Short-Term Tropisetron Treatment and Cognitive and P50 Auditory Gating Deficits in Schizophrenia
Abstract
Objective
The α7 nicotinic acetylcholine receptor (nAChR) is associated with cognitive and P50 auditory gating deficits in schizophrenia, and α7 nAChR agonists can potentially reverse these deficits. The authors examined multiple dosages of tropisetron, a partial agonist at the nAChR, for short-term effects on cognition and P50 deficits in schizophrenia.
Method
In a randomized double-blind design, 40 nonsmoking patients with schizophrenia who had P50 ratios greater than 0.5 and were stabilized on 3–6 mg/day of risperidone were randomly assigned to receive placebo (N=10) or oral tropisetron at 5 mg/day (N=10), 10 mg/day (N=10), or 20 mg/day (N=10). The authors measured P50 inhibitory gating and administered the Chinese-language version of the Repeatable Battery for the Assessment of Neuropsychological Status at baseline and after 10 days of treatment.
Results
After 10 days of treatment, all three daily doses of tropisetron significantly improved overall cognitive deficits, with 10 mg showing the greatest improvement for the immediate memory index score and 20 mg for the delayed memory index score on the cognitive battery. The P50 deficits were also improved, and that improvement was significantly correlated with cognitive improvement. Two patients in the 20 mg/day group dropped out because of adverse effects, but the other dosages were well tolerated.
Conclusions
The improvement of cognition with tropisetron appeared to be associated with normalization in P50 deficits. Thus, α7 nAChR agonists appear to be a promising therapeutic approach for the treatment of cognitive deficits that are related to abnormal P50 suppression in schizophrenia.
Compromised neurocognitive function is a core feature of schizophrenia, affecting processing speed, learning, memory, attention, executive function, and social cognition (1, 2). The cognitive impairment associated with schizophrenia is severe, widespread, and apparent long before overt signs of psychosis emerge (3, 4). Cognitive dysfunction in schizophrenia also persists and leads to poor social functioning regardless of changes in the patient’s symptom state after treatment (5–7). Overall quality of life suffers from this impairment (8). Hence, cognition has become the focus of recent research and a prime treatment target in schizophrenia (8). However, the pathophysiological mechanisms underlying these cognitive deficits are unclear. A sensory gating deficit that has been repeatedly found in schizophrenia patients appears to be a sensitive biomarker for cognitive impairment in this disorder (9–14). This measure of sensory gating abnormalities, diminished inhibition of the P50 evoked response to repeated auditory stimuli, has been linked to the chromosome 15q14 locus of the α7 nicotinic receptor gene. Polymorphisms in the core promoter of the gene are associated with schizophrenia and also with diminished inhibition of the P50 response (15–22). Because of this finding, α7 nicotinic acetylcholine receptor (nAChR) agonists have been suggested as a potential therapy for normalizing deficient inhibitory processing and improving cognition in schizophrenia (12, 16, 19–21, 23–27). The National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative identified classes of drugs most likely to act as cognitive enhancers in schizophrenia. These drugs included cholinergic agents, such as α7 nAChR agonists and M1 muscarinic acetylcholine receptor (mAChR) agonists; dopaminergic D1 receptor agonists; glutamatergic agents acting on both ionotropic and metabotropic receptors; α2 adrenergic receptor agonists; agents acting on the GABA system; and various serotonin receptor agents (26, 28).
Tropisetron is both a potent serotonin-3 receptor antagonist (it is widely used as such in the treatment of patients with chemotherapy-induced or postoperative nausea and vomiting [29]) and a high-affinity partial agonist of the nAChR (29–31). Hashimoto et al. reported that tropisetron improved both the deficits in inhibitory processing of the auditory evoked potential (32) and cognitive deficits in mice (33). Furthermore, they confirmed that tropisetron’s action was due to its nAChR activity (34). They also found that a single oral dose of 10 mg of tropisetron improved the deficits in auditory P50 suppression in Japanese patients with schizophrenia (35). Their most recent randomized double-blind placebo-controlled trial showed that tropisetron (10 mg/day) significantly improved P50 deficits and aspects of cognitive performance in nonsmoking patients with schizophrenia (30). These results support the need for multiple-dosage studies of tropisetron to improve deficits in P50 suppression and cognition in schizophrenia through nAChR agonism.
