High-Field Magnetic Resonance Imaging of Suicidality in Patients With Major Depressive Disorder
Abstract
Objective:
Suicide is a major social and public health problem, but its neurobiology in major depressive disorder is poorly understood. The purpose of this study was to use magnetic resonance diffusion tensor imaging to characterize abnormalities of white matter integrity in major depressive disorder patients with and without a history of suicide attempts.
Method:
Participants were 52 patients with major depressive disorder, with (N=16) and without (N=36) a history of suicide attempts, and 52 healthy comparison subjects matched for age, gender, education, and ethnicity. Diffusion tensor imaging in a 3.0 Tesla magnetic resonance scanner was performed. Whole-brain voxel-based analysis was used to compare fractional anisotropy across the three groups and analyze the correlation with symptom severity. A region-of-interest analysis was applied to the bilateral hippocampus, thalamus, and lentiform nucleus
Results:
Fractional anisotropy was decreased in the left anterior limb of the internal capsule in suicide attempters relative to both nonattempters and healthy comparison subjects, in the right frontal lobe relative to comparison subjects only, and in the right lentiform nucleus relative to nonattempters only. There was no significant correlation with symptom severity.
Conclusions:
Decreased fractional anisotropy in the left anterior limb of the internal capsule appears to characterize patients with major depressive disorder who have a history of attempting suicide. Longitudinal studies are required to validate this as a potential marker that may inform the development of strategies for reducing suicide.
Suicide is a major global social and public health problem. It is commonly associated with depressive disorder, which carries a 2%–12% lifetime risk of dying by suicide (1). Suicide attempts, typically defined as self-destructive acts with some intent to end life (2), are strongly correlated with major depressive disorder (2), and a history of attempts is the strongest predictor of eventual completed suicide (3). Thus, suicide attempts represent an important intervention point, and understanding their neural correlates may inform this intervention. However, the neurobiology of suicide attempts in depression is largely unknown. Despite some inconsistencies among reports, meta-analysis has found suicidal behaviors to be associated with polymorphisms in the gene for tryptophan hydroxylase 1 and the serotonin (5-HT) transporter gene (4). Both genes mediate serotonin function, and suicide attempters also show serotonin-related abnormalities, such as decreased CSF 5-hydroxyindoleacetic acid, decreased serum serotonin, reduced serotonin transporter numbers in the prefrontal cortex, and upregulation of postsynaptic 5-HT1A and 5-HT2A receptors (5). Structural neuroimaging in depressed suicide attempters has shown periventricular white matter hyperintensities (6), while suicide risk in unipolar depression is related to basal ganglia gray matter hyperintensities (7). Thus, there are biological differences between patients who do and do not attempt suicide. However, they are poor predictors of individual suicide risk (6).
Although the studies of suicidal behaviors published so far have focused on gray matter differences, several lines of evidence suggest that white matter may also be affected. First, gray matter differences between suicidal and nonsuicidal depression are likely to be the result of or lead to white matter differences over time (8). Second, abnormalities in white matter (9, 10) and functional integration (11) have been reliably reported in patients with major depressive disorder. It is therefore possible that these abnormalities are more pronounced in suicidal depression relative to nonsuicidal depression. Third, the 5-HT transporter gene, which mediates suicidal behavior, has been shown to affect both gray and white matter (12, 13). Thus, it is plausible that genetic vulnerability to suicidal behavior may result in both gray and white matter differences between suicidal and nonsuicidal depression. Advances in high-field magnetic resonance neuroimaging provide new opportunities to characterize both gray and white matter alterations in suicidal behavior.
Diffusion tensor imaging combines a conventional magnetic resonance imaging (MRI) sequence with additional magnetic field gradients to quantify water diffusion; namely, fractional anisotropy, the degree to which diffusion is directionally hindered, reflects the integrity of the white matter tracts. Diffusion tensor imaging data can be quantified by either voxel-based analysis or region-of-interest methods. Whole-brain voxel-based analysis investigates region-specific changes in gray and white matter by averaging results, spatially normalizing (coregistering) brain images across subjects, and performing statistical tests at each voxel. It is highly reproducible, user-independent (for a specific algorithm), and can explore differences across the entire brain without anatomically specific prior hypotheses (14). In contrast, region-of-interest methods, often more sensitive, are used for testing anatomically specific hypotheses (14) and are usually applied in the original image space. Finally, voxel-based morphometry is a fully automated whole-brain image analysis technique involving voxel-wise comparison of segmented gray and white matter (15).
