Effect of a Nicotine Vaccine on Nicotine Binding to β2*-Nicotinic Acetylcholine Receptors In Vivo in Human Tobacco Smokers
Abstract
Objective
Nicotine promotes smoking partly by binding to β2-containing nicotinic acetylcholine receptors (β2*-nAChRs) in the brain. Smoking one tobacco cigarette results in occupation of 80% of β2*-nAChRs for more than 6 hours. This likely contributes to maintenance of smoking dependence and cessation difficulty. Developing nicotine vaccines could improve treatments. The authors used [123I]5-I-A-85380 single photon emission computed tomography (SPECT) to evaluate the effect of 3′-AmNic-rEPA on the amount of nicotine that binds to β2*-nAChRs in smokers’ brain cortical and subcortical regions.
Method
Eleven smokers who smoked an average of 19 cigarettes per day, had smoked for 10 years on average, and met criteria for nicotine dependence were given SPECT scans on two days: before and after immunization with 4–400 μg of 3′-AmNic-rEPA. On scan days, three 30-minute baseline emission scans were followed by intravenous administration of nicotine (1.5 mg/70 kg body weight) and up to nine 30-minute emission scans.
Results
β2*-nAChR availability was quantified as VT/fP (total distribution volume divided by free plasma concentration), and nicotine binding was derived by the Lassen plot approach. Immunization led to a 12.5% reduction in nicotine binding. Nicotine bound to β2*-nAChRs correlated positively with nicotine injected before but not after vaccination. The daily number of cigarettes and desire for a cigarette decreased after vaccination.
Conclusions
This proof-of-concept study demonstrates that immunization with nicotine vaccine can reduce the amount of nicotine binding to β2*-nAChRs and disrupt the relationship between administered nicotine and nicotine available to occupy β2*-nAChRs.
It is estimated that each year half a million individuals in the United States die of diseases related to tobacco smoking, but an equal number become dependent on tobacco yearly. Smoking cessation rates are low, and the currently available treatments approved by the Food and Drug Administration for tobacco addiction are 10%–30% effective at 1-year follow up. The past decade has seen a different approach emerge for treating tobacco addiction: vaccines to block brain entry of nicotine, possibly the most addictive constituent in tobacco cigarettes. Vaccines are designed to stimulate the production of antibodies specific to the nicotine molecule.
In the present study, we tested the efficacy of a nicotine conjugate vaccine in reducing nicotine’s entry into the brain and binding to nicotinic acetylcholine receptors (nAChRs), the primary binding sites of nicotine in the brain, in vivo in human smokers. The vaccine was 3′aminomethylnicotine conjugated to recombinant Pseudomonas exoprotein A (3′-AmNic-rEPA) (Nabi Biopharmaceuticals, Rockville, Md.). 3′-AmNic-rEPA has high affinity for nicotine (2) and prolongs nicotine elimination from the body in animal studies (3, 4). Four to five injections of 400 μg each are safe, and the expected therapeutic effect is antibodies of more than 25 μg/mL (5, 6). Preclinical studies suggest that immunization results in 30%–90% less nicotine entering the brain after acute nicotine exposure (3, 7–10), and this is related to the observed decrease in locomotor (7, 8) and behavioral (11, 12) responses to nicotine. There is evidence that immunization slows nicotine elimination from the body (3, 4), which may contribute to reduction in smoking. This would be consistent with the finding that slow nicotine metabolizers smoke fewer cigarettes, i.e., nicotine is available for a longer period of time.
Nicotinic acetylcholine receptors in which the β2 subunit is coupled with α4 or another subunit are known as β2*-nAChRs. Chronic administration of nicotine up-regulates the high-affinity β2*-nAChRs (13), and nicotine from smoking cigarettes or a nicotine inhaler occupies the majority of these receptors (14–16). Although use of a nicotine inhaler leads to a prolonged occupancy of the β2*-nAChRs similar to that after cigarette smoking, use of an inhaler does not alleviate craving symptoms, as does smoking one cigarette (16). This is in part due to the 10% lower nicotine binding at the β2*-nAChRs after use of a nicotine inhaler (14). Thus, in addition to the explicit differences between nicotine inhalers and regular cigarettes (e.g., lack of other tobacco smoke ingredients, social impact), the 10% difference in nicotine binding to the β2*-nAChRs likely contributes to the poor ability of nicotine inhalers to significantly reduce craving symptoms. The complexities of tobacco smoking dependence in human subjects and the current lack of highly efficacious treatments suggest that β2*-nAChRs may be an excellent target for smoking cessation therapies.
