schizophrenia; see 23 | Probands | 39, –14, –16 | |||||
| Bipolar disorder | |||||||
| Superior longitudinal fasciculus, right | 37, –25, 27 | 0.53g | |||||
| Schizophrenia; see 23 | Probands | 35, –22, 39 | X | ||||
| Bipolar disorder | Probands | 35, –39, 44 | X | ||||
| Tapetum, left | –31, –48, 16 | — | |||||
| Schizophrenia | Probands | –32, –46, 15 | |||||
| Bipolar disorder | Probands | −31, −50, 15 | |||||
| Posterior corona radiata, superior aspect, right | 17, –40, 57 | 0.46 | |||||
| Schizophrenia | Probandsf | 21, –45, 53 | X | ||||
| Bipolar disorder | Probands | 21, –44, 53 | X | ||||
| Posterior corona radiata, superior aspect, left | –17, –40, 56 | 0.57g | |||||
| Schizophrenia | Probands | –10, –35, 58 | X | ||||
| Bipolar disorder | Probands | –15, –37, 59 | |||||
| Anterior corona radiata, superior aspect, right | 15, 41, 34 | — | |||||
| Schizophrenia | Probandsf, relatives | 14, 37, 40 | X | ||||
| Bipolar disorder; see 30 | Probands | 14, 38, 39 | |||||
| Anterior corona radiata, superior aspect, left | –16, 42, 34 | — | |||||
| Schizophrenia | Probands | –20, 36, 25 | X | ||||
| Bipolar disorder | |||||||
| Anterior corona radiata, inferior aspect, right | 14, 52, –4 | 0.26 | |||||
| Schizophrenia | Probands | 12, 46, –16 | X | ||||
| Bipolar disorder | |||||||
| Anterior corona radiata, inferior aspect, left | –15, 52, –4 | 0.37 | |||||
| Schizophrenia | Probandsf | –18, 53, –1 | X | ||||
| Bipolar disorder | X | ||||||
| Anterior corona radiata, right, middle frontal gyrus white matter | 36, 19, 28 | 0.39 | |||||
| Schizophrenia | Probandsf | 33, 19, 23 | X | ||||
| Bipolar disorder | |||||||
| Anterior corona radiata, left, middle frontal gyrus white matter | –34, 21, 28 | 0.53g | |||||
| Schizophrenia | Probands | –33, 20, 25 | |||||
| Bipolar disorder | Probands | –33, 23, 25 | |||||
| Superior longitudinal fasciculus, right | 47, –2, 27 | — | |||||
| Schizophrenia | Probandsf | 51, 5, 33 | |||||
| Bipolar disorder | |||||||
| Superior longitudinal fasciculus, left | –46, –3, 28 | — | |||||
| Schizophrenia | Probandsf | –40, 2, 26 | X | ||||
| Bipolar disorder | |||||||
| Superior longitudinal fasciculus, posterior aspect, right | 41, –53, 35 | 0.35 | |||||
| Schizophrenia | Probandsf | 34, –66, 27 | |||||
| Bipolar disorder | X | ||||||
| Superior longitudinal fasciculus, posterior aspect, left | –42, –47, 37 | 0.27 | |||||
| Schizophrenia | Probandsf | –33, –52, 32 | |||||
| Bipolar disorder | |||||||
| Posterior corona radiata, right | 18, –70, 28 | 0.57g | |||||
| Schizophrenia | Probandsf | 25, –59, 20 | |||||
| Bipolar disorder | Probands | 18, –70, 39 | |||||
| Posterior corona radiata, left | –19, –69, 30 | — | |||||
| Schizophrenia | Probandsf | –25, –54, 20 | |||||
| Bipolar disorder | Probands | –28, –59, 20 | |||||
| Posterior thalamic radiation, right skeleton | 25, –81, –3 | 0.48 | |||||
| Schizophrenia | Probandsf | 32, –71, –3 | X | X | |||
| Bipolar disorder; see 25 | |||||||
| Posterior thalamic radiation, left skeleton | –24, –80, –1 | — | |||||
| Schizophrenia | Probands | –25, –81, 0 | |||||
| Bipolar disorder | |||||||
| Superior temporal gyrus white matter, left skeleton | –44, 4, –24 | 0.56g | |||||
| Schizophrenia | Probands | –44, 12, –24 | |||||
| Bipolar disorder | |||||||
Our criteria for axis 2 cluster A or B characteristics were met by 17 relatives of the schizophrenia probands and 18 relatives of the bipolar probands. This allowed us to analyze clinical regression along the following scale: healthy comparison subjects, 1; healthy relatives without cluster A or B symptoms, 2; relatives with cluster A or B symptoms, 3; probands, 4. Additional general linear model analyses (at both region-of-interest and voxel levels) correlated this status with fractional anisotropy.