The aim of the present study was to use diffusion tensor imaging and optimized voxel-based morphometry with a 3.0 Tesla scanner to characterize microstructure abnormalities in individuals with major depressive disorder with and without a history of suicide attempts. We hypothesized that gray and white matter reductions would be specifically associated with suicide attempt in major depressive disorder, which would provide insight into the neurobiology of suicidal behavior and inform the possible early identification of individuals at high risk of suicide.
Method
Subjects
The study was approved by the local research ethics committee, and written informed consent was obtained from all participants. The patients were of Han Chinese ethnicity and recruited from the Department of Psychiatry at the West China Hospital of Sichuan University, Chengdu, China. They were assessed by an experienced psychiatrist and met the diagnostic criteria for major depressive disorder as determined using DSM-IV (16). All patients were medication-free for at least 2 weeks before the study. Severity of depression was quantified using the 17-item Hamilton Depression Rating Scale (HAM-D) (17). On the day of the magnetic resonance examination, all patients were in a depressive state, with a HAM-D total score ≥18. Patients were divided into the following two groups: suicide attempters with a history of at least one suicide attempt, defined as a self-destructive act with some degree of intent to die (2), and nonattempters with no such history. Subjects were not considered suicide attempters if their self-injurious behavior was determined to have no suicidal intention or ideation. Exclusion criteria were any other DSM-IV axis I comorbidities; any current significant medical problems, including a CNS disorder; any history of neurological illness or severe head trauma with loss of consciousness; substance or alcohol abuse/dependence within the past 12 months; and any conditions affecting the ability to participate in assessment, including illiteracy.
Healthy comparison subjects were recruited from the local area by poster advertisement and assessed with the Structured Clinical Interview for DSM-IV, Nonpatient Edition. Exclusion criteria for healthy subjects were a history or present diagnosis of any DSM-IV axis I diagnosis, any neurological illness, history of head trauma with loss of consciousness, and a history of psychiatric disorders or suicide among first-degree relatives.
Data Acquisition
Images were acquired using a 3.0 Tesla General Electric magnetic resonance scanner (EXCITE, General Electric Medical Systems, Milwaukee, Wisc.) employing a single-shot echo planar imaging sequence with an eight-channel phased array head coil. Subjects were fitted with soft ear plugs, positioned comfortably in the coil, and instructed to relax and remain still. Head motion was minimized with foam pads. For each slice, 15 images were collected with high-diffusion weighting along 15 noncolinear and noncoplanar directions (repetition time=10,000 msec, echo time=70.8 msec, slice thickness=3.0 mm, field of view=240×240 mm, voxel dimensions=1×1×3 mm3, scan matrix=128×128, b value=1,000 sec/mm2). High-resolution three-dimensional T1-weighted images were acquired using a spoiled gradient-recalled sequence (repetition time=8.5 msec, echo time=3.4 msec, fractional anisotropy=12°, 156 axial slices with thickness of 1 mm, axial field of view=240×240 mm, data matrix=256×256).
An experienced neuroradiologist (Dr. Lui), blinded to the group allocation, reviewed all scans to exclude any participant with obviously gross abnormalities.
Imaging Processing
Fractional anisotropy maps and three eigenvalues (λ1, λ2, λ3) were generated from each individual using DTIstudio software (Johns Hopkins Medical Institute, Laboratory of Brain Anatomical MRI, Baltimore [http://cmrm.med.jhmi.edu/]). Image preprocessing and statistical analysis were carried out using SPM2 software (Wellcome Trust Centre for Neuroimaging, London [http://www.fil.ion.ucl.ac.uk/spm/software/]). Each subject's echo planar image was spatially normalized to the Montreal Neurological Institute echo planar image template using parameters determined from the normalization of the image with a b value of 0 sec/mm2 and the echo planar image template in SPM2. Images were resampled with a final voxel size of 2×2×2 mm3. Normalized maps were spatially smoothed using an isotropic Gaussian filter (6-mm full-width half-maximum).
Voxel-Based Analysis
Voxel-based analysis was performed using SPM2 software. Fractional anisotropy maps were compared among the three groups using analysis of covariance (ANCOVA), with post hoc t tests for age, gender, and disease duration as covariates of no interest. The comparison between all depressed patients and healthy comparison subjects was performed using two-sample t tests. Statistical inferences were made with a voxel-level threshold of p<0.05, after family-wise error correction for multiple comparisons, and an extent threshold of p<0.05 (uncorrected), with a minimum cluster size of 50 voxels. For clusters identified in the ANCOVA, we performed follow-up between-group voxel-wise t tests to characterize group differences using the same thresholds. Montreal Neurological Institute coordinates were transformed to Talairach coordinates using MNI2tal (http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/). Statistical maps were overlaid on a canonical brain in the standard Montreal Neurological Institute atlas using MRIcro software (www.sph.sc.edu/comd/rorden/mricro.html). Results are presented using the voxel of peak significance. To quantify changes in affected regions, fractional anisotropy values were extracted using SPM2. Correlation between the microstructure abnormalities and HAM-D scores was examined using standard statistical software (SPSS, Release, 11.5, SPSS Inc., Chicago). A p value <0.05 after correction for multiple comparisons was deemed significant.