The present proof-of-concept study evaluated whether immunization with 3′-AmNic-rEPA reduces the amount of nicotine that reaches the brain and occupies, or binds, to β2*-nAChRs in healthy human tobacco smokers. We used [123I]5-I-A-85380 ([123I]5-IA) and single photon emission computed tomography (SPECT) imaging to quantify β2*-nAChRs. We administered nicotine intravenously to each subject at a dose of 1.5 mg/70 kg body weight, which is equivalent to the nicotine delivered from 1.5 cigarettes. We hypothesized that vaccination with 3′-AmNic-rEPA would be associated with a significant decrease in nicotine binding to β2*-nAChRs, indicating reduced entry into the brain by nicotine.
Method
Eleven non-treatment-seeking tobacco smokers (seven men, four women) signed consent statements and completed this study, which was approved by the institutional review boards of the Yale University School of Medicine, the Department of Veterans Affairs Connecticut Healthcare System, and the University of Toronto. Eligibility was evaluated through a structured interview, behavioral assessments, a physical examination, laboratory blood tests, a urine drug screen, and an electrocardiogram.
Study Design
All subjects participated in two [123I]5-IA SPECT scans on separate days 20 weeks apart. They received four injections of 3′-AmNic-rEPA between the two SPECT scan days; the injections were 4 weeks apart. The subjects were instructed to abstain from tobacco cigarettes or any nicotine products for the 5 days before each SPECT scan day to allow for any nicotine or metabolites to clear the brain, because they may compete with radiotracer binding (17). Smoking abstinence was confirmed as previously described (14). For the remainder of the study, the subjects were instructed to smoke ad libitum but not to use any medications or nicotine replacement therapies. Smoking characteristics were recorded at each visit.
Assessments
The severity of nicotine dependence was assessed by using the Fagerström Test of Nicotine Dependence (18) at intake. Nicotine withdrawal symptoms were assessed with the Minnesota Nicotine Withdrawal Scale (19), and craving was assessed by using the Tiffany Questionnaire on Smoking Urges (20) at intake, during each period of smoking abstinence, and on each scan day before and after intravenous nicotine administration. The Tiffany Questionnaire on Smoking Urges brief version (21) was used on the SPECT scan days before and after nicotine challenge. Two factors of the Tiffany questionnaire were employed: desire (positive symptoms associated with wanting a cigarette) and relief (withdrawal relief expected if cigarette is smoked). Subsyndromal depressive symptoms were measured with the Center for Epidemiological Studies Depression Scale (CES-D) (22), and state and trait anxiety symptoms were measured with the Spielberger State-Trait Anxiety Inventory (23) at intake and on both scan days.
3′-AmNic-rEPA
The active investigational product was purified 3′-aminomethylnicotine conjugated to P. aeruginosa r-exoprotein A (rEPA) (3′-AmNic-rEPA). Each single-use syringe contained 3′-aminomethylnicotine conjugated to 400 μg of rEPA adsorbed to 1.1 mg aluminum (Alhydrogel 85, InvivoGen, San Diego) in 1 mL of phosphate-buffered saline (0.15 M NaCl, 0.002 M NaPO4, pH 7.2, 0.01% polysorbate 80). All subjects were administered vaccines from the same lot.
Antinicotine antibody concentrations were measured by using enzyme-linked immunosorbent assay (ELISA) as described previously (6). Because no national or international reference standards exist for nicotine antibodies, reference standards were developed by Nabi Biopharmaceuticals and prepared from pools of serum from human volunteers who were immunized. Nicotine-specific IgG antibody was quantitated by an ELISA in which antibody bound to nicotine-coated plates was quantitated against antibody bound by anti-Fab–coated plates (plates coated to resist binding with antigen-binding fragments of antibody). Here we report absolute concentrations of antibodies, which are in units of mass per volume (µg/ml). Side effects of the vaccine were monitored as done previously (5). Subjects’ vital signs (blood pressure, temperature, pulse, and respiration rate) were collected before and 30 minutes after vaccination. Following each vaccine appointment, each subject filled out a reactogenicity diary for 7 consecutive days to keep a record of local and systemic reactions and temperature. The diary was reviewed at the next administration date unless there was a notable reaction. Every subject was followed for 2 weeks after the last study date to review any symptoms or side effects.
Nicotine and Analysis of Nicotine and Cotinine
Vials of nicotine bitartrate were prepared by mixing the nicotine with saline to a concentration of 1 mg/ml nicotine base. They were administered intravenously over 10 minutes.