The Schizo-Bipolar Scale (2) was designed to investigate overlap between schizophrenia and psychotic bipolar disorder by using continuous measures based on phenomenology and illness course. The correlation between fractional anisotropy values and scores on the Schizo-Bipolar Scale were calculated in a separate general linear model analysis.
Additional general linear model analyses assessed white matter differences possibly attributable to medications. The subjects were assigned to eight medication groups (Table 1). A general linear model analysis was performed separately for each diagnostic group, contrasting individuals using medications from each of the eight categories with all other subjects.
Heritability analyses were conducted by using Sequential Oligogenic Linkage Analysis Routines (SOLAR) (31). This software determines the relative importance of familial and environmental influences on a phenotype by modeling the covariance among family members as a function of kinship. Scanner was used as a covariate.
Results
Differences Between Diagnostic Groups in Probands and Relatives
We first analyzed global mean fractional anisotropy averaged over the whole white matter skeleton. Fractional anisotropy in both proband groups was significantly lower (p<0.001) than in the healthy comparison subjects (t=4.82, df=227, for schizophrenia; t=3.69, df=184, for psychotic bipolar disorder; effect size: d=0.64 and 0.54, respectively), with no differences between proband groups (effect size: d=0.04). Fractional anisotropy was lower in 29 regions (of 76 analyzed) in probands (Table 2); none differed significantly between schizophrenia and psychotic bipolar disorder. For all regions, effect sizes for the differences between proband groups were less than 0.2. The global fractional anisotropy average showed significantly higher (p<0.01) variance in the bipolar probands than in either the schizophrenia probands or the comparison subjects and nonsignificantly higher variance in the relatives of the bipolar probands (p<0.06). Figure 1 (top left) shows the distribution of differences between the healthy comparison subjects and the clinical groups (p<0.01, corrected). Many regions revealed fractional anisotropy differences for schizophrenia but not psychotic bipolar disorder. With the exception of one cluster, all voxels showing differences in psychotic bipolar disorder also showed differences in schizophrenia. None of the fractional anisotropy values was higher in a clinical group than in the comparison subjects.
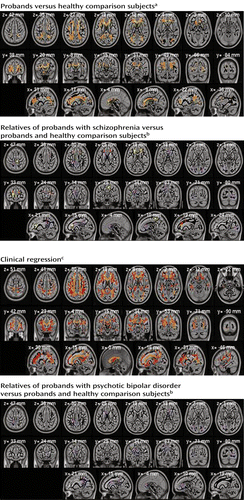
a Results are from voxel-based randomized analysis. Orange voxels show lower fractional anisotropy in both schizophrenia and bipolar probands, red in schizophrenia probands only (p<0.01, whole-brain correction by threshold-free cluster enhancement). Yellow voxels show lower fractional anisotropy both in schizophrenia and bipolar probands and in unaffected relatives of schizophrenia probands (p<0.05, corrected).
b Regions were anatomically predefined. Red and yellow show regions (right anterior corona radiata, superior aspect, and left anterior corona radiata) where schizophrenia relatives differ significantly from comparison subjects (resemble probands). Violet shows regions where fractional anisotropy of relatives is significantly higher than that of probands (thus resembles value of comparison subjects).
c Voxels show regression of fractional anisotropy values to the clinical score (0, healthy comparison subjects; 1, unaffected relatives; 2, relatives with cluster A personality traits; 3, probands) significant at p<0.01 after whole-brain correction by threshold-free cluster enhancement. Yellow regions show differences for both schizophrenia and psychotic bipolar disorder; orange voxels show significant differences in schizophrenia only.