Axial and Radial Diffusivity Analysis
In five regions of decreased fractional anisotropy, voxel-wise comparisons were made between the key measures of diffusivity (axial diffusivity: λ‖=λ1, and radial diffusivity: λ⊥=[λ2+λ3]/2) using marsbar-0.38.2, an automated tool (http://marsbar.sourceforge.net) (18). Results were analyzed using ANCOVA, with age, gender, and disease duration as covariates. A p value <0.05 after correction for multiple comparisons was deemed significant.
Region-of-Interest Analysis
Region-of-interest analysis was carried out using marsbar-0.38.2. The following subcortical areas were selected: bilateral lentiform nucleus, bilateral hippocampus, and bilateral thalamus. These are part of the limbic-striatal-pallidal-thalamic circuit implicated in mood regulation and have been shown to be anatomically and functionally altered in previous neuroimaging studies of depressed patients (19, 20). For accurate localization, the normalized diffusion-weighted imaging map was used as a reference to manually outline these areas. Regions-of-interest were measured using a semiautomated segmentation method in marsbar-0.38.2, which involved manually drawing around the target, followed by application of a pixel-based threshold to shrink the boundaries. Interobserver variability was assessed by two raters, and intraclass correlation coefficients were good (range: 0.82–0.91). Results were analyzed using a design model of ANCOVA, with age, gender, and disease duration as covariates of no interest. Post hoc comparisons were used to determine significant differences among the three groups. A p value <0.05 after correction for multiple comparisons was deemed significant.
Voxel-Based Morphometry
To identify possible morphological differences among the three groups, T1-weighted images were analyzed using optimized voxel-based morphometry implemented in SPM2 (21). ANCOVA was performed, with age, gender, and disease duration as covariates of no interest. The same statistical threshold used in the voxel-based analysis was applied.
RESULTS
Demographic and Clinical Comparisons
Table 1 summarizes and compares demographic and clinical characteristics of the subjects. Suicide attempters did not differ from nonattempters in sex, age, education, and HAM-D scores, although disease duration was greater among the suicide attempters (p<0.05). There were no significant differences between the patient groups and healthy comparison subjects in age, gender, and education.
| Characteristic | Major Depressive Disorder Patients | Healthy Comparison Subjects (N=52) | ||||
|---|---|---|---|---|---|---|
| Nonattempters (N=36) | Suicide Attempters (N=16) | |||||
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 34.7 | 12.5 | 34.2 | 13.7 | 37.1 | 16.0 |
| Education (years completed) | 12.8 | 2.5 | 13.4 | 2.8 | 13.1 | 2.3 |
| Disease duration (months) | 21 | 25 | 80 | 90 | ||
| HAM-D score | 22.3 | 4.3 | 24.6 | 3.8 | ||
| N | % | N | % | N | % | |
| Female | 16 | 44 | 11 | 69 | 28 | 54 |
TABLE 1. Demographic and Clinical Characteristics of Major Depressive Disorder Patients and Healthy Comparison Subjectsa
Voxel-Based Analysis
Three-group comparisons
The three groups differed in white matter fractional anisotropy in the left subgyral white matter of the parietal lobe, right cerebellum (culmen), left anterior limb of the internal capsule, and right lentiform nucleus and in the right subgyral white matter of the frontal lobe (Figure 1, Table 2).