Venous blood samples for the nicotine and cotinine analyses were drawn at intake and on each scan day. On the scan days, samples were drawn before radiotracer administration and after intravenous nicotine administration at 2, 5, 10, 20, 30, 60, 90, 120, 180, 240, and 300 minutes. The samples were processed as described previously (24). Plasma nicotine, cotinine (metabolite of nicotine), and 3-hydroxycotinine (metabolite of cotinine) were measured. Free nicotine was measured because it can cross the brain-blood barrier and act on nicotinic receptors and because nicotine glucuronide is a minor metabolite that is rapidly cleared, resulting in only a small fraction of the total nicotine in plasma being in the conjugated form. Free nicotine was measured by liquid chromatography/tandem mass spectrometry (24).
Using the sample data over time, we determined whether there were changes in nicotine’s half-life, volume of distribution, and clearance as a result of treatment. Systematic clearance was determined by dividing the nicotine dose by the plasma area under the curve for t0-∞ (AUCt0-∞), extrapolated by using terminal time points. The nicotine half-life was estimated by means of a regression analysis of the concentration versus time. Nicotine's apparent volume of distribution was estimated by multiplying its half-life by clearance then dividing by 0.693.
Immunogenicity Samples
Serum samples were collected for immunogenicity measurements at five time points: before each of the four vaccine administrations and on the second SPECT scan day. Antinicotine antibody concentrations were measured by using ELISA, and the subjects reported any adverse events, as described previously (6).
MRI and [123I]5-IA SPECT Imaging
Each subject participated in one magnetic resonance imaging (MRI) scan before SPECT scanning on a Signa 1.5-T system (General Electric, Milwaukee, Wis.) as described previously (14).
All SPECT emission scans were obtained on a Phillips PRISM 3000 XP SPECT camera (Phillips Healthcare, Cleveland), and [123I]5-IA was synthesized and administered as done previously (25). We used a bolus plus constant infusion paradigm with a ratio of 7.0 hours (i.e., bolus was equivalent to 7 hours of infusion) (SD=0.0) on scan day 1 and a ratio of 7.0 hours (SD=0.0) on scan day 2 and with a total injected dose (accounting for decay) of 358.7 MBq (SD=30.1) on scan day 1 and 352.6 MBq (SD=31.4) on scan day 2. Another antecubital venous catheter was placed into the opposite arm or hand to collect blood for measuring protein binding and metabolism. Six hours after the [123I]5-IA injection, a simultaneous transmission/emission protocol scan and three 30-minute equilibrium emission scans were obtained. The subject was removed from the camera, and intravenous nicotine was administered through a butterfly catheter. Thereafter, up to nine additional 30-minute emission scans were acquired to evaluate nicotine-induced displacement of [123I]5-IA. Blood samples were collected at the midpoint of each set of postnicotine scans to quantify the total parent concentration and free plasma concentration in order to obtain the outcome measure VT/fP, which corrects for individual differences in metabolism and protein binding of [123I]5-IA.
Image Analysis
SPECT emission images were analyzed as described previously (25). Regional [123I]5-IA uptake was measured as VT/fP for the following brain regions: frontal, parietal, anterior cingulate, temporal and occipital cortices, thalamus, striatum, and cerebellum.
The VT/fP data from the prenicotine and postnicotine scans were analyzed by use of Lassen plots (14, 15, 26). Receptor occupancy (Ro) by nicotine was derived for each subject across all brain regions for each postnicotine scan (compared with baseline) on each scan day, and the final result represents the average across scans for each subject. The difference in nicotine binding to the receptor between scan 2 and scan 1 was calculated as %Δ = (1 – [VT/fP 2]/[VT/fP 1]) * 100.
Determination of Nicotine Reduction
The concentration of nicotine in tissue can be calculated as where C is the concentration of nicotine in tissue and IC50 is the concentration of nicotine in tissue at Ro=50%. In order to obtain the percentage reduction of nicotine in tissue from time 1 (before immunization) to time 2 (after immunization), we divided the concentration at time 2 (C2) by the concentration at time 1 (C1) and subtracted the result from 1: %Δ=(1 – C2/C1)*100.
where C is the concentration of nicotine in tissue and IC50 is the concentration of nicotine in tissue at Ro=50%. In order to obtain the percentage reduction of nicotine in tissue from time 1 (before immunization) to time 2 (after immunization), we divided the concentration at time 2 (C2) by the concentration at time 1 (C1) and subtracted the result from 1: %Δ=(1 – C2/C1)*100.