Among the relatives, the whole-brain average for fractional anisotropy was lower in the schizophrenia relatives than in the healthy comparison subjects (t=2.62, df=221, p<0.01, d=0.35). Two anatomically defined regions (left anterior corona radiata and superior right corona radiata) differed significantly (p<0.05 corrected) between the schizophrenia relatives and comparison subjects (Table 2). Table S1 and Figures 1S and 2S in the online data supplement shows results of the focused post hoc analyses of the regions of interest. In the secondary analyses, fractional anisotropy in the schizophrenia relatives was significantly lower than in the healthy comparison subjects in 10 regions and higher than in the schizophrenia probands in 11 regions. Figure 1 (top right) shows the spatial distribution of those differences. In many regions, the relatives in the schizophrenia group closely resembled the probands. Only the corpus callosum genu in the schizophrenia relatives differed significantly from that region in both the schizophrenia probands and the healthy comparison subjects.
The relatives of the probands with psychotic bipolar disorder showed fewer significant effects, with only one region where their fractional anisotropy was lower than that of the healthy comparison subjects (superior aspect of the left posterior corona radiata) and five regions where their values were higher than those of the bipolar probands. No analyzed region showed significant differences between schizophrenia and psychotic bipolar disorder for either the probands or the relatives.
Most of the 29 regions differing between probands and healthy comparison subjects also manifested a significant clinical regression effect for schizophrenia; in psychotic bipolar disorder this effect was restricted to the corpus callosum genu (Table 2). Similarly, in voxel-based analysis (Figure 1, bottom left) most regions showing differences between probands and comparison subjects manifested similar clinical regression effects. As with these clinical contrasts, correlations between fractional anisotropy and clinical status were higher in schizophrenia than in psychotic bipolar disorder.
Fractional anisotropy values are displayed in Figure 2 for the anatomically defined genu of the corpus callosum, the region showing the strongest differences among groups.

To study effects of psychosis common to both diseases, we analyzed group differences (probands and relatives versus comparison subjects) and clinical regression correlations with the data for schizophrenia and psychotic bipolar disorder combined. The region-of-interest analysis of clinical regression and the analysis of probands versus comparison subjects showed that all regions showing significant differences for schizophrenia (Table 2) also showed lower values in the joint group analysis. In the comparisons of relatives and comparison subjects, only one region (left anterior corona radiata) showed significant difference in the combined analysis.
Cluster A or B Traits in Relatives
Few relatives met our criteria for cluster A or B personality disorders, so their comparisons with relatives lacking those personality characteristics had lower statistical power. Nevertheless, in the analysis comparing cluster A relatives to other relatives, two regions (Table 2) showed significant (p<0.05) differences for the relatives of probands with schizophrenia, and one region showed a difference for the relatives of the bipolar probands. Both the corpus callosum genu and right posterior thalamic radiation skeleton showed significant stepwise progressions of fractional anisotropy values from the healthy comparison subjects to relatives to cluster A relatives to schizophrenia probands, with the cluster A relatives resembling the probands. Similar trends were seen in other regions where probands differed from comparison subjects. No such differences were observed for relatives with cluster B traits.
Heritability
Whole-brain mean fractional anisotropy was highly heritable (h2=0.45, p<0.001), as was fractional anisotropy for many anatomically defined regions (Table 2, Figure 3), not restricted to those showing between-group effects (see Table S1 in the online data supplement).
