Poor Nutrition at Age 3 and Schizotypal Personality at Age 23: The Mediating Role of Age 11 Cognitive Functioning
Abstract
Objective:
Poor prenatal nutrition has been associated with schizophrenia spectrum disorders in the Netherlands and China, and it has been suggested that perinatal and postnatal nutritional factors lead to the development of schizophrenia and the exhibition of schizotypal traits later in life. There appears to be no prior research on the existence of possible factors that may mediate the relationship between malnutrition and schizophrenia spectrum disorders or whether this association is a direct one. The authors tested the hypothesis that low IQ mediates the relationship between early childhood malnutrition and adult schizotypal personality.
Method:
Participants were drawn from a birth cohort of 1,795 boys and girls who were followed prospectively. Objective indicators of malnutrition (anemia and stunting) were assessed at age 3. Verbal and performance intelligence were assessed at age 11, and schizotypal personality was assessed at age 23.
Results:
Both stunting and anemia at age 3 were associated with low IQ at age 11. Low performance IQ at age 11 was associated with increased interpersonal and disorganized features of schizotypal personality at age 23. Poor performance IQ was found to mediate the relationship between poor nutrition at age 3 and interpersonal and disorganized features of schizotypy at age 23. Findings in female participants were replicated in male participants.
Conclusions:
Given that poor nutrition is an alterable risk factor, these findings suggest that nutritional enhancements may improve brain functioning and possibly reduce some features of schizotypal personality disorder.
Studies of populations in China (1) and the Netherlands (2) have demonstrated that maternal exposure to discrete periods of famine during pregnancy results in the birth of children who are twice as likely to develop schizophrenia or exhibit schizoid personality disorder than those who are not exposed. However, conditions that cause prenatal malnutrition may continue into the postnatal period and can be conceptualized as dimensional risk factors for physical and mental illnesses. The rate of postnatal growth may be considered as a continuum from fetal development (3), and the growth trajectory from birth to 2½ years among female schizophrenia patients has been reported to be slower than that for comparison subjects (4). In addition to birth disturbances, thinness in childhood (attributed to malnutrition) has been associated with later development of schizophrenia (5). Furthermore, a study of 31-year-old women reported that both perinatal and postnatal disturbances are related to increased schizotypal traits in later life (6). Surprisingly, however, there have been no studies, to our knowledge, of the relationship between malnutrition directly assessed postnatally and any schizophrenia spectrum disorder.
If poor nutrition is indeed a risk factor for schizotypal personality disorder, then an important question concerns whether this factor operates directly or whether a third factor mediates this relationship. A key requirement of a mediator is that it must relate to both the independent variable (malnutrition) and the dependent variable (schizotypal personality). One such possible candidate is low IQ, given its linkage with schizophrenia. Meta-analyses have demonstrated that children who later develop schizophrenia have lower IQs than comparison subjects (7). In a study of Swedish Army recruits, individuals with low IQ had an increased risk of developing schizophrenia, and it was mainly a nonverbal test of mechanical ability that revealed this association (8). The authors indicated that this test requires executive control, which in turn may be reliant on frontal lobe functioning. Furthermore, individuals with clinically assessed schizotypal personality disorder (particularly those with negative and disorganized features) have demonstrated poorer performance on the arithmetic subtest of the Wechsler Adult Intelligence Scale (9). Given these findings, IQ meets one criterion for status as a mediator.
IQ also meets a second requirement for status as a mediator. Comprehensive reviews of risk factors that affect child development (10, 11) have demonstrated that childhood undernutrition can cause stunting and iron deficiency anemia, which are common risk factors in developing countries and lead to deficits in intelligence. Since both factors give rise to continuous variables, they are suitable for use in the present context.
While poor nutrition may be a risk factor for later development of schizotypal personality and IQ may be a possible mediator, one further unresolved issue is with regard to whether any relationship between malnutrition and schizotypy applies to all forms of schizotypy or whether such a relationship is specific to certain subfactors. One comprehensive review of schizotypal personality suggests that two fundamentally different forms of schizotypy exist (12). One form, referred to as “neuroschizotypy,” is hypothesized to be a neurodevelopmental disorder, with its origins in genetic, prenatal, and early postnatal influences (including malnutrition), and consists of interpersonal and disorganized subfactors of schizotypal personality (13). A second form, referred to as “pseudoschizotypy,” is hypothesized to have origins in psychosocial adversity, including childhood abuse and neglect, and consists of the cognitive-perceptual features of schizotypal personality. Given this model, it might be expected that malnutrition would be relatively more associated with interpersonal and disorganized features rather than cognitive-perceptual features.