Our primary goal in this study was to compare cognition and sensory gating changes after 10 days of treatment with one of three tropisetron doses (5, 10, and 20 mg/day) as an adjunctive treatment to risperidone. We chose the daily doses of 5 and 20 mg to bracket the 10 mg dose that has been shown to improve these deficits in auditory P50 suppression in patients with schizophrenia (35), and we selected risperidone as an antipsychotic because it does not exhibit significant nAChR agonism or 5-HT3 receptor antagonism (30). A second goal was to examine any relationships between the changes in sensory gating and cognition in order to test the hypothesis that the sensory gating deficit is associated with cognitive impairments in schizophrenia through activation of nAChRs.
Method
Participants
We recruited nonsmoking inpatients with schizophrenia from the Mental Health Center of Shantou University and confirmed their DSM-IV diagnosis using the Structured Clinical Interview for DSM-IV Axis I Disorders administered by trained psychiatrists. All patients were Han Chinese, and they ranged in age from 20 to 55 years. All had been receiving a stable dosage of oral risperidone (3–6 mg/day) for at least 1 month prior to entry into the study, without any other antipsychotic drugs, and all had deficits in sensory gating, with P50 ratios greater than 0.5. The exclusion criteria were cardiovascular or neurological disease, a history of a head injury with loss of consciousness, physical abnormalities, and meeting DSM-IV criteria for substance dependence or current mood or anxiety disorders. All participants had normal hearing acuity (a subjective hearing threshold of 40 dB).
All patients underwent a complete medical history and physical examination, laboratory tests, and ECG, and all reported that they were not dependent on alcohol or other substances. All participants gave signed, informed consent to take part in the study after the procedure had been fully explained to them. The institutional review board of the Mental Health Center of Shantou University approved the study.
Clinical Treatment
The trial consisted of a 2-week placebo lead-in followed by 10 days of double-blind placebo-controlled tropisetron treatment. After the 2-week lead-in, 62 patients were screened out with P50 suppression testing. Forty patients had a P50 ratio above 0.5. Demographic characteristics, the duration of illness, and symptom severity on the Positive and Negative Syndrome Scale (PANSS) (36) did not differ between patients who had P50 deficits and those who did not (all p values, >0.05). These 40 patients were randomly assigned to four groups of 10 receiving either placebo or oral tropisetron at 5, 10, or 20 mg/day (Navoban, Novartis Pharmaceuticals, Ltd., Beijing). All study drugs were capsulized and identical in appearance and administered once daily. We maintained patients on a fixed daily dose of 3–6 mg of risperidone throughout the study and permitted no concomitant psychotropic medications except chloral hydrate as needed for insomnia during the study.
Because the proposed effects on P50 gating ratio and neurocognition reflect agonism at a ligand-gated ion channel, biological effects were expected immediately (24). In a previous similar study (24), single-day administration of the partial α7 nAChR agonist 3-[(2,4-dimethoxy)benzylidene]anabaseine (DMXB-A) was associated with significant neurocognitive improvement on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (37) total scale score, as well as improvement in P50 inhibition. Therefore, short-term effects on P50 inhibition and cognition were expected, and we were testing whether these effects would be sustained with sustained treatment.
P50 Testing
P50 auditory evoked potentials were recorded at baseline and on day 10. At baseline, P50 auditory evoked potentials were used as a screening test to eliminate subjects whose P50 gating ratio was below 0.5 when recorded without administration of tropisetron. On day 10 the P50 test was recorded 1 hour after administration of tropisetron or placebo.