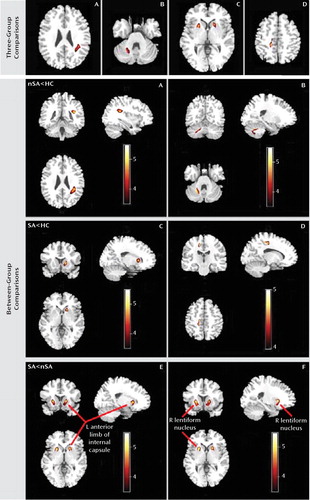
FIGURE 1. White Matter Fractional Anisotropy Differences in Voxel-Based Analysis Comparisons Among Major Depressive Disorder Patients With and Without a History of Suicide Attempts and Healthy Comparison Subjectsa
a Images are presented in radiological orientation. The three groups differed in regions of fractional anisotropy in A) the left subgyral white matter of the parietal lobe, B) the right cerebellum (culmen), C) the left anterior limb of the internal capsule and the right putamen of the lentiform nucleus, and D) the right subgyral white matter of the frontal lobe. In nonattempters relative to healthy comparison subjects, fractional anisotropy was reduced in the left subgyral white matter of the parietal lobe and the right culmen of the cerebellum. In suicide attempters relative to healthy comparison subjects, fractional anisotropy was reduced in the left anterior limb of the internal capsule and the right subgyral white matter of the frontal lobe. In suicide attempters relative to nonattempters, fractional anisotropy was reduced in the E) left anterior limb of the internal capsule and the F) right putamen of the lentiform nucleus. Statistical inferences were made with a voxel-level statistical threshold (p<0.05) after family-wise error correction for multiple comparisons and an extent threshold (p<0.05) (uncorrected) with a minimum cluster size of 50 voxels. Abbreviations: nSA=depressed patients without a history of suicide attempts; SA=depressed patients with a history of suicide attempts; HC=healthy comparison subjects; L=left; R=right.
| Anatomical Region | Talairach Coordinate (x, y, z)a | Cluster Size (Voxels) | Fb | Comparisonc | ||
|---|---|---|---|---|---|---|
| Nonattempters < Healthy Comparison Subjects | Suicide Attempters < Healthy Comparison Subjects | Suicide Attempters < Nonattempters | ||||
| Left parietal lobe (subgyral) | −38, −38, 24 | 134 | 14.51 | 4.80 | ||
| Right cerebellar anterior lobe (culmen) | 24, −56, −23 | 113 | 14.38 | 4.47 | ||
| Left anterior limb of internal capsule | −18, 16, 1 | 95 | 14.77 | 4.61 | 4.87 | |
| Right lentiform nucleus (putamen) | 22, 12, 1 | 115 | 14.43 | 4.98 | ||
| Right frontal lobe (subgyral) | 14, −19, 47 | 85 | 17.22 | 4.60 | ||
TABLE 2. Differences in Fractional Anisotropy Among Depressed Patients With and Without a History of Suicide Attempts and Healthy Comparison Subjects
Between-group comparisons
Suicide attempters showed reduced fractional anisotropy in white matter of the left anterior limb of the internal capsule relative to nonattempters and comparison subjects (p=0.005 and p=0.02, respectively) (Table 2, Figure 1), and this region revealed decreased axial diffusivity (p=0.02) but unchanged radial diffusivity. No significant difference was found in this region between nonattempters and comparison subjects. Suicide attempters also showed reduced fractional anisotropy in subgyral white matter of the right frontal lobe relative to comparison subjects (p=0.03) and of the right lentiform nucleus relative to nonattempters (p=0.008). Right subgyral white matter of the frontal lobe and right lentiform nucleus revealed increased radial diffusivity (p=0.006 and p=0.02, respectively) but unchanged axial diffusivity. Furthermore, nonattempters showed reduced fractional anisotropy in subgyral white matter of the left parietal lobe and right putamen of the cerebellum relative to comparison subjects (p=0.01 and p=0.01, respectively). No other difference was found between groups in this region. Left subgyral white matter of the parietal lobe revealed increased radial diffusivity (p=0.01) but unchanged axial diffusivity, and the right cerebellum revealed decreased axial diffusivity (p=0.01) but unchanged radial diffusivity. Figure 2 quantifies the change of fractional anisotropy value in these affected regions. No association was found with HAM-D scores.
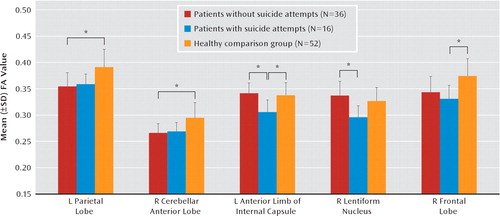
FIGURE 2. Fractional Anisotropy in Major Depressive Disorder Patients With and Without a History of Suicide Attempts and Healthy Comparison Subjectsa
a Brain regions differed significantly among the three groups in the left parietal lobe and right cerebellum anterior lobe between nonattempters and healthy comparison subjects, in the left anterior limb of the internal capsule and right lentiform nucleus between suicide attempters and nonattempters, and in the left anterior limb of the internal capsule and right frontal lobe (subgyral) between suicide attempters and healthy comparison subjects. Abbreviations: FA=fractional anisotropy; L=left; R=right.*p<0.05.