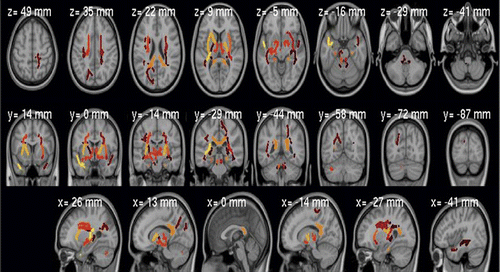
a Significant at p<0.05 corrected for multiple comparisons.
Correlation With Schizo-Bipolar Scale Scores
Analysis of anatomically defined regions of interest showed a weak association between scores on the Schizo-Bipolar Scale and fractional anisotropy values (t=2.11, df=181, p<0.05, uncorrected) in the left superior longitudinal fasciculus, peaking at MNI coordinates –33, –19, 41 (see Figure 3S in the online data supplement).
Demographic Measures
While the general linear model approach removes effects of confounding variables, interactions among sex, race or ethnicity, age, and diagnosis are relevant for understanding pathologic and clinical applications. Separate general linear model analyses were performed to examine those interactions. Age was the most important confounding factor; fractional anisotropy values decreased significantly with age in all groups and regions of interest. For the global whole-brain average, age interacted with disorder. The interaction was negative in the probands with psychotic bipolar disorder and their relatives (less decrease with age than in comparison subjects). In schizophrenia it was positive for the whole-brain average and for the superior posterior corona radiata, where fractional anisotropy values decreased more steeply with age than they did among the healthy comparison subjects.
In the analysis of whole-brain mean fractional anisotropy, sex and race showed significant differences, with men and European Americans showing higher values. The effects of sex and race on global fractional anisotropy did not interact with clinical diagnosis. At the regional level, sex showed an interaction (t>3.0, p<0.01) in five regions in the comparison of bipolar probands and healthy comparison subjects: the corpus callosum body (MNI coordinates 13, 3, 33), right and left anterior limbs of the internal capsule (MNI coordinates 23, 6, 20 and –12, 14, 17), right anterior corona radiata (MNI coordinates 23, 27, 5), and right inferior fronto-occipital fasciculus (MNI coordinates 29, 14, –7). The direction of the interaction was such that in women the decrease of fractional anisotropy in the bipolar probands was larger than in men.
Other DTI Measures
We also analyzed radial diffusivity, mean diffusivity, and axial diffusivity. They showed no significant differences at the threshold used for fractional anisotropy. We additionally investigated data pooled in all voxels showing significant changes in fractional anisotropy. As expected, fractional anisotropy decreases were accompanied by increases in all three diffusivity measures. A significant (p<0.01) difference was observed; differences in radial diffusivity were larger than those for mean and axial diffusivity in both the schizophrenia and bipolar probands, suggesting that demyelination (not axonal injury) causes the observed white matter differences.
Scanner Effects and Reproducibility
After we adjusted for age, mean whole-brain fractional anisotropy did not differ between scanners. Site showed no interaction with clinical diagnosis, and the spatial map of scanner effects did not correlate with clinical effect maps. Quantifying between-scanner reproducibility as a stand-alone measure is difficult. To estimate it, we ran general linear model analyses for each scanner separately and calculated general linear model contrast maps for each site. To quantify their agreement, we developed a randomization procedure in which data from both scanners were randomly split into groups the size of the scanned subjects at each site; the general linear model was run for each data set separately, and the degrees of reproducibility in the subsamples were compared. The between-scanner correlation (r) values were 0.60 for age, 0.12–0.18 for the main contrast of probands and healthy comparison subjects, 0.05–0.10 for the main contrast of relatives and comparison subjects, and 0.06–0.12 for the contrast of schizophrenia and bipolar probands. In randomization procedures, real comparisons fell between 25% (schizophrenia probands versus comparison subjects) and 6% (relatives of bipolar probands versus comparison subjects) of randomized splits. Thus, the between-scanner data agreement was not significantly worse (p<0.05) than would be expected from two data sets derived from the same scanner. General linear model analyses performed separately for each scanner showed the same, but less significant, findings than in the combined analysis.