We performed the electrophysiological examinations using a signal generator and the data acquisition system of a fully functional digital 40-channel EBNeuro Sirius EEG system (EBNeuro, Florence, Italy). A full description of the measurement of P50 suppression is available elsewhere (38). Briefly, participants were seated in a comfortable examination chair and instructed to relax and to focus their eyes on a fixation point. We generated a stimulus signal of 90-dB pulses of 0.1 ms in duration and recorded the event-related potential waveforms. We presented 32 pairs of auditory clicks every 10 seconds, with a 500-ms interclick interval. The event-related potential responses were amplified and band-pass filtered with a 0.1- to 300-Hz analog filter and no 50-Hz notch filter, at a sampling rate of 512 Hz, for a total of 1000 ms (100 ms before to 400 ms after the stimuli with a 500-ms gap between stimulus 1 and stimulus 2). After data acquisition, those trials that contained artifacts (±50 μV wave amplitude deflection) were not included in the waveform averaging. Data were analyzed offline and band-pass filtered (10–50 Hz, 12 dB/octave roll-off). Eye movements were recorded via electro-oculography with Ag-AgCl disc electrodes. All of the electrode resistances were less than 5 kΩ. We presented 70-dB(A) broadband white noise continuously throughout the session to control background noise during stimulus presentation.
The conditioning P50 wave (S1) was identified as the most positive peak between 30 and 90 ms after the conditioning stimulus. The test P50 wave (S2) was identified as the positive peak after the test stimulus that was closest in latency to the conditioning P50. Amplitude was defined as the difference between the positive peak and the preceding negative trough for both waves. The data from the vertex (CZ site) are reported because that is the best site for distinguishing patients with schizophrenia from healthy subjects when using this electrode array (39). The P50 gating ratios were calculated as the ratio of the test P50 amplitude to the conditioning P50 amplitude, and to minimize skewed distributions, ratios greater than 2 were assigned the value 2 (38).
Two independent neurophysiologists who were evoked potential specialists and who remained blind to the patient’s diagnosis and treatment analyzed the P50 testing results. When the findings of the two examiners differed, the results were reanalyzed by both to reach a consensus value.
Cognitive Performance
Our main measure of cognitive functioning was the individually administered RBANS at baseline and on day 10. The RBANS, a widely used screening instrument in neuropsychological assessment, includes 12 subtests (most of which are similar to individual neuropsychological measures) that produce five age-adjusted index scores and a total score (37). The indexes include immediate memory (including list learning and story memory tasks); visuospatial/constructional (including figure copy and line orientation tasks); language (including picture naming and semantic fluency tasks); attention (including digit span and coding tasks); and delayed memory (including list recall, story recall, figure recall, and list recognition tasks). Our group previously translated this instrument into Chinese and established its clinical validity and test-retest reliability in healthy subjects and in patients with schizophrenia (40); the translated version also had relatively good reliability and validity in a sample of community-dwelling elderly subjects (41). At the time we conducted the present study, neither the Cambridge Neuropsychological Test Automated Battery nor the MATRICS Consensus Cognitive Battery was available in China.
Side Effects
A psychiatrist used the Treatment Emergent Symptom Scale to record side effects in all patients after 10 days of treatment.
Statistical Analysis
Several considerations guided our statistical analysis plan. The principal outcome analysis consisted of repeated-measures multivariate analyses of variance (MANOVAs) for the RBANS total and index scores, with a between-subject factor of drug (placebo, 5 mg, 10 mg, 20 mg) and a within-subject factor of time (baseline and day 10). If the drug or drug-by-time interaction effects were significant in the repeated-measures MANOVA, the effects of age, education level, sex, duration of illness, risperidone dosage, and chloral hydrate use were tested by adding these variables to the model as covariates. Chloral hydrate use was assessed as a dichotomous variable (presence/absence), which did not change the results of the analysis (see Results section). If the overall treatment effect was significant, the three planned comparisons for the effects of each dosing level of tropisetron on the cognitive test battery total and index scores compared with placebo were performed by analysis of covariance (ANCOVA), with baseline score as a covariate, followed by Fisher’s least significant difference test to compare the differences between the dosage groups. The P50 data were analyzed by a similar repeated-measures analysis of variance. Correlations between the changes in the RBANS and P50 gating ratios used Pearson’s correlation coefficients. Additional statistical analyses included t tests, analyses of variance (ANOVA) for continuous variables, and chi-square tests for categorical variables. To adjust for multiple testing, a Bonferroni correction was applied. The Bonferroni procedure refers to all applied independent statistical tests. This approach was chosen because it allows for stringent control for multiple testing and a reduction of the probability of false positives (type I errors). Since we had six comparisons among four groups, the Bonferroni-adjusted significance level was set at p=0.05/6=0.0083. All statistical analyses were conducted in PASW Statistics, version 18.0 (SPSS, Inc., Chicago).