Depressed patients and healthy comparison subjects
Depressed patients showed reduced fractional anisotropy in subgyral white matter of the bilateral parietal lobe, paracentral lobule of the right frontal lobe, and anterior lobe of the right cerebellum (Table 3, Figure 3).
| Extent of Cluster | Cluster Size (Voxels) | Montreal Neurological Institute Coordinate Voxel (x, y, z)b | Analysis | |
|---|---|---|---|---|
| t | z | |||
| Left parietal lobe (subgyral) | 245 | −38, −38, 24 | 5.37 | 5.02 |
| Right parietal lobe (subgyral) | 157 | 38, −36, 24 | 4.78 | 4.53 |
| Right frontal lobe (paracentral lobule) | 137 | 18, −59, −21 | 5.88 | 5.44 |
| Right cerebellum (anterior lobe) | 367 | 24, −56, −23 | 5.37 | 5.02 |
TABLE 3. Reduced Fractional Anisotropy Regions in Patients With Major Depression Relative to Healthy Comparison Subjectsa
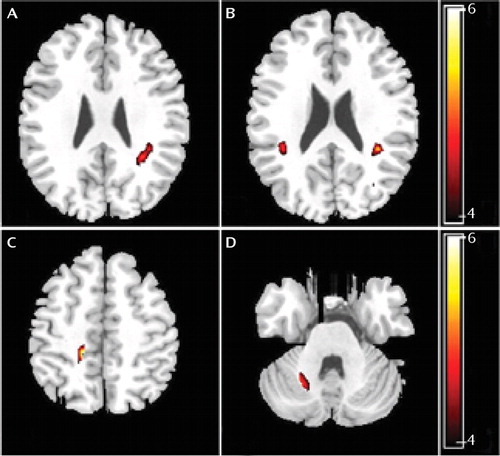
FIGURE 3. White Matter Fractional Anisotropy Differences Between Major Depressive Disorder Patients and Healthy Comparison Subjectsa
a Images are presented in radiological orientation. There were significant differences between the two patient groups (depressed subjects with and without a history of suicide attempts) and healthy comparison subjects in the A) left subgyral white matter of the parietal lobe, B) right subgyral white matter of the parietal lobe, C) right paracentral lobule of the frontal lobe, and D) right anterior lobe of the cerebellum. Statistical inferences were made with a voxel-level statistical threshold (p<0.05) after family-wise error correction for multiple comparisons and an extent threshold (p<0.05) (uncorrected) with a minimum cluster size of 50 voxels.
Region-of-Interest
ANCOVA revealed a significant difference in the fractional anisotropy of the right lentiform nucleus across groups (F=3.71, df=2, 101, p=0.03), which post hoc between-group t tests showed to be driven by differences between suicide attempters and nonattempters (p=0.003) but not between either of the patient groups and comparison subjects (Figure 4). No statistically significant differences in fractional anisotropy were found in the other regions of interest, including the left lentiform nucleus, bilateral hippocampus, and bilateral thalamus.
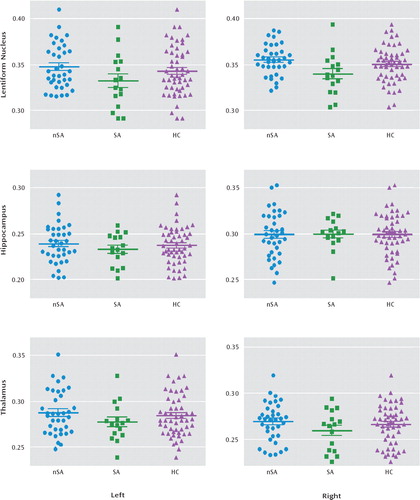
FIGURE 4. Scatter Plots of Fractional Anisotropy by Region-of-Interest Among Major Depressive Disorder Patients With and Without a History of Suicide Attempts and Healthy Comparison Subjectsa
a Significant difference was observed in the right lentiform nucleus among patients with major depressive disorder with (SA) and without (nSA) a history of suicide attempts and healthy comparison (HC) subjects (p=0.03), revealed by analysis of covariance. Post hoc t tests showed a significant difference between depressed patients with and without a history of suicide attempts (p=0.003) but not between either patient group and healthy comparison subjects. No significant differences between groups in fractional anisotropy were found in the left lentiform nucleus, bilateral hippocampus, and bilateral thalamus. The bold horizontal line represents the mean; error bars represent one standard deviation.