In this study, we used a prospective, longitudinal design assessing nutrition, IQ, and schizotypy in order to establish a temporal ordering between these variables and to assess the mechanisms of action. We hypothesized that 1) poor nutrition at age 3 would be associated with lower IQ at age 11, 2) low IQ at age 11 would be associated with increased schizotypy at age 23, 3) poor nutritional status at age 3 would be associated with higher rates of schizotypal personality at age 23, particularly with respect to cognitive-perceptual and disorganized features, and 4) this latter relationship would be mediated by low IQ.
Method
Participants
Participants were drawn from a birth cohort of 1,795 children from the Mauritius Child Health Project. Complete details of the study are described elsewhere (14). All children born in 1969 and 1970 in two main towns in Mauritius were enrolled in the study when they were 3 years old. The ethnic distribution of the sample was as follows: Indian, 68.5%; Creole (African origin), 25.7%; and other (Chinese, English, or French origin), 5.8%. Boys were 51.4% of the sample, and girls were 48.6%. Analyses were restricted to Indian and Creole participants because of the small number of children in the “other” ethnic category. After complete description of the study was provided, oral informed consent was obtained in the early phases (at ages 3 and 11) from the participants' mothers, while written informed consent was obtained from the participants themselves at age 23. Early-phase research activities were performed in accordance with the principles outlined in the Declaration of Helsinki (15), current in 1972, and in the Belmont report (16), applicable from 1979 onward. Institutional review board approval for the latter research phases was granted by the University of Southern California, Los Angeles.
Malnutrition at Age 3
Stunting.
Stunting is defined as the observed height as a percentage of the expected height for a child's particular age (17). It has been suggested (18) that growth norms should be developed based on healthy populations from different ethnic groups. Because Mauritius was a developing country in 1972 and height at age 3 was positively associated with socioeconomic status (r=0.24, p<0.0001; N=1,008), growth norms were based on the heights in the subsample, which were 1.5 standard deviations above the mean on socioeconomic status. Because the age range of the children was from 2.78 to 3.15 years at the time of measurement and height was correlated with age (r=0.22, p<0.0001; N=1,728), height was regressed to what it would have been at age 3. Furthermore, because there was a significant difference in height between the sexes as well as the two ethnic groups, norms for the expected heights were calculated separately for Indian and Creole male and female participants, and the results were combined. Measures were z-transformed and converted so that positive values indicate greater stunting.
Anemia.
Hemoglobin levels were assessed via laboratory testing of blood drawn by medical practitioners who also carried out other assessments. The mean hemoglobin level was 9.78 g/dL (SD=1.38). Data were z-transformed and converted so that positive values indicate greater anemia.
Psychosocial Adversity at Age 3
Psychosocial adversity was based on nine variables collected by social workers who visited the homes of the children and interviewed caregivers when the children were 3 years old (for complete details, see reference 19). The adversity index was created by adding 1 point for each of the variables, which were as follows: uneducated father, uneducated mother, semiskilled father or father in unskilled occupation, single-parent status, separation from parents, large family size, poor health of mother, teenage mother, and overcrowded home.
Intelligence at Age 11
Estimates of verbal and performance IQ were assessed at age 11 using seven subtests of the Wechsler Intelligence Scale for Children (20). Raw scores on the subscales were normalized and standardized. The similarities test and digit span task formed an estimate of verbal IQ, while block design, object assembly, coding, maze, and picture completion tests formed an estimate of performance IQ. This two-factor division has been established previously by confirmatory factor analysis, and its validity and reliability have been described (21).
Schizotypal Personality at Age 23
Schizotypal personality was measured using the Schizotypal Personality Questionnaire (22), which assesses the nine DSM-III-R features of schizotypal personality disorder. Questions were translated into patois Creole, checked via back translation, and administered by trained research assistants. Criterion validity for this self-report instrument is supported by a previously reported point-biserial correlation (0.60) between clinical diagnosis of schizotypal personality disorder and questionnaire scores (22). Confirmatory factor analysis of this scale has demonstrated that three main factors—cognitive-perceptual, interpersonal, and disorganized features—underlie individual differences in schizotypal personality (13). This same three-factor model has been confirmed in the Mauritius sample and generalizes across gender and different ethnic groups (23). The distribution of the nine DSM criteria among the three factors, along with clinical vignettes highlighting these factors, is summarized in Table 1.