Results
No significant differences were observed between treatment groups on any demographic characteristic (see Table S1 in the data supplement that accompanies the online edition of this article). Two patients in the 20 mg group dropped out because of adverse events (see below), and 38 patients completed the trial (see Figure S1 in the data supplement). Thirteen patients took chloral hydrate during the study. There were no significant differences between groups in use of risperidone and adjunctive chloral hydrate during the study.
Effects on Cognitive Functioning
The RBANS total and index scores (Table 1) did not differ between treatment groups at baseline (all p>0.05). Repeated-measures MANOVA on the total and index scores showed a significant drug-by-time effect for the total score (F=3.19, df=3, 34, p=0.04) and the immediate memory score (F=3.55, df=3, 34, p=0.02). When the effects of age, education level, sex, duration of illness, risperidone dosage, and chloral hydrate use were examined by adding them to the repeated-measures MANOVA as covariates, there was still a significant drug-by-time effect for the total (F=4.20, df=3, 28, p=0.01) and immediate memory (F=3.43, df=3, 28, p=0.03) scores; the effect for the delayed memory score fell just short of significance (F=2.79, df=3, 28, p=0.06).
| Baseline | Day 10 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tropisetron | Tropisetron | Dose-by-Treatment Contrast | ||||||||||||||||
| RBANS Scores | Placebo (N=10) | 5 mg (N=10) | 10 mg (N=10) | 20 mg (N=10) | Placebo (N=10) | 5 mg (N=10) | 10 mg (N=10) | 20 mg (N=8) | F | pa | ||||||||
| Immediate memory | 61.1 | 8.2 | 65.0 | 14.3 | 64.2 | 17.0 | 64.3 | 7.2 | 65.5 | 8.4 | 71.6 | 13.4 | 78.6 | 18.1 | 74.9 | 8.0 | 3.43 | 0.03 |
| Visuospatial/constructional | 97.8 | 8.6 | 98.5 | 7.9 | 100.4 | 17.5 | 95.3 | 9.2 | 98.3 | 7.5 | 102.2 | 10.7 | 100.6 | 11.3 | 101.9 | 12.7 | 0.92 | 0.44 |
| Language | 75.7 | 8.7 | 70.4 | 9.3 | 69.3 | 14.2 | 75.5 | 2.1 | 75.5 | 8.1 | 73.2 | 7.0 | 66.6 | 11.1 | 75.5 | 2.1 | 1.14 | 0.35 |
| Attention | 62.2 | 8.2 | 63.9 | 7.7 | 69.9 | 12.5 | 63.9 | 8.2 | 67.9 | 8.8 | 74.7 | 9.9 | 76.2 | 13.3 | 68.8 | 11.4 | 1.60 | 0.21 |
| Delayed memory | 80.6 | 13.7 | 75.8 | 17.7 | 72.2 | 17.8 | 78.4 | 14.1 | 81.2 | 8.5 | 86.6 | 7.9 | 87.1 | 12.4 | 90.8 | 5.4 | 2.98 | <0.05 |
| Total | 69.1 | 8.6 | 69.4 | 9.0 | 67.3 | 13.1 | 68.4 | 6.4 | 71.8 | 7.1 | 76.4 | 6.1 | 76.4 | 12.4 | 77.4 | 5.0 | 4.20 | 0.014 |
Further ANCOVA showed that after 10 days of treatment, the RBANS total score was significantly higher in the 20 mg (p=0.002; effect size=0.95), 10 mg (p=0.006; effect size=0.48), and 5 mg (p=0.04; effect size=0.73) tropisetron groups than in the placebo group. Immediate memory was better in the 10 mg group compared with the placebo group (p=0.005; effect size=0.98). The 20 mg group performed better than the placebo group on delayed memory (p=0.007; effect size=1.39) (Table 1). The effects of all three dosages of tropisetron on cognitive tests remained significant after Bonferroni correction for multiple testing except the comparison between placebo and 5 mg on the RBANS total score (Bonferroni-corrected p=0.0083). However, the three tropisetron dosage groups did not differ from each other on the test battery.