Statistical Analyses
All statistical analyses were performed by using SPSS version 17.0 (SPSS, Chicago). To assess whether immunization reduces the overall amount of nicotine that reaches the brain and binds to receptors, we performed an analysis of variance with repeated measures at the time of maximal displacement of the radioligand (3–4 hours after intravenous nicotine injection). Statistical significance was set at p≤0.05, two-tailed. Paired-sample t tests were also used to assess within-subject differences in mood, smoking, and craving variables after immunization and to assess differences in nicotine pharmacokinetic characteristics. Nonparametric correlational analyses (using the Spearman rho correlation coefficient) were used to examine the relationship between receptor occupancy and nicotine variables on the SPECT scan days.
Results
Before Immunization
Participant characteristics.
The healthy tobacco smokers had a mean age of 36.1 years (SD=12.9), smoked an average of 19.5 cigarettes/day (SD=11.2), had smoked for 8.7 years (SD=6.2), and were moderately dependent on nicotine (Fagerström Test of Nicotine Dependence score: mean=5.3, SD=2.9). Smoking status was verified by plasma nicotine (mean=9.1 ng/mL, SD=5.0), urine cotinine (mean=909 ng/mL, SD=126), and breath carbon monoxide (mean=17.3 ppm, SD=5.3) levels at screening. Scores for mood and smoking craving are presented in Table 1.
| Score on Scan Day 1 (before immunization) | Score on Scan Day 2 (after immunization) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Score | Before Intravenous Nicotine | After Intravenous Nicotine | Before Intravenous Nicotine | After Intravenous Nicotine | ||||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Center for Epidemiologic Studies Depression Scale | 5.7 | 8.9 | 6.0 | 9.1 | 5.5 | 7.8 | 5.1 | 7.4 | 4.5 | 6.8 |
| Spielberger State-Trait Anxiety Inventory state subscale | 28.4 | 9.4 | 27.5 | 8.0 | 29.0 | 7.0 | 30.3 | 12.7 | 30.2 | 11.0 |
| Minnesota Nicotine Withdrawal Scale | 4.5 | 7.2 | 3.8 | 3.7 | 1.6 | 1.6 | 3.5 | 7.8 | 2.1 | 3.7 |
| Tiffany Questionnaire on Smoking Urges | ||||||||||
| Desire | 30.1 | 7.2 | 31.5 | 6.8 | 25.6 | 11.6 | 24.0 | 10.8 | 20.5 | 10.1 |
| Relief | 16.2 | 8.6 | 16.7 | 8.3 | 14.5 | 8.8 | 13.1 | 7.8 | 9.8 | 4.2 |
| Questionnaire for Smoking Urges–Brief | ||||||||||
| Desire | 9.3 | 4.5 | 9.2 | 3.3 | 7.0 | 4.4 | 7.2 | 4.6 | 4.2 | 3.5 |
| Relief | 6.5 | 4.2 | 7.2 | 4.5 | 6.1 | 4.3 | 5.9 | 3.7 | 4.2 | 1.8 |
The participants abstained from smoking for a mean of 4.9 days (SD=0.8) before the first SPECT scan day; abstinence was verified by a mean urine cotinine level of 214 ng/mL (SD=346) and a carbon monoxide level of 2.9 ppm (SD=2.5). One participant was not able to abstain from smoking and smoked the night before the SPECT scan (hence, the average urine cotinine level was higher than in our previous studies), but we proceeded with the scan day procedures. This same subject was also not able to abstain from smoking for the second scan day and smoked the night before that scan as well. Since the study had a within-subject design and the nicotine plasma level before the intravenous nicotine challenge on both SPECT days was below 0.1 ng/mL (analyzed as described in Method), we included this subject in the analyses.
Plasma nicotine level.
The nicotine concentrations for each subject are presented in Table 2 and Figure 1. After nicotine administration, the mean maximal plasma nicotine concentration (Cmax) was 9.6 ng/mL (SD=2.8) at a mean of 17.0 minutes (SD=10.3) and the AUC was 1,722 ng⋅min/ml (SD=951).