Voxel-Based Morphometry
Voxel-based morphometric analysis revealed no statistically significant volumetric differences among the three groups in gray or white matter.
Discussion
We have previously demonstrated differences in brain microstructure and perfusion between the subclasses of refractory depressive disorder and nonrefractory depressive disorder (22, 23). In the present study, we have extended this by using the magnetic resonance technique of diffusion tensor imaging to compare patients with major depressive disorder (but no other axis I psychiatric comorbidities) with and without a history of suicide attempts and healthy comparison subjects.
The main findings are that suicide attempters had lower fractional anisotropy in the left anterior limb of the internal capsule relative to both nonsuicidal patients and healthy comparison subjects. In addition, suicide attempters had lower fractional anisotropy in the white matter of the right frontal lobe relative to healthy comparison subjects and in the right lentiform nucleus relative to nonattempters. Furthermore, nonattempters had reduced fractional anisotropy in the white matter of the left parietal lobe and right cerebellum relative to healthy comparison subjects.
Optimized voxel-based morphometric analysis detected no morphological changes in gray or white matter comparison among the three groups. This appears to be inconsistent with voxel-based analysis of fractional anisotropy, for which there are several possible reasons. Voxel-based morphometry allows unbiased and objective comparison of local gray matter concentration between groups (24, 25). However, it is susceptible to normalization and segmentation confounds that may affect sensitivity (15). For example, nonlinear distortions in MRI may compromise registration accuracy, affecting the group comparison (15). Furthermore, group-specific patterns of abnormal anatomy may result in group-specific misregistration (24, 25). Finally, one experimental group may move more in the scanner than the other, resulting in group-specific motion artifact, which may interact with the segmentation to produce systematic classification differences (24, 25). Alternatively, diffusion tensor imaging may be more sensitive to subtle white matter abnormalities without volumetric changes detectable by structural MRI, for example, in neurological disorders (26).
Fractional anisotropy, the fraction of directional diffusion in white matter, is influenced by fiber organization, myelination, and integrity. We found that suicide attempters had significantly lower fractional anisotropy than healthy comparison subjects in white matter of the left anterior limb of the internal capsule, while nonattempters did not. Microstructure abnormalities in this region are likely to involve demyelination or axonal damage and might be expected to trigger malfunctions in other areas of the emotional regulatory circuit (27). Axonal damage leads to a marked decrease in axial diffusivity and only modest decreases in radial diffusivity. In contrast, demyelination (e.g., in multiple sclerosis) leads to an increase in radial diffusivity without change in axial diffusivity (28). The reduced fractional anisotropy in the left anterior limb of the internal capsule was associated with decreased axial diffusivity but unchanged radial diffusivity, suggesting that it was not a result of demyelination but of gross reduction in axonal number and/or size.
In addition, both voxel-based and region-of-interest analysis revealed that suicide attempters had reduced fractional anisotropy in the right lentiform nucleus relative to nonattempters, although neither patient group differed significantly from healthy comparison subjects in this area. This is consistent with a previous report that showed major depressive disorder patients with a history of suicide attempts had significantly more subcortical gray matter hyperintensities than nonsuicidal patients (7). The lentiform nucleus, which is the part of the corpus striatum lateral to the internal capsule, has been reported to be associated with experiencing sad events or unpleasant stimuli (29). This area is dense with dopamine innervation and has a moderate amount of serotonin innervation (30). Dopamine and serotonin are thought to be connected with suicidal behavior in depressed patients (31). It is therefore possible that altered white matter integrity in the right lentiform nucleus is related to abnormalities in dopamine and serotonin function, although the causal direction of such association is yet to be established. The lentiform nucleus also belongs to the limbic-striatal-pallidal-thalamic circuit, which plays an important role in the regulation of affect and cognition (19). Different parts of the lentiform nucleus have distinct afferent and efferent connections with other regions of this network. Thus, it is possible that a localized abnormality of the lentiform nucleus may affect other regions of the network, contributing to the pathogenesis in those major depressive disorder patients who intend to commit suicide.
Further, voxel-based analysis also revealed fractional anisotropy in the subgyral white matter of the right frontal lobe to be significantly reduced in suicide attempters relative to healthy comparison subjects, although not in nonattempters. Reduced fractional anisotropy in the frontal lobe was associated with increased radial diffusivity and unchanged axial diffusivity, which suggests reduced axonal myelination and/or alterations in the axonal cyto-skeleton, increasing water diffusion perpendicular to the primary axonal axis. An earlier diffusion tensor imaging study reported an association between impulsivity and the reduction of microstructural integrity of frontal white matter systems in other psychiatric diseases characterized by disturbed serotonin function (32). The study also suggested that decreased serotonin activity in the frontal lobes may contribute to the pathogenesis of major depressive disorder patients who attempt suicide (33).