The testers also noted that at day 10 of treatment, about half of the 28 treated patients provided spontaneous comments about their cognition, whereas none of the placebo patients did so, and the providing of comments did not differ among the three dosing groups. Specific comments were relatively similar across dosage groups; patients remarked that they could think more clearly or concentrate better and could remember things easier or better. When the testers asked for more details, the patients simply said that they could pay attention better and respond or react to questions from others more easily.
P50 Sensory Gating
The four groups exhibited no significant differences at baseline in conditioning amplitude (S1), test amplitude (S2), and P50 gating ratio (S2/S1). After the 10-day treatment, a repeated-measures ANOVA showed a significant overall effect on the P50 gating ratio (F=6.80, df=3, 34, p=0.001). Effects of sex, age, education level, duration of illness, risperidone dosage, and chloral hydrate use were also examined. These covariates had no significant effects (F=7.31, df=3, 28, p=0.001). ANCOVA analysis showed that the P50 gating ratio was significantly lower in the 10 mg (p=0.001; effect size=2.01), 5 mg (p=0.001; effect size=1.39), and 20 mg (p=0.004; effect size=1.41) tropisetron groups than in the placebo group (see Table 2; see also Figure S2 in the online data supplement). The effects of all three dosages on the P50 gating ratio remained significant after Bonferroni correction for multiple testing (Bonferroni-corrected p=0.0083). However, the analysis did not establish a significant difference between the three tropisetron dosages.
| Tropisetron | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time Point and P50 Parameters | Placebo | 5 mg | 10 mg | 20 mg | Analysis | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | |
| Baseline | ||||||||||
| Amplitude (μV) | ||||||||||
| Conditioning | 1.70 | 1.40 | 2.03 | 1.50 | 1.48 | 0.98 | 1.78 | 1.38 | 0.28 | 0.84 |
| Test | 2.42 | 2.19 | 2.65 | 1.11 | 2.81 | 1.37 | 2.98 | 2.43 | 0.15 | 0.93 |
| P50 gating ratio (%) | 124.5 | 59.5 | 160.0 | 73.7 | 160.0 | 46.7 | 146.5 | 58.8 | 0.79 | 0.51 |
| Day 10 | ||||||||||
| Amplitude (μV) | ||||||||||
| Conditioning | 1.84 | 1.41 | 2.26 | 1.30 | 2.46 | 1.04 | 2.49 | 1.11 | 0.56 | 0.65 |
| Test | 2.27 | 1.66 | 1.35 | 0.86 | 1.07 | 0.77 | 1.74 | 1.15 | 1.98 | 0.14 |
| P50 gating ratio (%) | 142.5 | 58.8 | 69.4 | 51.7 | 48.4 | 36.9 | 69.9 | 49.3 | 6.79 | 0.001 |
The grand averages of our P50 recordings at baseline (N=40) and at day 10 for the four groups are shown in Figure S3 in the online data supplement. The baseline P50 ratio (S2/S1) for all patients was 1.59 (S2=2.7 μv, S1=1.7 μv); at day 10, the P50 ratio for the active treatment groups had decreased to the range of 0.48–0.70 (S2=1.1–1.7 μv, S1=2.3–2.5 μv), but it remained almost the same as at baseline for the placebo group, at 1.28 (S2=2.3 μv, S1=1.8 μv). Hence, the P50 ratio and the S2 P50 waveform showed a marked difference between tropisetron and placebo treatment.
Relationship Between P50 Gating Ratio and Cognitive Functioning
After treatment, the improvement in the RBANS total score was associated with improvement in the P50 gating ratio (baseline minus day 10 scores) (r=0.33, p<0.05) (Figure 1). The two index scores showing significant improvement also were associated with improvement in the P50 gating ratio. Immediate memory had a significant association (r=0.38, p<0.01), while delayed memory fell just short of significance (r=0.31, p=0.06). In addition, as shown in Figure 2, the 10 and 20 mg dosages of tropisetron yielded a flattened dose-response curve on the P50 gating ratio and the RBANS total score, which is characteristic of a partial agonist at higher dosages.
