ADHD Pharmacotherapy: Rates of Stimulant Use and Cardiovascular Risk
Attention deficit hyperactivity disorder (ADHD) is one of the most common of the pediatric neuropsychiatric disorders. With ADHD diagnoses in 9% of U.S. children and adolescents 6–17 years of age (1) and more than 2.7 million children receiving ADHD pharmacotherapy each year (2), this is a significant public health issue. Clinicians, patients, parents, and the general public are all interested in the prevalence and safety of ADHD medications. Some are concerned with excessive use, while others worry about underutilization of pharmacotherapy when clearly warranted, and all parties want a better understanding of the safety of these agents. Three articles in this issue of the Journal provide important data on ADHD medications; one describes U.S. rates of stimulant prescriptions, and two address pediatric and adult cardiovascular safety.
Zuvekas and Vitiello (3), using a nationally representative database, the Medical Expenditure Panel Survey, analyzed extensive data on stimulant use from 1996 to 2008 in children under age 19. A steady annual increase in the number treated with stimulants was reported, with 2.9% of all U.S. children and adolescents treated with stimulants in 1996, rising to 3.5% in 2008 (Figure 1). Rates remained relatively steady over the study period for children ages 6–12, while the numbers of adolescents (ages 13–18), who as a group had been more likely to be underdiagnosed and less likely to be treated, expanded significantly. In 2008, the rates of stimulant treatment nearly converged for children and adolescents, at 5.1% for children ages 6–12 and 4.9% for adolescents. The most controversial age group, preschoolers (up to age 5), saw a decrease in rates of stimulant treatment, from 0.3% in 1996 to 0.1% in 2008. This drop may have been at least in part a response to the Preschool ADHD Treatment Study (PATS) published in 2006, which demonstrated that while methylphenidate was effective in the treatment of preschoolers with ADHD, its tolerability and efficacy were less than seen in older children.
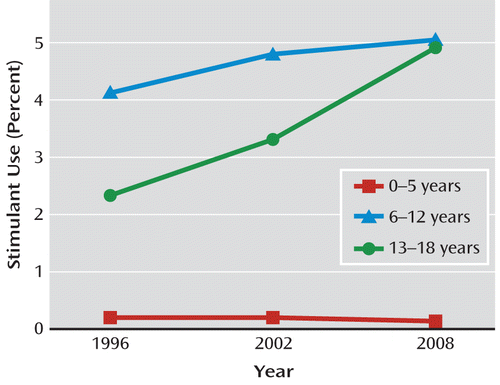
FIGURE 1. Stimulant Use in the U.S. Pediatric Population, by Age Group
In another article in this issue, Schelleman et al. (4) examine the cardiovascular safety of methylphenidate in adults. This is one of three articles assessing ADHD drugs and serious cardiovascular events released in rapid succession in three different medical journals; it joins an article by Cooper et al. published in the New England Journal of Medicine (5) and one by Schelleman et al. in Pediatrics (6). In their article in this issue, Schelleman and colleagues report on their analysis of a database that included 43,999 new methylphenidate users and 175,955 nonusers, all adults. While the authors found no statistically significant association of methylphenidate use with stroke, myocardial infarction, or a composite endpoint of stroke or myocardial infarction, they observed 1.8 times the risk of ventricular arrhythmia or sudden death and 1.7 times the risk of all-cause death among methylphenidate users compared with nonusers. The authors note, however, that the inverse association with dose observed in the study may argue against a causal association. In Pediatrics, Schelleman et al. reported that the rate of cardiovascular events in 241,417 children exposed to ADHD pharmacotherapy was low and in general no higher than in unexposed comparison children (6). The number of events was so low overall, however, that the ability to identify relative increases in rate is limited. The Cooper et al. study reported in the New England Journal of Medicine included 1,200,438 children and young adults 2–24 years of age, with 2,579,104 person-years of follow-up, which included 373,667 person-years of ADHD pharmacotherapy. The authors concluded that there was no increased risk for serious cardiovascular events for current users of ADHD medications.
While the studies described above looked at aggregate data from large databases, the Multimodal Treatment Study of Children With ADHD (MTA) is a clinical trial that provides a much smaller but detailed data set regarding cardiovascular issues. In the article by Vitiello et al. in this issue (7), the authors assess whether stimulant pharmacotherapy in MTA participants over a 10-year period was associated with increased heart rate, increased blood pressure, or blood pressures within the prehypertension or hypertension ranges. The MTA study randomly assigned 579 children 7–9 years of age to 14 months of behavioral therapy, pharmacotherapy (generally methylphenidate), the combination of the two, or usual community treatment, followed by several years of naturalistic treatment. The investigators did not identify a treatment effect on either systolic or diastolic blood pressure, although at the end of 14 months of initial study treatment, the children who received stimulants had higher heart rates (84.2 bpm [SD=12.4] for medication alone and 84.6 bpm [SD=12.2] for medication plus behavioral therapy) than those treated with behavioral therapy alone (79.1 bpm [SD=12.0]). Greater stimulant exposure was correlated with a higher heart rate if therapy was continued, but the authors did not identify a risk for prehypertension or hypertension with stimulant treatment over the 10 years of the study. While useful, these MTA analyses have several limitations, including the exclusion of children with identified cardiovascular disease, a lack of standardization of blood pressure and pulse measurements, attrition over time, and the fact that abnormal blood pressure values were not confirmed over three separate assessments as would be required in order to make a diagnosis of prehypertension or hypertension.
In light of the controversies surrounding the increase in the number of children, adolescents, and adults being diagnosed with ADHD, the broad use of stimulant medications in these patients, and cardiovascular concerns about such medications, these articles address topics of community interest and clinical significance. For many, the usage data will be seen as reassuring, with pharmacotherapy rates well below epidemiological sample rates of ADHD. Others will continue to struggle with acceptance of both the rate of diagnosis of ADHD and the rate of stimulant use. The articles in this issue on cardiovascular safety, coupled with the recent articles in Pediatrics and the New England Journal of Medicine, are generally reassuring and demonstrate movement in the right direction, with systematic retrospective analyses better informing us of issues related to cardiovascular safety with ADHD pharmacotherapy. While gaps persist in the methodical and comprehensive assessments of the safety of ADHD medications, these studies add valuable information to our already large repository of safety and efficacy data of ADHD pharmacotherapies and better inform the risk-benefit analysis of their use.
Perhaps the massive resources currently being devoted to establishing robust electronic health records nationally will result in larger and more accessible databases, containing detailed and discrete data elements that will allow us to improve our identification and understanding of rare but serious adverse effects and better address these questions of public health significance.
1. : Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010; 49:980–989Crossref, Medline, Google Scholar
2.
3. : Stimulant medication use in children: a 12-year perspective. Am J Psychiatry 2012; 169:160–166Link, Google Scholar
4. : Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry 2012; 169:178–185Link, Google Scholar
5. : ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med 2011; 365:1896–1904Crossref, Medline, Google Scholar
6. : Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics 2011; 127:1102–1110Crossref, Medline, Google Scholar
7. : Blood pressure and heart rate over 10 years in the Multimodal Treatment Study of Children With ADHD. Am J Psychiatry 2012; 169:167–177Link, Google Scholar