| Traita | Clinical Vignetteb |
|---|---|
| Cognitive perceptual | |
| Ideas of reference, magical thinking, unusual perceptual experiences, paranoid ideation | “Participant C” is from a middle-income Creole family and presents primarily with cognitive-perceptual features. She has the sense that there is a force around her that she cannot see, and she believes in telepathy. When high winds blow, she is afraid of being attacked by a loup-garou (French name for werewolf). She feels as though she has to be on her guard, even with close friends. She feels that the things she sees on television sometimes have a special meaning exclusively for her. |
| Interpersonal | |
| No close friends, constricted affect, excessive social anxiety | “Participant B” is a machinist from a lower-income Muslim family and presents primarily with interpersonal features. He prefers to keep to himself, and he has no close friends outside of his family. He tends to avoid eye contact with others and rarely laughs or smiles. He feels very uncomfortable when he is with unfamiliar people. |
| Disorganization | |
| Odd/eccentric behavior, odd speech | “Participant J” is from a poor Hindu family of sugarcane workers and presents primarily with disorganized features. He dresses oddly in clothes that are of different colors and materials. He keeps chickens and talks to them as if they were human. He admits that other people find him strange. His speech is rambling, and he sometimes forgets what he is trying to say. |
TABLE 1. DSM-III-R Features of Schizotypal Personality Disorder
Representativeness of Data
Complete data for all three study phases (at ages 3, 11, and 23) were available for 815 of 1,795 participants in the total sample. This attrition rate was partly because the sample analyzed was restricted to Indian and Creole participants, but mainly because in the latter two phases, it was not possible to contact all of the original participants. Many of those in the original sample had moved after a hurricane or had migrated, resulting in incomplete data collection. As summarized in Table 2, there were no significant differences between the 815 participants for whom complete data were available and the remaining participants on any of the variables examined except performance IQ. Consequently, a sensitivity analysis was run for those in the analyzed group selected to produce a mean performance IQ equal to that for the unanalyzed group. This analysis revealed that there was no substantial departure from the main findings reported (see the data supplement that accompanies the online edition of this article). There was no significant difference with regard to ethnicity (Creole and Indian) between the two samples. There was also no difference with regard to social class (high, low) between the samples. However, there was a statistically significant difference in gender distribution between the groups, with fewer female than male participants in the analyzed group (χ2=12.15, p<0.001). In view of this sex difference, the data were analyzed separately for male and female participants.
| Analyzed Sample (N=815) | Nonanalyzed Sample (N=980) | ||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p |
| Standardized stunting | –0.0046 | 1.019 | 0.0042 | 0.984 | 0.85 |
| Standardized anemia | –0.0201 | 1.385 | 0.0221 | 1.372 | 0.54 |
| Psychosocial adversity | 1.88 | 1.41 | 1.87 | 1.34 | 0.84 |
| Verbal IQ | 100.16 | 14.59 | 99.78 | 15.74 | 0.67 |
| Performance IQ | 100.50 | 14.53 | 99.00 | 15.78 | 0.09 |
| Schizotypal Personality Questionnaire score | |||||
| Cognitive perceptual | 11.14 | 6.09 | 11.13 | 6.10 | 0.99 |
| Interpersonal | 12.41 | 6.67 | 12.32 | 6.69 | 0.85 |
| Disorganization | 4.58 | 3.88 | 4.71 | 3.92 | 0.59 |
TABLE 2. Comparison Between Analyzed and Nonanalyzed Samples on Variables Associated With Schizotypal Personality in a Mauritius Birth Cohort
Statistical Analysis
Given the prospective longitudinal design of the study and the fact that all variables examined were continuous, we used structural equation modeling, as recommended by Iacobucci et al. (24), for all mediation analyses, which were implemented using the SPSS Amos, 18.0, statistical software package (25). The conceptual model framing all analyses is illustrated in Figure 1. This model allows for assessment of the extent to which the independent variable (malnutrition) influences the dependent variable (schizotypal personality) directly via pathway c or whether the variance in the independent variable is determined wholly or partially by the mediator variable (IQ) via pathways a and b. In the mediation model, if the direct path taking account of a and b (c′) is not significant, the mediation effect is full. However, if c′ is significant, the mediation effect is partial. The direct effect of the independent variable on the dependent variable in the absence of the mediator variable is referred to as pathway c. An elaboration of the simple mediation model, including three independent variables (stunting, anemia, and psychosocial adversity) and two mediator variables (verbal and performance IQ) is presented in Figure 2. Analyses were first performed separately for each of the three Schizotypal Personality Questionnaire factors (cognitive perceptual, interpersonal, and disorganization) as dependent variables and then separately for both genders. Subsequently, the extent of mediation (partial or full) was examined using the bootstrap method (26), a conservative method that is independent of the distribution of the variables. Finally, to examine the effect of other previously unexamined variables on the mediation effect, we followed the logical implications of the structure described by VanderWeele (27), and the data were reanalyzed. Because this reanalysis did not produce any substantial change in the pattern of results, the findings are presented in the online data supplement.