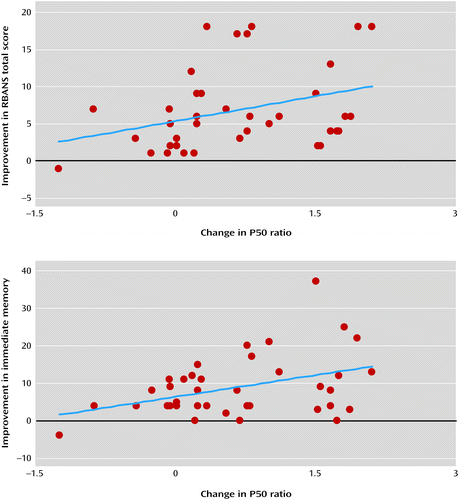
a RBANS=Repeatable Battery for the Assessment of Neuropsychological Status. The reduction in P50 gating ratio after treatment had a significant correlation with improvement on the RBANS total score (r=0.33, df=38, p<0.05) and the immediate memory index score (r=0.38, df=38, p<0.01).
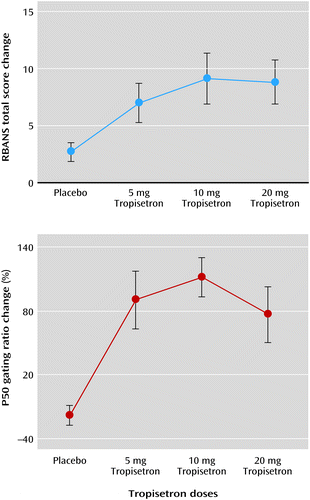
a RBANS=Repeatable Battery for the Assessment of Neuropsychological Status. The changes in RBANS total score and in P50 gating ratio after 10-day treatment with placebo or tropisetron show flattened dose-response curves.
Adverse Events
The frequency of adverse events (Table 3) appeared to increase with tropisetron dosage. However, the three dosages of tropisetron showed no significant effects on vital signs, ECG, hematology, or serum biochemistry tests. One patient in the 20 mg group complained of moderate leg edema and decided to discontinue the trial. Shortly after discontinuation, his symptoms resolved without any medical intervention. A second patient at 20 mg discontinued because of abdominal pain and constipation. Three others in the 20-mg group and two patients in the 10-mg group complained of moderate constipation and needed to take a laxative. The other adverse events were mild and required no additional treatments.
| Tropisetron | ||||
|---|---|---|---|---|
| Characteristic | Placebo (N=10) | 5 mg (N=10) | 10 mg (N=10) | 20 mg (N=10) |
| Dry mouth | 0 | 1 | 2 | 3 |
| Constipation | 0 | 1 | 3 | 5 |
| Blurred vision | 0 | 0 | 0 | 1 |
| Nausea | 0 | 0 | 0 | 1 |
| Hypertension | 0 | 0 | 0 | 2 |
| Leg edema | 0 | 0 | 0 | 2 |
Discussion
We found that short-term treatment with three different daily doses of tropisetron (5, 10, and 20 mg) was associated with significant improvement in cognitive performance in patients with schizophrenia, as measured with the RBANS, and that these improvements were significantly correlated with improvement in the P50 gating ratio. Treatment with 10 mg/day of tropisetron for 8 weeks was found in a recent study by Shiina et al. (30) to be associated with improvement in cognitive performance in patients with schizophrenia, specifically improving rapid visual information processing (sustained visual attention) as measured with the Cambridge Neuropsychological Test Automated Battery. Our study additionally found that 10 mg/day of tropisetron was associated with improved immediate memory but had less effect on attention and that 20 mg/day was associated with improved delayed memory. Overall, these two studies show excellent agreement, and ours further demonstrates that the cognitive enhancements associated with tropisetron in schizophrenia are related to its dosing. Furthermore, our study showed no significant differences in the dosage of risperidone used across the four study groups, indicating a balanced randomization. Thus, the differences in the treatment effects on cognition and P50 gating ratio among the four groups appeared to be associated with tropisetron itself.