Medication
We compared probands receiving a particular medication class with all other probands. Patients with psychotic bipolar disorder who took second-generation antipsychotic drugs showed significantly higher fractional anisotropy in the corpus callosum genu and body (t=2.93, df=80, p<0.01 uncorrected) than did the remaining bipolar probands. For schizophrenia, a significant effect was observed in the corpus callosum splenium for anticonvulsant mood stabilizers (t=–3.31, df=123, p<0.01), such that probands with schizophrenia who were taking these medications had lower fractional anisotropy values than other schizophrenia probands.
Discussion
We compared fractional anisotropy values of healthy comparison subjects to those of a large group of probands with schizophrenia or psychotic bipolar disorder and their first-degree relatives. After identifying between-group differences, we assessed whether deviations observed in probands might qualify as candidate endophenotypes (i.e., were also observed in their relatives). Our results show that despite scanner or site differences, DTI studies from multiple sites can be successfully combined to achieve increased statistical power for detecting differences between clinical groups.
The white matter abnormalities in schizophrenia and psychotic bipolar disorder were similar in spatial distribution. The corpus callosum genu and body showed the most significant deficits in fractional anisotropy in both proband groups, in agreement with previous reports (32). Like others (23), we found no significant differences between the probands with schizophrenia and those with psychotic bipolar disorder. The close agreement between the spatial distributions of maps of fractional anisotropy differences, similar magnitudes of those differences, and higher variance in the bipolar probands suggest that this might result, in part, from greater heterogeneity of white matter abnormalities in bipolar disorder (4, 25) than in schizophrenia.
In comparison to findings based on diagnostic categories, ratings on the dimensional Schizo-Bipolar Scale (2) revealed a modest correlation with fractional anisotropy in the left superior longitudinal fasciculus, suggesting that this region distinguishes schizophrenia and psychotic bipolar disorder. This fasciculus, connecting Broca’s and Wernicke’s areas, has been reported as abnormal in both schizophrenia (2) and bipolar disorder (33). As no differences were found between the schizophrenia and bipolar probands in fractional anisotropy, scores on the Schizo-Bipolar Scale may capture subtle differences between those diseases better than clinical diagnosis.
Many abnormalities present among probands were observed, in attenuated form, in first-degree relatives. Indeed, there was a linear, stepwise progression from healthy comparison subjects to noncluster relatives to cluster A relatives to probands. The schizophrenia relatives generally showed fractional anisotropy deviations similar to, but less significant than, those seen in the schizophrenia probands. Fractional anisotropy decreased with age in all groups, an effect that was exaggerated in schizophrenia but not psychotic bipolar disorder, suggesting more pronounced progressive white matter abnormalities in schizophrenia. In psychotic bipolar disorder, fractional anisotropy decreases may occur early and not progress thereafter.
The subgroups of relatives with cluster A or B symptoms resembled the probands more than the other relatives did. This effect was most pronounced in the corpus callosum genu and posterior thalamic radiation skeleton of the schizophrenia relatives, but it was also seen in the bipolar probands’ relatives in the corpus callosum genu. While the schizophrenia and bipolar probands showed similar differences from the comparison subjects, the relatives showed weaker, more diverse differences. This was illustrated by the analysis that combined data from the schizophrenia and bipolar relatives and compared them to the healthy subjects, which showed less significant effects than analyses in each group of relatives separately. Thus, the differences in relatives were smaller in effect size and more disease specific. The relatives’ fractional anisotropy values fell between those of the healthy comparison subjects and the probands, but abnormal regions differed for each disorder. Overall, our findings point to similarity in the distributions of white matter deficits in schizophrenia and bipolar probands, with similar but attenuated abnormalities observed in their relatives. This suggests that genetic risk factors present in the relatives differ from those in the probands, perhaps indicating that protective genetic variants are exerting effects visible at a DTI level.