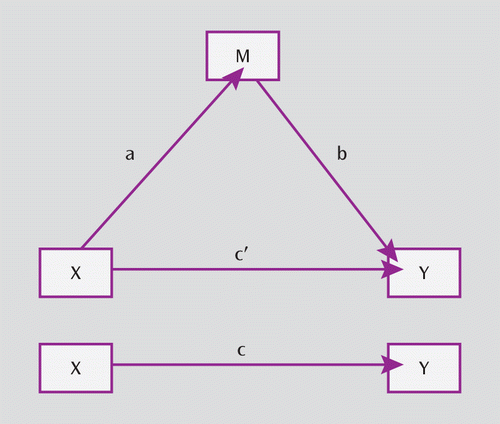
FIGURE 1. Conceptual Framing Modela
a The top portion of the model (triangle) depicts X, the independent variable (malnutrition); M, the mediator (IQ); and Y, the dependent variable (schizotypy). The indirect relationship between X and Y is via pathways a and b, while the direct path taking account of a and b is indicated by c′. The lower portion of the diagram shows the direct relationship between X and Y not taking account ofthe mediator and is indicated by c.
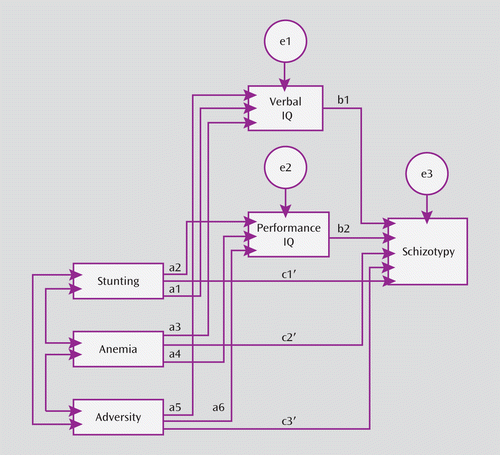
FIGURE 2. Elaborated Mediation Model Underlying Analysesa
a The path diagram used in the analyses carried out to test mediation depicts the pathways for stunting, anemia, and adversity (independent variables); verbal and performance IQ (mediator variables); and schizotypy (dependent variable).
Results
Analyses were aimed at establishing 1) the candidacy of IQ as a potential mediator of the relationship between malnutrition and schizotypy and 2) whether the extent of any mediation was full or partial. The beta weights for the regression slopes of IQ on malnutrition (pathway a) are listed in Table 3. Across gender, all regressions were statistically significant for both indicators of malnutrition (stunting and anemia). Negative slopes indicated that increased malnutrition was associated with low verbal and performance IQ. Subsequent testing revealed that there were no significant differences between the regression slopes for verbal and performance IQ.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Pathway and Mediation Relationship | Beta | SE | p | Beta | SE | p |
| Pathway a | ||||||
| Stunting-verbal IQ | –3.1 | 0.61 | <0.001 | –1.9 | 0.57 | <0.001 |
| Stunting-performance IQ | –3.8 | 0.56 | <0.001 | –2.3 | 0.56 | <0.001 |
| Anemia-verbal IQ | –1.1 | 0.47 | 0.02 | –1.2 | 0.40 | 0.002 |
| Anemia-performance IQ | –0.9 | 0.44 | 0.05 | –1.6 | 0.40 | <0.001 |
| Psychosocial adversity-verbal IQ | –1.8 | 0.44 | <0.001 | –3.0 | 0.42 | <0.001 |
| Psychosocial adversity-performance IQ | –2.0 | 0.41 | <0.001 | –3.3 | 0.42 | <0.001 |
| Pathway b | ||||||
| Verbal IQ-cognitive perceptual | –0.003 | 0.02 | 0.89 | 0.04 | 0.02 | 0.07 |
| Performance IQ-cognitive perceptual | 0.02 | 0.02 | 0.54 | 0.01 | 0.02 | 0.52 |
| Verbal IQ-interpersonal | –0.01 | 0.02 | 0.52 | 0.00 | 0.02 | 0.99 |
| Performance IQ-interpersonal | –0.05 | 0.02 | 0.04 | –0.05 | 0.02 | 0.02 |
| Verbal IQ-disorganization | –0.008 | 0.01 | 0.51 | –0.008 | 0.01 | 0.51 |
| Performance IQ-disorganization | –0.03 | 0.01 | 0.03 | –0.03 | 0.01 | 0.03 |
| Pathway c′ | ||||||
| Stunting-cognitive perceptual | –0.08 | 0.27 | 0.76 | 0.28 | 0.286 | 0.33 |
| Stunting-interpersonal | –0.29 | 0.30 | 0.32 | 0.35 | 0.30 | 0.25 |
| Stunting-disorganization | –0.10 | 0.17 | 0.54 | 0.23 | 0.19 | 0.22 |
| Anemia-cognitive perceptual | –0.07 | 0.19 | 0.74 | 0.20 | 0.20 | 0.32 |
| Anemia-interpersonal | 0.10 | 0.21 | 0.63 | 0.37 | 0.21 | 0.08 |
| Anemia-disorganization | –0.02 | 0.12 | 0.85 | 0.20 | 0.13 | 0.13 |
| Psychosocial adversity-cognitive perceptual | 0.29 | 0.19 | 0.12 | 0.20 | 0.23 | 0.51 |
| Psychosocial adversity-interpersonal | 0.50 | 0.20 | 0.01 | 0.16 | 0.24 | 0.50 |