We also found that relative to healthy comparison subjects, nonattempters had lower fractional anisotropy in the left subgyral parietal lobe and right cerebellum. Although there was no significant difference between suicide attempters and comparison subjects in these regions, this decrease in fractional anisotropy is perhaps related to major depressive disorder per se. The abnormality in white matter of the left parietal lobe is consistent with a previous report of structural abnormalities in treatment-naive young adults with major depressive disorder (34).
The cerebellum is anatomically and functionally connected to the prefrontal cortex, subcortical limbic structures, and monoamine producing brainstem nuclei (35). Disruption of the functional connections between the cerebellum and frontal lobes impairs expression of emotion (36). The cerebellum is also implicated in dysregulation of affect (36). A recent study using repetitive transcranial magnetic stimulation showed that inhibition of cerebellar function leads to impaired regulation of emotion and increased negative mood (37). It is possible that the reduction in fractional anisotropy in the cerebellum is related to the reductions observed in the cerebellar levels of glial fibrillary acidic protein in individuals with major depressive disorder (38).
Neither voxel-based nor region-of-interest analysis yielded any evidence of reduced fractional anisotropy in the bilateral hippocampus and thalamus in either patient group, which was unexpected, since these parts of the limbic system are thought to play a crucial role in the early pathogenesis of major depressive disorder (39). Nevertheless, this finding is consistent with a previous study that reported no significant differences in the volume of the hippocampus and thalamus in suicidal and nonsuicidal female patients with major depressive disorder (40).
Our study has some limitations. First, the subgroup of suicide attempters was relatively small, which may have limited statistical power. However, we maximized sensitivity in crucial areas by performing a region-of-interest analysis in conjunction with the whole-brain voxel-based analysis. Second, there was no psychiatric comparison group (e.g., schizophrenia patients with a history of suicide attempts). Therefore, it cannot be concluded from our study that reduced fractional anisotropy in the left anterior limb of the internal capsule, right frontal lobe, and right lentiform nucleus is specific to suicidality in major depressive disorder. Indeed, a recent study of structural correlates of past suicidality in schizophrenia implicates the fronto-temporo-limbic circuits (41). To clarify this, future studies should include subjects with different psychiatric disorders with and without a history of suicide attempts. Third, specific modes, such as violent versus nonviolent suicide behaviors, are believed to be associated with particular phenotypes (42). It may be of interest to examine their changes in fractional anisotropy, for which a large cohort study would be required. Finally the present results cannot establish the direction of causality (i.e., whether reduced fractional anisotropy causes a predisposition to suicide attempts or vice versa or whether both are the result of a separate cause). To clarify this, a longitudinal approach would be required to examine patients before and after a suicide attempt.
The frontal-striatal-thalamic-cortical circuits, connected by the anterior limb of the internal capsule, play an important role in behavioral regulation (43), and the anterior limb of the internal capsule is the most frequent therapeutic target in obsessive-compulsive disorder and depression (44, 45). The decreased fractional anisotropy we observed in the frontal lobe, anterior limb of the internal capsule, and lentiform nucleus in suicide attempters suggests a possible abnormality of neuroanatomic pathways in frontal-striatal (lentiform nucleus) circuits passing via the anterior limb of the internal capsule. Specific lesions or disrupted connections involving areas regulating affect and behaviors might trigger the onset of depression and confer a biological vulnerability, which in combination with environmental stressors results in suicidal behaviors.
In summary, the present study suggests that fractional anisotropy reductions in the left anterior limb of the internal capsule characterize those patients with major depressive disorder who have made suicide attempts. In order to identify those at greatest risk and eventually develop more effective preventive strategies, future longitudinal studies should use neuroimaging techniques in conjunction with genetic approaches for a multidimensional characterization of predisposition to suicidality in major depressive disorder.