Current medications showed different effects in the clinical groups. Interpretation of those effects is difficult, as the study design did not allow us to differentiate among premedication patterns of symptoms or severity, prescription bias, and medication effects. Comparisons of the medication groups seemed to exclude medications as the main cause of lower fractional anisotropy. Analysis of the sex and race interactions showed lower fractional anisotropy values in women and in African American subjects, with sex-by-diagnosis interactions stronger for psychotic bipolar disorder.
Fractional anisotropy values decreased significantly with age in all groups and most brain regions. In the whole brain and one region, this finding interacted with diagnosis for schizophrenia: age-related fractional anisotropy decreases were significantly greater in the schizophrenia probands, consistent with findings in other studies (34). This may support the hypothesis (35) that schizophrenia is a progressive brain disorder, perhaps related to accelerated aging (36) and/or medication effects. The probands with psychotic bipolar disorder and their relatives showed opposite effects: the differences from the comparison subjects were strongest for younger probands and relatives, disappearing with increasing age. This may explain why the present study did not replicate the widespread fractional anisotropy decreases in relatives of bipolar probands that were reported earlier (28) in younger subjects. Our global analysis showed that the age-related fractional anisotropy decrease was smaller for the bipolar probands and their relatives than for schizophrenia probands and healthy subjects. In addition, limiting the group to younger subjects yielded a stronger difference in fractional anisotropy among the relatives of bipolar probands. Thus, the fractional anisotropy differences between relatives of bipolar probands and healthy subjects may have been driven by the proportion of younger subjects in this study group. Perhaps the younger relatives of the bipolar probands included subjects who will later develop clinical symptoms, or perhaps endophenotypic white matter features in relatives of individuals with psychotic bipolar disorder manifest early and do not progress with age.
Table 2 lists previous fractional anisotropy reports that agree with our findings. We consulted three recent meta-analyses (4, 23, 37) of fractional anisotropy findings for schizophrenia and eight for bipolar disorder (23, 25–27, 29, 30, 38, 39). Of 18 coordinates reported in the schizophrenia reviews, 15 fell within regions found in our study. Of 21 coordinates reported in studies of bipolar disorder, 10 were located within regions we detected. Major regions reported in these reviews but not identified in our study included the left posterior retrolenticular internal capsule (23), left superior corona radiata (23), left cingulum (25), right fornix (23), right superior (23) and inferior (40) fronto-occipital fasciculus, and left superior anterior corona radiata (27).
Possible study limitations should be noted. The large number of subjects was obtained at the cost of combining studies from two different scanners and slightly different pulse sequences. Between-scanner effects were present but were not correlated with clinical contrast results and were successfully removed in the general linear model as confirmed by randomized comparison of maps obtained from each scanner. The study design did not allow full analysis of medication effects. The study groups were not precisely matched demographically, and so age and sex effects had to be accounted for by the model, reducing power. Neither disease severity nor patient medication response was assessed or included in the analyses.
Overall, these findings from a large multisite study show strong agreement with existing reports of differences in fractional anisotropy between healthy comparison subjects and probands with schizophrenia or bipolar disorder. Abnormalities in fractional anisotropy were remarkably similar in spatial distribution in the two diseases. Relatives showed similar, attenuated changes in some affected regions, most pronounced in the relatives of schizophrenia probands. Differences in the relatives of the bipolar probands were limited to younger subjects and were not significant. The endophenotypic importance of fractional anisotropy for psychosis is further supported by heritability. Fractional anisotropy was highly heritable, both for the whole-brain average and for many regions, not only those showing differences between clinical groups. Although fractional anisotropy measurements currently cannot be used clinically to diagnose psychosis, especially in individual subjects, better understanding of the relationship between white matter deficits and clinical symptoms will likely translate to improved subtyping, gene discovery, and improved future drug trials and/or other interventions. A logical next step is to combine DTI connectivity measures with functional MRI resting-state connectivity, as we did previously (41).