| Psychosocial adversity-disorganization | 0.25 | 0.12 | 0.03 | 0.06 | 0.15 | 0.70 |
| Pathway c | ||||||
| Stunting-cognitive perceptual | –0.14 | 0.25 | 0.59 | 0.18 | 0.29 | 0.53 |
| Stunting-interpersonal | –0.08 | 0.28 | 0.77 | 0.49 | 0.30 | 0.10 |
| Stunting-disorganization | 0.003 | 0.16 | 0.99 | 0.34 | 0.19 | 0.06 |
| Anemia-cognitive perceptual | –0.05 | 0.20 | 0.79 | 0.14 | 0.20 | 0.50 |
| Anemia-interpersonal | 0.21 | 0.22 | 0.34 | 0.43 | 0.21 | <0.05 |
| Anemia-disorganization | 0.03 | 0.12 | 0.82 | 0.25 | 0.13 | 0.06 |
| Psychosocial adversity-cognitive perceptual | 0.25 | 0.18 | 0.16 | –0.02 | 0.21 | 0.933 |
| Psychosocial adversity-interpersonal | 0.61 | 0.20 | 0.002 | 0.34 | 0.22 | 0.12 |
| Psychosocial adversity-disorganization | 0.32 | 0.11 | 0.004 | 0.19 | 0.13 | 0.15 |
TABLE 3. Regression Beta Weights for Pathways Relating Stunting, Anemia, and Psychosocial Adversity at Age 3 to Verbal and Performance IQ at Age 11 and to Schizotypal Features at Age 23 in Male and Female Participantsa
The beta weights for the regression slopes of the two measures of IQ associated with the three schizotypy factors (pathway b) are also listed in Table 3. Increased scores for interpersonal and disorganized schizotypy were significantly associated with reduced performance IQ among both male and female participants. There were no such associations with verbal IQ or with cognitive-perceptual schizotypy. These results suggest that while performance IQ may be a candidate as a mediator, any mediation effect may be specific to interpersonal and disorganized features of schizotypy. Regarding pathway c′, the regression slopes relating malnutrition to schizotypy while accounting for the influence of the mediator indicated no significant direct pathways. Thus, the mediation effect was full. The results for pathway c, which accounts for the relationship between malnutrition and schizotypy without the influence of the mediator, revealed that with one exception, there were no significant effects.
Psychosocial adversity at age 3 was found to be related to both intelligence measures as well as to interpersonal and disorganized schizotypy (Table 3). Consequently, while there was a mediation effect of performance IQ on the relationship between psychosocial adversity and schizotypy, a significant c′ pathway indicated that this effect was partial and that the relationship was present in the absence of the mediator variable. Since psychosocial adversity was present in the modeling of the relationships between malnutrition and schizotypy, it was taken into account as a potential confounding variable of those relationships.
Additional analyses of the data confirmed the results (Table 4). Regression analyses were used to generate the modified Sobel index and bootstrap analyses, as described by Preacher and Hayes (26). Bootstrap analyses provide results that are independent of the form of the distribution of the data and thus represent strong confirmation of the finding that performance IQ is a mediator of the relationship between malnutrition and schizotypy. The results were further confirmed by analyses using the structural models described by VanderWeele (27) (see the online data supplement).