Our finding of improvement in auditory sensory gating P50 deficits with tropisetron treatment is consistent with the Shiina et al. study (30), which reported a significant improvement in P50 deficits in nonsmoking schizophrenia patients treated with tropisetron. Moreover, another study from the same group showed that a single administration of tropisetron (10 mg) improved deficits in P50 suppression (35). These findings suggest that administration of tropisetron may improve P50 deficits in patients with schizophrenia. Our finding of a significant correlation between improved RBANS total score and improved P50 sensory gating is consistent with another study with low-dose DMXB-A treatment that found a correlation between a decrease in P50 ratio and an increase in the RBANS total score (p=0.06) (23). While Shiina et al. (30) did not observe any significant correlation between P50 changes and improved cognitive functioning in schizophrenia, this was probably due to their small sample size. Thus, stimulation of the nAChRs appears to improve both auditory P50 deficits and cognitive performance such as attention (30) and immediate and delayed memory, as shown in the present study.
Tropisetron’s partial agonism at α7 nAChRs appears to be its mechanism for improving both sensory gating and cognitive deficits (12, 16, 19–21, 23–27). Hashimoto et al. (32) reported that tropisetron improved the inhibitory processing deficits through the nAChR in mice. The nAChR agonist DMXB-A was also found to improve auditory P50 deficits as well as cognitive performance, including attention/vigilance and working memory, in patients with schizophrenia (23, 24). Since tropisetron is also a potent antagonist at 5-HT3 receptors, its effects on cognitive deficits might be mediated via antagonism of 5-HT3 receptors. However, using phencyclidine-induced cognitive deficits in mice as a model for cognitive deficits in schizophrenia, tropisetron or the selective nAChR agonist SSR180711, but not the selective 5-HT3 receptor antagonist ondansetron, ameliorated the phencyclidine-induced cognitive deficits (33, 34). Furthermore, the effects of tropisetron or SSR180711 were blocked by coadministration of methyllycaconitine, a selective α7 nAChR antagonist (33, 34). Thus, tropisetron’s improvement in cognitive deficits is more likely mediated through α7 receptors than 5-HT3 antagonism. Finally, a recent PET neuroimaging study using [11C]CHIBA-1001 found that a single oral dose of tropisetron (5, 10, or 20 mg) bound to brain α7 nAChRs in a dose-dependent manner, but the functional activity of tropisetron appeared to follow a typical partial agonist pattern with a flattened dose-response curve above 10 mg daily (30).
Our study’s limitations include the relatively small sample size and the short duration of treatment in this trial. However, the results strongly support the need for a double-blind, placebo-controlled study with a longer duration of tropisetron and risperidone treatment using a larger sample, which is currently under way. Our initial analysis from this larger study found that both the placebo and tropisetron groups showed significant improvement between baseline and week 10 on the PANSS total score and its subscores as well as on the Hamilton Depression Rating Scale (HAM-D) score (all p values, <0.05). Furthermore, we found that the PANSS total score and its negative symptom subscore as well as the HAM-D score at weeks 5 and 10 showed a significant difference between the tropisetron and placebo groups (all p values, <0.05), suggesting that tropisetron treatment may enhance the effectiveness of risperidone in schizophrenia, especially for negative and depressive symptoms.
Another limitation of the present study was the possible presence of practice effects for the RBANS. Freedman et al. (23) observed practice effects in a study using the MATRICS battery to investigate the efficacy of DMXB-A. Also, Shiina et al. (30) found evidence of practice effects for the Cambridge Neuropsychological Test Automated Battery, since the improvement of cognitive performance was noted in the placebo group of their study. However, our study found no significant improvement in the RBANS total and index scores for the placebo group, supporting the conclusion that practice effects did not confound tropisetron’s improvement of cognitive performance in schizophrenia.