| Subject and Scan | Antinicotine Antibody Level (μg/mL) | Intravenous Nicotine Dose (mg) | Average Nicotine Binding (%)a | Area Under Plasma Nicotine Concentration Time Curve (ng⋅min/ml)b | Maximal Plasma Nicotine Concentration (Cmax) (ng/mL)c | Time to Reach Cmax (min) | Nicotine Clearance Rate (mL/min)d | Total Distribution Volume (L)e | Half-Life (min) |
|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | 1.66 | ||||||||
| Scan 1 | 0.0 | 56.9 | 920 | 9.7 | 20.0 | 1,804 | 233,511 | 90 | |
| Scan 2 | 59.8 | 56.2 | 1,994 | 9.3 | 10.0 | 833 | 154,433 | 129 | |
| Subject 2f | 1.90 | ||||||||
| Scan 1 | 0.0 | 54.4 | 742 | 4.8 | 30.0 | 2,560 | 351,398 | 95 | |
| Scan 2 | 45.3 | 30.3 | 1,165 | 7.5 | 30.0 | 1,630 | 213,721 | 91 | |
| Subject 3 | 2.76 | ||||||||
| Scan 1 | 0.0 | 65.5 | 2,496 | 9.1 | 30.0 | 1,106 | 263,278 | 165 | |
| Scan 2 | 85.4 | 51.5 | 2,979 | 22.3 | 10.0 | 926 | 141,204 | 106 | |
| Subject 4 | 1.86 | ||||||||
| Scan 1 | 0.0 | 56.6 | 1,514 | 15.5 | 10.0 | 1,228 | 78,755 | 44 | |
| Scan 2 | 64.6 | 49.7 | 2,413 | 46.1 | 10.0 | 771 | 132,457 | 119 | |
| Subject 5 | 1.46 | ||||||||
| Scan 1 | 0.0 | 46.4 | 1,421 | 10.7 | 10.0 | 1,027 | 172,453 | 116 | |
| Scan 2 | 75.9 | 47.6 | 2,622 | 20.1 | 20.0 | 557 | 75,759 | 94 | |
| Subject 6 | 1.75 | ||||||||
| Scan 1 | 0.0 | 66.6 | 2,097 | 10.0 | 30.0 | 835 | 295,992 | 246 | |
| Scan 2 | 127.9 | 59.6 | 3,994 | 34.2 | 10.0 | 438 | 159,289 | 252 | |
| Subject 7 | 1.35 | ||||||||
| Scan 1 | 0.0 | 39.1 | 4,001 | 8.3 | 20.0 | 337 | 161,987 | 333 | |
| Scan 2 | 64.6 | 31.5 | 15,718 | 28.4 | 30.0 | 86 | 46,904 | 378 | |
| Subject 8 | 1.62 | ||||||||
| Scan 1 | 0.0 | 60.3 | 1,489 | 8.0 | 5.0 | 1,088 | 261,298 | 166 | |
| Scan 2 | 41.7 | 62.7 | 2,083 | 8.5 | 10.0 | 778 | 177,543 | 158 | |
| Subject 9 | 1.70 | ||||||||
| Scan 1 | 0.0 | 59.5 | 1,205 | 11.8 | 5.0 | 1,411 | 151,182 | 74 | |
| Scan 2 | 65.0 | 49.7 | 1,524 | 15.0 | 5.0 | 1,115 | 154,616 | 96 | |
| Subject 10g | 1.74 | ||||||||
| Scan 1 | 52.8 | ||||||||
| Scan 2 | 49.1 | ||||||||
| Subject 11 | 1.39 | ||||||||
| Scan 1 | 0.0 | 48.0 | 1,337 | 7.6 | 10.0 | 1,040 | 216,448 | 144 | |
| Scan 2 | 129.2 | 56.2 | 2,625 | 20.1 | 20.0 | 530 | 72,835 | 95 | |
| Mean | 1.9 | ||||||||
| Scan 1 | 0.0 | 54.9 | 1,722 | 9.6 | 17.0 | 1,244 | 218,630 | 147 | |
| Scan 2 | 75.9 | 49.1 | 3,712 | 21.2 | 15.5 | 766 | 132,876 | 152 | |
| SD | 0.5 | ||||||||
| Scan 1 | 0.0 | 8.7 | 951 | 2.8 | 10.3 | 596 | 79,657 | 87 | |
| Scan 2 | 30.5 | 10.1 | 4,291 | 12.4 | 9.0 | 418 | 52,169 | 93 |
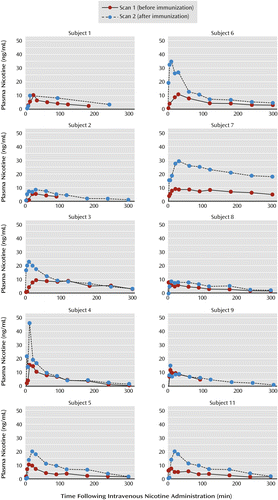
a Subject 10 is not shown because this participant had poor venous access during and after the intravenous nicotine challenge and no plasma levels were obtained on either scan day.
b After immunization the average maximal nicotine concentration across subjects was significantly higher: 21.2 ng/mL versus 9.6 before immunization. The difference in the area under the curve approached statistical significance: 3,712 versus 1,722 ng·min/mL.