| Mediation Effect Normal Analysis | Mediation Effect Bootstrap Analysis | |||||
|---|---|---|---|---|---|---|
| Mediation Relationship | Sex | Mean | z | p | Mean | 95% CI |
| Stunting-performance IQ-cognitive perceptual | Male | –0.04 | 0.50 | 0.61 | –0.05 | –0.23 to 0.13 |
| Stunting-performance IQ-cognitive perceptual | Female | –0.08 | 0.96 | 0.36 | –0.08 | –0.26 to 0.08 |
| Stunting-performance IQ-interpersonal | Male | 0.30 | 2.66 | 0.007 | 0.30 | 0.09 to 0.54 |
| Stunting-performance IQ-interpersonal | Female | 0.28 | 2.77 | 0.006 | 0.28 | 0.09 to 0.50 |
| Stunting-performance IQ-disorganization | Male | 0.14 | 2.10 | 0.03 | 0.13 | 0.02 to 0.25 |
| Stunting-performance IQ-disorganization | Female | 0.20 | 3.05 | 0.002 | 0.20 | 0.08 to 0.34 |
| Anemia-performance IQ-cognitive perceptual | Male | 0.02 | 0.58 | 0.56 | 0.02 | –0.10 to 0.05 |
| Anemia-performance IQ-cognitive perceptual | Female | 0.08 | 1.36 | 0.17 | 0.08 | –0.19 to 0.03 |
| Anemia-performance IQ-interpersonal | Male | 0.11 | 2.18 | 0.03 | 0.11 | 0.03 to 0.22 |
| Anemia-performance IQ-interpersonal | Female | 0.12 | 1.96 | 0.05 | 0.13 | 0.01 to 0.27 |
| Anemia-performance IQ-disorganization | Male | 0.06 | 2.01 | 0.04 | 0.06 | 0.01 to 0.11 |
| Anemia-performance IQ-disorganization | Female | 0.11 | 2.59 | 0.009 | 0.11 | 0.03 to 0.21 |
| Psychosocial adversity-performance IQ-cognitive perceptual | Male | –0.06 | 1.06 | 0.28 | –0.06 | –0.17 to 0.04 |
| Psychosocial adversity-performance IQ-cognitive perceptual | Female | –0.09 | 1.05 | 0.29 | –0.09 | –0.25 to 0.07 |
| Psychosocial adversity-performance IQ-interpersonal | Male | 0.13 | 2.04 | 0.04 | 0.13 | 0.01 to 0.26 |
| Psychosocial adversity-performance IQ-interpersonal | Female | 0.27 | 2.85 | 0.004 | 0.27 | 0.09 to 0.48 |
| Psychosocial adversity-performance IQ-disorganization | Male | 0.08 | 2.11 | 0.03 | 0.08 | 0.01 to 0.15 |
| Psychosocial adversity-performance IQ-disorganization | Female | 0.18 | 3.07 | 0.002 | 0.18 | 0.06 to 0.31 |
TABLE 4. Regression Analyses Relating Stunting, Anemia, and Psychosocial Adversity to Schizotypy Measures in Male and Female Participantsa
Discussion
The results of our study broadly support our hypotheses. IQ was established as a mediator of the malnutrition-schizotypy relationship. The mediation model supports the suggestion that poor nutrition early in childhood results in poorer cognitive performance in later childhood, which in turn is associated with the development of two factors of schizotypy in early adulthood. Importantly, performance but not verbal IQ at age 11 was found to mediate this relationship. This mediation effect was specific to the interpersonal and disorganized features of schizotypy and was not observed in cognitive-perceptual schizotypy (also see reference 28). These mediation effects applied to both male and female participants. Our findings remained robust when they were reanalyzed for both the effects of attrition in sample size and the effects of additional hypothesized moderating variables.
To provide a hypothesis for future brain imaging studies, our findings suggest that malnutrition may predispose individuals to hippocampal and frontal brain impairments, which in turn predisposes them to schizotypy. There is evidence that the hippocampus and the prefrontal cortex are dysfunctional in schizophrenia (29), although the role of the prefrontal cortex in schizotypy is more controversial (12). One study using rats demonstrated that excitability of the prefrontal cortex is lowered by early postnatal malnutrition, and this prefrontal deficit persisted even when adequate feeding was later introduced (30). Neuronal and synaptic loss in the hippocampus has also been found to result from postnatal protein deprivation (31). Prenatal dietary deprivation affects subregions of the hippocampus (32), while prenatal protein malnutrition alters the response of medial prefrontal neurons to stress (33). The only study of the effect of malnutrition on discrete brain areas in humans reported that frontal and prefrontal lobe volumes were lower in children who were malnourished in early life (34). Taken together, these prenatal and postnatal studies provide initial evidence that those areas of the brain that are dysfunctional in individuals with schizophrenia and schizotypy personality disorder are regions that are also affected by malnutrition.
Another question is with regard to the extent to which particular brain areas are related to measures of intelligence. One MRI study demonstrated a positive relationship between IQ and gray matter density in both the orbitofrontal cortex and the cingulate gyrus (35), while another study reported a positive relationship between IQ (mainly performance IQ) and temporal gray matter, temporal white matter, and frontal white matter volumes (36). These findings support some linkage between low IQ and frontal dysfunction (8). Also consistent with the finding of performance IQ as a mediator of the malnutrition-schizotypy relationship are meta-analytic and later empirical findings demonstrating that early performance IQ is more predictive than verbal IQ of later schizophrenia (37, 38).