1 : Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry 2004; 65:361–372Crossref, Medline, Google Scholar
2 : What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev 2009; 19:365–384Crossref, Medline, Google Scholar
3 : Cognition at illness onset as a predictor of later functional outcome in early psychosis: systematic review and methodological critique. Schizophr Res 2011; 125:221–235Crossref, Medline, Google Scholar
4 : Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Res 2004; 129:45–53Crossref, Medline, Google Scholar
5 : Determinants of work outcome in schizophrenia and schizoaffective disorder: role of cognitive function. Psychiatry Res 2009; 169:178–179Crossref, Medline, Google Scholar
6 : Neuropsychological predictors of functional outcome in Cognitive Behavioral Social Skills Training for older people with schizophrenia. Schizophr Res 2008; 100:133–143Crossref, Medline, Google Scholar
7 : Pharmacological cognitive enhancement in schizophrenia. Neuropsychol Rev 2009; 19:324–335Crossref, Medline, Google Scholar
8 : Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 2011; 36:316–338Crossref, Medline, Google Scholar
9 : Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17:639–654Medline, Google Scholar
10 : Sensory gating in schizophrenics and normal controls: effects of changing stimulation interval. Biol Psychiatry 1989; 25:549–561Crossref, Medline, Google Scholar
11 : Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry 2004; 161:1822–1828Link, Google Scholar
12 : Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry 1990; 47:181–188Crossref, Medline, Google Scholar
13 : Schizophrenia and nicotinic receptors. Harv Rev Psychiatry 1994; 2:179–192Crossref, Medline, Google Scholar
14 : The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep 2003; 5:155–161Crossref, Medline, Google Scholar
15 : Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 1998; 24:189–202Crossref, Medline, Google Scholar
16 : Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587–592Crossref, Medline, Google Scholar
17 : The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat 2000; 20:299–306Crossref, Medline, Google Scholar
18 : Nicotinic receptor function in schizophrenia. Schizophr Bull 1996; 22:431–445Crossref, Medline, Google Scholar
19 : Association of promoter variants in the α7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 2002; 59:1085–1096Crossref, Medline, Google Scholar
20 : Genetics of chromosome 15q13-q14 in schizophrenia. Biol Psychiatry 2006; 60:115–122Crossref, Medline, Google Scholar
21 : α-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004; 174:54–64Crossref, Medline, Google Scholar
22 : Nicotinic agonists and psychosis. Curr Drug Targets CNS Neurol Disord 2002; 1:149–162Crossref, Medline, Google Scholar
23 : Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry 2008; 165:1040–1047Link, Google Scholar
24 : Proof-of-concept trial of an α7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry 2006; 63:630–638Crossref, Medline, Google Scholar
25 : Treating schizophrenia symptoms with an α7 nicotinic agonist, from mice to men. Biochem Pharmacol 2007; 74:1192–1201Crossref, Medline, Google Scholar
26 : Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull 2007; 33:1120–1130Crossref, Medline, Google Scholar
27 : α7 Nicotinic receptor agonists: potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and Alzheimer’s disease. Open Med Chem J 2010; 4:37–56Medline, Google Scholar
28 : The NIMH-MATRICS project for developing cognition-enhancing agents for schizophrenia. Dialogues Clin Neurosci 2006; 8:109–113Medline, Google Scholar
29 : Tropisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 2000; 59:1297–1315Crossref, Medline, Google Scholar
30 : A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry 2010; 9:27Crossref, Medline, Google Scholar
31 : The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective α7 nicotinic receptor partial agonist. Bioorg Med Chem Lett 2001; 11:319–321Crossref, Medline, Google Scholar
32 : Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of alpha 7 nicotinic acetylcholine receptors. Psychopharmacology (Berl) 2005; 183:13–19Crossref, Medline, Google Scholar
33 : Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the novel selective alpha7 nicotinic receptor agonist SSR180711. Biol Psychiatry 2008; 63:92–97Crossref, Medline, Google Scholar
34 : Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of α7 nicotinic receptors. Eur J Pharmacol 2006; 553:191–195Crossref, Medline, Google Scholar
35 : Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res 2005; 76:67–72Crossref, Medline, Google Scholar
36 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
37 : The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998; 20:310–319Crossref, Medline, Google Scholar
38 : Neuroleptic effects on P50 sensory gating in patients with first-episode never-medicated schizophrenia. Schizophr Res 2009; 108:151–157Crossref, Medline, Google Scholar
39 : Multiple site evaluation of P50 suppression among schizophrenia and normal comparison subjects. Schizophr Res 1998; 30:71–80Crossref, Medline, Google Scholar
40 : Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) as a screening test in Chinese: reliability and validity. Chin Ment Health J 2009; 28:865–869Google Scholar
41 : Reliability and validity of the Repeatable Battery for the Assessment of Neuropsychological Status in community-dwelling elderly. Arch Med Sci 2011; 7:850–857Crossref, Medline, Google Scholar