Receptor occupancy by nicotine.
Equilibrium, defined as ≤5% change in receptor availability per hour, was achieved between 6 and 8 hours after injection on each scan day. The subjects were placed back in the camera at an average of 59.4 minutes (SD=21.9) after initiation of the intravenous nicotine challenge. Maximal displacement of [123I]5-IA was achieved 3–4 hours after nicotine administration (mean displacement=56.2%, SD=11.1) (Figure 2). The range of maximal occupancy was 47.1%–68.3% across subjects (Table 2). There was a significant positive correlation between the amount of nicotine injected and the proportion of nicotine bound (Figure 3).
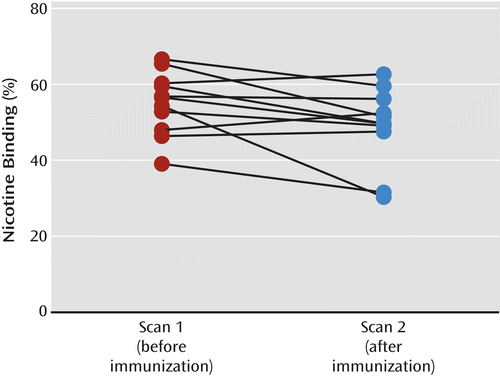
a The change in binding, or receptor occupancy (Ro), was calculated as Roafter/Robefore * 100. There was a significant average 12.5% decrease in binding after immunization (F=5.2, df=1, 9, p<0.05).
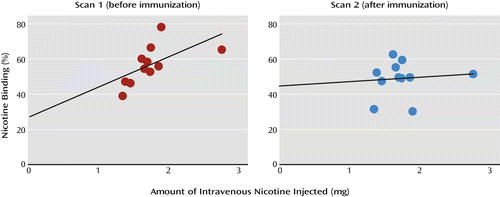
a Each subject received the same amount of intravenous nicotine (1.5 mg/70 kg body weight) on both scan days.
b Nicotine binding correlated positively with the amount of intravenous nicotine administered on the first scan day, before immunization (r=0.60, N=11, p=0.05), but not on the second scan day, after immunization (r=0.01, N=11, p=0.98).
Plasma antibody level.
Blood samples for measurement of titer levels were collected before administration of the vaccine at each vaccination appointment and on SPECT scan day 2 (Table 2). Before initiation of the vaccinations, none of the subjects had a detectable antibody level. There was a significant increase in antibody level over the course of treatment, with an average of 75.9 μg/mL (SD=30.5) on scan day 2 (2 weeks after the fourth injection), and all of the subjects reached a level above 25 μg/mL after the vaccinations. No unexpected issues or adverse events were reported. Most commonly reported were the expected mild tenderness and ache at the injection site. None of the subjects required follow-up past the standard 2 weeks at the end of the study.
After Immunization
Smoking characteristics.
Plasma nicotine concentration.
From scan day 1 to scan day 2 there was a significant increase in maximal plasma nicotine concentration after intravenous nicotine administration (t=–3.5, df=9, p=0.007) but not in the AUC or time to maximal concentration (Table 2). We also observed a significant effect of vaccine treatment on the total volume of distribution (t=4.2, df=9, p=0.002) and nicotine clearance (t=5.6, df=9, p<0.001) such that both decreased from scan 1 to scan 2. The ratio of free nicotine to antibody-bound nicotine immediately after nicotine administration was not significantly different from the ratio 3 hours after nicotine challenge. To examine the differences in nicotine metabolism from before to after vaccination, AUCs were calculated for cotinine and 3-hydroxycotinine on each scan day to determine the ratio of 3-hydroxycotinine to cotinine. There was no difference in the ratio of the AUCs between scan day 1 (mean=0.12, SD=0.07) and scan day 2 (mean=0.12, SD=0.07) (p=0.34) and no significant change in the overall half-life of nicotine from scan 1 (mean=147 minutes, SD=87) to scan 2 (mean=152 minutes, SD=93) (p>0.05). There were also no significant correlations between any of the nicotine outcome measures and the antibody level on scan day 2.
Receptor occupancy by nicotine.