Mediation effects were found for interpersonal and disorganized features of schizotypy but not for cognitive-perceptual features. These findings are consistent with a model of schizotypal personality in which interpersonal and disorganized (but not cognitive perceptual) features are hypothesized to be elements of a form of schizotypy (neuroschizotypy) that is neurodevelopmentally based, with its antecedent in early prenatal and early postnatal factors. Poor early nutrition was specifically hypothesized to be one contributing factor to this form of schizotypy (12). No significant relationship was observed between cognitive-perceptual schizotypy and IQ, a finding that was also observed recently by other investigators (28). The heightened self-awareness of thought processes reflected in cognitive-perceptual schizotypy (Table 1) may partly account for this null effect and may partly explain why few, if any, studies have reported IQ deficits in cognitive-perceptual schizotypy (12).
Our finding of a linkage between early malnutrition and adult schizotypy as demonstrated through reduced performance ability is broadly consistent with a growing body of evidence linking nutritional status to risks for schizophrenia spectrum disorders as well as to prevention of cognitive breakdown. Using this same sample, we previously demonstrated that an experimental environmental enrichment intervention from ages 3 to 5 years consisting of better nutrition, more physical exercise, and cognitive stimulation resulted in a significant reduction in schizotypy at age 23 in participants who had poor nutritional status prior to the beginning of the intervention (39). The fact that there was a significant interaction between nutritional status and the experimental enrichment intervention suggests that nutrition was a key component in this better outcome. Because the children in the enrichment program received portions of fish that were 2.5 times greater per week than the portions received by comparison subjects, increased levels of omega-3 fatty acids in their diet may have contributed to the beneficial effect. Recent evidence for the efficacy of omega-3 in preventing cognitive breakdown in schizophrenia can be found in a randomized controlled trial (40). Our findings, combined with those of prior studies demonstrating that poorer prenatal nutrition is associated with schizophrenia spectrum disorders, contribute to growing empirical evidence suggesting that nutritional factors may be taken into account in future risk and prevention studies of schizotypal personality disorder.
Several limitations to our study need to be recognized. First, while the observed associations are consistent with a causal model, the findings cannot establish causality. Second, the findings cannot be generalized from individual differences in schizotypal personality in the community to schizotypal personality disorder in a clinical population, although the Schizotypal Personality Questionnaire measure was validated against a clinically defined schizotypal personality disorder sample. Third, further studies are required to assess the generalizability of these findings from Mauritius to other regions. Fourth, we caution that postnatal malnutrition may also reflect prenatal malnutrition, and consequently we cannot argue for any temporal specificity to the postnatal period. Fifth, the measure of psychosocial adversity was based on data collected in the initial study phase and thus could not reflect more recent approaches, which might include family assets, although such a variable was included in the later analysis, which is presented in the online data supplement. Sixth, the main analyses were carried out in a sample consisting of some participants who were also involved in the intervention study (39). However, analyses presented in the data supplement demonstrated that elimination of these participants did not change the results. Seventh, the position put forward is likely a simplification of a more complex reality. For example, there could be other potential processes that result in variations in IQ, which in turn is likely to be only one of a number of possible moderators of the malnutrition-schizotypy relationship. Null findings for some alternatives that we had the capacity to examine (birth complications, birth weight, temperament, and psychosocial adversity at ages 11 and 23) are presented in the data supplement. Finally, it is recognized that low IQ is an indirect proxy for brain dysfunction, and consequently we caution that these findings cannot directly identify specific brain mechanisms that mediate the malnutrition-schizotypy relationship.
Despite these limitations, our findings bring initial research on nutrition and schizophrenia spectrum disorders a step further. The prospective longitudinal design enabled us to identify the temporal ordering of variables, moving from malnutrition at age 3 to poor cognitive functioning at age 11 to schizotypy at age 23. In addition, findings in a sample of male participants were replicated in a sample of female participants, indicating the robustness of the malnutrition-schizotypy relationship. Furthermore, of the few prior studies of the relationship between nutrition and schizotypy, none appear to have assessed mediating factors. A unique contribution of our study is that it goes beyond establishing a longitudinal link between malnutrition and schizotypy and moves toward an understanding of the mechanism of action. Given that poor nutrition is an alterable risk factor, the future challenge raised by these findings concerns whether nutritional enhancements are capable of improving brain functioning and reducing some features of schizotypal personality disorder.
1. : Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull 2009; 35:568–576Crossref, Medline, Google Scholar
2. : Schizoid personality disorder after prenatal exposure to famine. Am J Psychiatry 1996; 153:1637–1639Link, Google Scholar
3. : The determinants of stunting: can we regard the linear growth performance as a continuum of fetal development. Asia Pacific J Clin Nutr 1996; 5:56–69Google Scholar
4. : Growth trajectory during early life and risk of adult schizophrenia. Br J Psychiatry 2007; 191:512–520Crossref, Medline, Google Scholar
5. : Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry 2001; 58:48–52Crossref, Medline, Google Scholar
6. : Early life origins of schizotypal traits in childhood. Br J Psychiatry 2009; 195:132–137Crossref, Medline, Google Scholar
7. : Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry 2008; 165:579–587Link, Google Scholar
8. : IQ and risk for schizophrenia: a population based cohort study. Psychol Med 1997; 27:1311–1323Crossref, Medline, Google Scholar
9. : Cognitive function and symptoms in adolescents with schizotypal personality disorder. Schizophr Bull 2006; 32:489–497Crossref, Medline, Google Scholar
10. : Inequality in early childhood: risk and protective factors for early child development. Lancet 2011; 378:1325–1338Crossref, Medline, Google Scholar
11. : Child development: risk factors for adverse outcomes in developing countries. Lancet 2007; 369:145–157Crossref, Medline, Google Scholar
12. : Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol 2006; 2:291–336Crossref, Medline, Google Scholar
13. : Cognitive-perceptual, interpersonal, and disorganized features of schizotypal personality. Schizophr Bull 1994; 20:191–201Crossref, Medline, Google Scholar
14. : Cohort profile: the Mauritius Child Health Project. Int J Epidemiol 2010; 39:1441–1451Crossref, Medline, Google Scholar
15.
16. : The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Biomedical and Behavioral Research. Washington, DC, US Department of Health and Human Services, 1979Google Scholar
17. : Malnutrition in man, in Early Malnutrition and Mental Development. Edited by Cravioto JHambraeus LVahlquist B. Uppsala, Almqvist & Wiskell, 1974, pp 13–26Google Scholar
18. : The National Center for Health Statistics reference and the growth of Indian adolescent boys. Am J Clin Nutr 2001; 74:248–253Crossref, Medline, Google Scholar
19. : Spatial but not verbal cognitive defects at age 3 in persistently antisocial individuals. Dev Psychopathol 2002; 14:25–44Crossref, Medline, Google Scholar
20. : Wechsler Intelligence Scale for Children. New York, Psychological Corporation, 1949Google Scholar
21. : Stimulation seeking and intelligence: a prospective longitudinal study. J Pers Soc Psychol 2002; 82:663–674Crossref, Medline, Google Scholar
22. : The SPQ: a scale for the assessment of schizotypal personality disorder based on DSM-III-R criteria. Schizophr Bull 1991; 17:555–564Crossref, Medline, Google Scholar
23. : Three factor model of schizoptypal personality: invariance across culture, gender, religious affiliation, family adversity and psychopathology. Schizophr Bull 2000; 26:603–618Crossref, Medline, Google Scholar
24. : A meditation on mediation: evidence that structural equations models perform better than regressions. J Consum Psychol 2007; 17:139–153Crossref, Google Scholar
25. : Amos 18 User's Guide. Chicago, SPSS, 2009Google Scholar
26. : SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 2004; 36:717–731Crossref, Medline, Google Scholar
27. : Mediation and mechanism. Eur J Epidemiol 2009; 24:217–234Crossref, Medline, Google Scholar
28. : Relationships between season of birth, schizotypy, temperament, character and neurocognition in a non-clinical population. Psychiatry Res 2012; 195:69–75Crossref, Medline, Google Scholar
29. : Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890–897Link, Google Scholar
30. : Prefrontal cortex excitability in early postnatally malnourished rats. Int J Neurosci 1986; 29:119–124Crossref, Medline, Google Scholar
31. : Time scale and extent of neuronal and synaptic loss in the hippocampal formation of malnourished rats. Brain Res 1996; 718:1–12Crossref, Medline, Google Scholar
32. : Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience 2008; 152:859–866Crossref, Medline, Google Scholar
33. : Prenatal protein malnutrition in rats alters the c-Fos response of neurons in the anterior cingulate and medial frontal region to behavioral stress. Nutr Neurosci 2004; 7:281–289Crossref, Medline, Google Scholar
34. : [Measurement of the frontal and prefrontal lobe volumes in children with malnutrition by three dimensional magnetic resonance imaging scan]. No To Hattatsu 2002; 34:398–403Medline, Google Scholar
35. : Mapping IQ and gray matter density in healthy young people. Neuroimage 2004; 23:800–805Crossref, Medline, Google Scholar
36. : Associations between IQ, total and regional brain volumes and demography in a large normative sample of healthy children and adolescents. Dev Neuropsychol 2010; 15:296–317Crossref, Google Scholar
37. : Premorbid performance IQ deficit in schizophrenia. Acta Psychiatr Scand 2000; 102:414–422Crossref, Medline, Google Scholar
38. : Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
39. : Effects of environmental enrichment at ages 3–5 years on schizotypal personality and antisocial behavior at ages 17–23. Am J Psychiatry 2003; 160:1627–1635Link, Google Scholar
40. : Long chain omega-3 fatty acids for indicated prevention of psychotic disorders. Arch Gen Psychiatry 2010; 67:146–154Crossref, Medline, Google Scholar