1 : Manifestation of insanity. Z Gesamte Neurol Psychiatr 1920; 62:1–29Crossref, Google Scholar
2 : A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res 2011; 133:250–254Crossref, Medline, Google Scholar
3 : Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry 2007; 62:179–186Crossref, Medline, Google Scholar
4 : Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108:3–10Crossref, Medline, Google Scholar
5 : Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry 2006; 14:47–63Crossref, Medline, Google Scholar
6 : Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry 2010; 67:168–177Crossref, Medline, Google Scholar
7 : Endophenotypes, dimensions, risks: is psychosis analogous to common inherited medical illnesses? Clin EEG Neurosci 2008; 39:73–77Crossref, Medline, Google Scholar
8 : Schizophrenia: a disconnection syndrome? Clin Neurosci 1995; 3:89–97Medline, Google Scholar
9 : Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 2009; 106:1279–1284Crossref, Medline, Google Scholar
10 : A large scale (N=400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res 2008; 101:95–105Crossref, Medline, Google Scholar
11 : Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry 2010; 68:61–69Crossref, Medline, Google Scholar
12 : Brain connectivity and gyrification as endophenotypes for schizophrenia: weight of the evidence. Curr Top Med Chem 2012; 12:2393–2403Crossref, Medline, Google Scholar
13 : White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disord 2011; 13:604–613Crossref, Medline, Google Scholar
14 : Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry 2012; 71:881–889Crossref, Medline, Google Scholar
15 : Affective traits in schizophrenia and schizotypy. Schizophr Bull 2008; 34:856–874Crossref, Medline, Google Scholar
16 : The emergence of the bipolar spectrum: validation along clinical-epidemiologic and familial-genetic lines. Psychopharmacol Bull 2008; 40:99–115Google Scholar
17 : The disease entity in psychiatry: fact or fiction? Epidemiol Psychiatr Sci 2012; 21:255–264Crossref, Medline, Google Scholar
18 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
19 : Structured Interview for DSM-IV Personality. Arlington, Va, American Psychiatric Publishing, 1997Google Scholar
20 : Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31:1487–1505Crossref, Medline, Google Scholar
21 ;
22 : Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44:83–98Crossref, Medline, Google Scholar
23 : White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord 2009; 11:11–18Crossref, Medline, Google Scholar
24 : Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr Res 2008; 106:125–131Crossref, Medline, Google Scholar
25 : A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1820–1826Crossref, Medline, Google Scholar
26 : Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disord 2011; 13:414–424Crossref, Medline, Google Scholar
27 ACombined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci 2011; 36:391–401Crossref, Medline, Google Scholar
28 : White matter integrity in individuals at high genetic risk of bipolar disorder. Biol Psychiatry 2011; 70:350–356Crossref, Medline, Google Scholar
29 : Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 2011; 127:46–57Crossref, Medline, Google Scholar
30 : White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry 2009; 194:527–534Crossref, Medline, Google Scholar
31 : Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998; 62:1198–1211Crossref, Medline, Google Scholar
32 : Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients' nonpsychotic relatives. Front Hum Neurosci 2009; 3:35Crossref, Medline, Google Scholar
33 : Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry 2010; 68:560–567Crossref, Medline, Google Scholar
34 : Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res 2009; 174:9–16Crossref, Medline, Google Scholar
35 : Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 2000; 57:637–648Crossref, Medline, Google Scholar
36 : Is schizophrenia a syndrome of accelerated aging? Schizophr Bull 2008; 34:1024–1032Crossref, Medline, Google Scholar
37 : Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res 2010; 117:1–12Crossref, Medline, Google Scholar
38 : Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry 2008; 65:1041–1052Crossref, Medline, Google Scholar
39 : Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol Psychiatry 2011; 69:309–317Crossref, Medline, Google Scholar
40 : The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry 2005; 162:1911–1918Link, Google Scholar
41 : Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage 2008; 43:554–561Crossref, Medline, Google Scholar