Baseline β2*-nAChR availability did not differ significantly between the two scans (p>0.10). After baseline scans were obtained and intravenous nicotine was administered, the subjects were placed back in the camera an average of 62.1 minutes (SD=3.6) after initiation of the intravenous nicotine challenge. Maximal displacement of the radioligand was achieved 3–4 hours after nicotine administration. The mean displacement was 49.1% (SD=10.1) (Figure 2), and the range across subjects was 30.3%–62.7% (Table 2). Immunization was associated with a significant 12.5% decrease in receptor occupancy by nicotine, with an estimated reduction in brain nicotine of 23.6%. After removal of subject 2 (who was not able to abstain from smoking before each scan), the statistical significance of the decrease in receptor occupancy by nicotine fell below significance (F=4.5, df=1, 8, p=0.07).
Discussion
This was a proof-of-concept study designed to evaluate the effect of the nicotine vaccine 3′-AmNic-rEPA on the ability of nicotine to enter the brain and bind to high-affinity β2*-nAChRs in healthy tobacco smokers. The primary findings confirm that immunization with 3′-AmNic-rEPA leads to a significant reduction in nicotine’s ability to enter the brain and bind to β2*-nAChRs. We observed a 12.5% decrease in β2*-nAChR occupancy by nicotine, associated with a 23.6% decrease in the amount of nicotine available to enter the brain after vaccination.
All subjects had titer levels indicating that antibodies for nicotine had been developed. Consistent with preclinical findings (4), administration of intravenous nicotine after immunization was associated with plasma nicotine concentrations at least twice as high as before immunization, as well as with altered nicotine clearance and total volume of distribution and with a decreased ability of nicotine to enter the brain. Unlike the rodent studies (4), our investigation did not show that immunization prolonged nicotine’s terminal half-life. This difference is likely due to three factors. First, nicotine clearance and volume of distribution changed proportionally, which would not alter nicotine half-life. In the rodent studies these variables were not proportional. Second, rats and humans have similar but not identical nicotine clearance and volume of distribution, and the effects of vaccination on half-life could differ. Third, in the rodent studies the titer levels achieved were much higher than in the current study, and this likely affected the pharmacokinetics of nicotine (27).
Maximal nicotine binding to the β2*-nAChRs before immunization was 56.2% and was lowered significantly to 49.4% after immunization (12.5% reduction). This reduction in receptor occupancy by nicotine was associated with an estimated 23.6% reduction in the available nicotine in the brain. Vaccination disrupted the straightforward association between the amount of nicotine administered and the percentage of nicotine bound to β2*-nAChRs, although we did not detect significant associations between the antibody level achieved and the reduction in receptor occupancy by nicotine after immunization. The lack of association between antibody level and the reduction in nicotine’s occupancy of the receptors could be due to several reasons, including the small number of subjects, the fact that all of the subjects achieved optimal antibody levels, or physiological differences between our subjects and those in the rodent studies. A significant positive relationship between the amount of nicotine administered and β2*-nAChR occupancy by nicotine has been previously shown by our group (14, 15) and others (16). Thus, the disruption in this association after immunization is remarkable and strongly suggests that the vaccine had a role in altering distribution of nicotine to the brain and occupancy of β2*-nAChRs. These results are in line with findings by Satoskar and colleagues (28) showing that the reduction in the amount of nicotine reaching the brain was significantly greater in vaccinated rats than in unvaccinated rats.
Clinical changes that accompanied the 12.5% reduction in bound nicotine were a 40% reduction in cigarette use and a significant reduction in craving for cigarettes from baseline to completion of immunization. This difference in the amount of bound nicotine from baseline to postimmunization is comparable to that in a previous study by us, where the 10% difference in bound nicotine partially contributed to the differential effects on craving (14). The clinical results in the present study may appear discrepant since phase III clinical trials for this vaccine did not show efficacy. There are several potential explanations for this. First, the differences between the outcomes may be due to the fact that the levels of antibody titers may have been suboptimal in the majority of the smokers in the clinical trial. Second, the 12.5% reduction in occupancy may not be sufficient to lead to improved abstinence rates. Third, the study group in the present study was composed of non-treatment-seeking smokers. Last, the clinical trials for 3′-AmNic-rEPA compared smoking cessation outcomes for vaccination and placebo months after the vaccination schedule, whereas the present study concentrated on the period immediately following immunization.