1. : Affective disorders and suicide risk: a reexamination. Am J Psychiatry 2000; 157:1925–1932Link, Google Scholar
2. : Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry 2004; 161:1433–1441Link, Google Scholar
3. : Attempted suicide in elderly Chinese persons: a multi-group, controlled study. Am J Geriatr Psychiatry 2005; 13:562–571Crossref, Medline, Google Scholar
4. : Genetics of suicide. Mol Psychiatry 2006; 11:336–351Crossref, Medline, Google Scholar
5. : Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J Psychiatry Neurosci 2000; 25:371–377Medline, Google Scholar
6. : Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1501–1507Crossref, Medline, Google Scholar
7. : MRI correlates of suicide attempt history in unipolar depression. Biol Psychiatry 2001; 50:266–270Crossref, Medline, Google Scholar
8. : Neurobiology of suicidal behaviour. Nat Rev Neurosci 2003; 4:819–828Crossref, Medline, Google Scholar
9. : Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord 2010; 120:240–244Crossref, Medline, Google Scholar
10. : Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry 2009; 66:245–252Crossref, Medline, Google Scholar
11. : Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62:429–437Crossref, Medline, Google Scholar
12. : Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J Neurosci 2009; 29:6229–6233Crossref, Medline, Google Scholar
13. : Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord 2009; 119:132–141Crossref, Medline, Google Scholar
14. : A study of diffusion tensor imaging by tissue-specific, smoothing-compensated voxel-based analysis. Neuroimage 2009; 44:870–883Crossref, Medline, Google Scholar
15. : What's new in neuroimaging methods? Ann N Y Acad Sci 2009; 1156:260–293Crossref, Medline, Google Scholar
16. : Structured Clinical Interview for DSM-IV Axis I Disorders-Clinical Version (SCID-CV). Washington, DC, American Psychiatric Publishing, 1997Google Scholar
17. : Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
18. : Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res 2008; 101:106–110Crossref, Medline, Google Scholar
19. : Effects of electroconvulsive therapy on frontal white matter in late-life depression: a diffusion tensor imaging study. Neuropsychobiology 2004; 50:48–53Crossref, Medline, Google Scholar
20. : Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry 2008; 165:238–244Link, Google Scholar
21. : A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001; 14:21–36Crossref, Medline, Google Scholar
22. : Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology 2009; 251:476–484Crossref, Medline, Google Scholar
23. : Magnetization transfer imaging reveals the brain deficit in patients with treatment-refractory depression. J Affect Disord 2009; 117:157–161Crossref, Medline, Google Scholar
24. : Voxel-based morphometry of the human brain: methods and applications. Curr Med Imag Rev 2005; 1:105–113Crossref, Google Scholar
25. : Voxel-based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. Neuroimage 2005; 25:1175–1186Crossref, Medline, Google Scholar
26. : Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 2004; 46:339–350Crossref, Medline, Google Scholar
27. : Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 2007; 57:688–695Crossref, Medline, Google Scholar
28. : Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005; 26:132–140Crossref, Medline, Google Scholar
29. : A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 2005; 57:201–209Crossref, Medline, Google Scholar
30. : The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 2000; 405:365–373Crossref, Medline, Google Scholar
31. : The role of dopa-mine and serotonin in suicidal behaviour and aggression. Prog Brain Res 2008; 172:307–315Crossref, Medline, Google Scholar
32. : Frontal white matter integrity in borderline personality disorder with self-injurious behavior. J Neuropsychiatry Clin Neurosci 2007; 19:383–390Crossref, Medline, Google Scholar
33. : Epilepsy, suicidal behaviour, and depression: Do they share common pathogenic mechanisms? Lancet Neurol 2006; 5:107–108Crossref, Medline, Google Scholar
34. : White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry 2007; 164:823–826Link, Google Scholar
35. : The role of the cerebellum in affect and psychosis. J Neurolinguist 2000; 13:189–214Crossref, Google Scholar
36. : The neuropsychiatry of the cerebellum: insights from the clinic. Cerebellum 2007; 6:254–267Crossref, Medline, Google Scholar
37. : The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. Cerebellum 2009; 8:28–34Crossref, Medline, Google Scholar
1. : Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr Res 2004; 69:317–323Crossref, Medline, Google Scholar
39. : Life events and hippocampal volume in first-episode major depression. J Affect Disord 2008; 110:241–247Crossref, Medline, Google Scholar
40. : Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry 2007; 12:360–366Crossref, Medline, Google Scholar
41. : Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1673–1676Crossref, Medline, Google Scholar
42. : Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol Psychiatry 2007; 62:72–80Crossref, Medline, Google Scholar
43. : Association between symptom severity and internal capsule volume in obsessive-compulsive disorder. Neurosci Lett 2009; 452:68–71Crossref, Medline, Google Scholar
44. : Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: w.orldwide experience. Mol Psychiatry 2010; 15:64–79Crossref, Medline, Google Scholar
45. : A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 2009; 65:276–282Crossref, Medline, Google Scholar