The study has limitations. The lack of a placebo control group limits clinical interpretation. However, this study was undertaken to test the concept that nicotine vaccine does reduce the amount of nicotine in the brain and affects nicotine pharmacokinetics in vivo in human subjects. The small number of subjects limits our ability to examine variables that may play a role in receptor response to the vaccine, such as gender. As described previously (15), use of radiotracer imaging limits interpretation of temporal findings. The slow kinetics of the radiotracer might not accurately model the correct time period for maximal occupancy of β2*-nAChRs by nicotine since [123I]5-IA is characterized by a slow dissociation of the receptor-ligand complex and slow clearance from the brain (29–31). This means that radioligand binding to the receptor does not instantaneously match the quantity of available receptors and that the maximal occupancy detected here 3–4 hours after nicotine administration is likely achieved sooner in the brain. Faster radioligands, which may provide a better representation of the effects of nicotine at the β2*-nAChRs, are currently under development.
In this study we found that immunization with 3′-AmNic-rEPA significantly reduced β2*-nAChR occupancy by nicotine by sequestering nicotine in the blood and reducing entry into the brain. Moreover, immunization was associated with significant reductions in cigarette use and craving in non-treatment-seeking smokers. These findings provide evidence for mechanisms involved in the use of vaccination against nicotine dependence in human tobacco smokers.
1 : Nicotine conjugate vaccine: is there a right to a smoking future? J Med Ethics 2004; 30:344–345Crossref, Medline, Google Scholar
2 : Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol 2008; 8:1589–1594Crossref, Medline, Google Scholar
3 : Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res 1999; 1:241–249Crossref, Medline, Google Scholar
4 : Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose- and affinity-response relationships. Drug Metab Dispos 2005; 33:1056–1061Crossref, Medline, Google Scholar
5 : Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 2011; 89:392–399Crossref, Medline, Google Scholar
6 : Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther 2005; 78:456–467Crossref, Medline, Google Scholar
7 : A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav 2000; 65:191–198Crossref, Medline, Google Scholar
8 : Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg Med Chem 2004; 12:563–570Crossref, Medline, Google Scholar
9 : Preclinical development of a vaccine ‘against smoking’. Onkologie 2002; 25:406–411Crossref, Medline, Google Scholar
10 : Active immunization against nicotine alters the distribution of nicotine but not the metabolism to cotinine in the rat. Naunyn Schmiedebergs Arch Pharmacol 2004; 370:299–304Crossref, Medline, Google Scholar
11 : Passive immunization against nicotine attenuates nicotine discrimination. Life Sci 2002; 70:2793–2798Crossref, Medline, Google Scholar
12 : Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006; 184:409–416Crossref, Medline, Google Scholar
13 : Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci 2006; 26:8707–8714Crossref, Medline, Google Scholar
14 : Brain β2*-nicotinic acetylcholine receptor occupancy after use of a nicotine inhaler. Int J Neuropsychopharmacol 2011; 14:389–398Crossref, Medline, Google Scholar
15 : Quantification of smoking-induced occupancy of β2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. J Nucl Med 2010; 51:1226–1233Crossref, Medline, Google Scholar
16 : Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 2006; 63:907–915Crossref, Medline, Google Scholar
17 : Beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry 2009; 66:666–676Crossref, Medline, Google Scholar
18 : The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86:1119–1127Crossref, Medline, Google Scholar
19 : Tobacco withdrawal symptoms: an experimental analysis. Psychopharmacology (Berl) 1984; 84:231–236Crossref, Medline, Google Scholar
20 : The development and initial validation of a questionnaire on smoking urges. Br J Addict 1991; 86:1467–1476Crossref, Medline, Google Scholar
21 : Investigating the factor structure of the Questionnaire on Smoking Urges1–Brief (QSU-Brief). Addict Behav 2006; 31:1231–1239Crossref, Medline, Google Scholar
22 : The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401Crossref, Google Scholar
23 : Manual for State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
24 : Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther 2004; 76:64–72Crossref, Medline, Google Scholar
25 : 123I-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: feasibility and reproducibility. J Nucl Med 2005; 46:1466–1472Medline, Google Scholar
26 : Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab 2010; 30:46–50Crossref, Medline, Google Scholar
27 : Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochem Pharmacol 2011; 81:1164–1170Crossref, Medline, Google Scholar
28 : Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. Int Immunopharmacol 2003; 3:957–970Crossref, Medline, Google Scholar
29 : 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol 2000; 57:642–649Crossref, Medline, Google Scholar
30 : In vivo studies with [125I]5-I-A-85380, a nicotinic acetylcholine receptor radioligand. Neuroreport 1998; 9:2311–2317Crossref, Medline, Google Scholar
31 : Assessment of dynamic neurotransmitter changes with bolus or infusion delivery of neuroreceptor ligands. J Cereb Blood Flow Metab 1998; 18:1196–1210Crossref, Medline, Google Scholar



