Relationship Between Cingulo-Insular Functional Connectivity and Autistic Traits in Neurotypical Adults
Abstract
Objective: The Social Responsiveness Scale—Adult Version (SRS-A) measures autistic traits that are continuously distributed in the general population. Based on increased recognition of the dimensional nature of autistic traits, the authors examined the neural correlates of these traits in neurotypical individuals using the SRS-A and established a novel approach to assessing the neural basis of autistic characteristics, attempting to directly relate SRS-A scores to patterns of functional connectivity observed in the pregenual anterior cingulate cortex, a region commonly implicated in social cognition. Method: Resting state functional magnetic resonance imaging scans were collected for 25 neurotypical adults. All participants provided SRS-A ratings completed by an informant who had observed them in natural social settings. Whole brain-corrected connectivity analyses were then conducted using SRS-A scores as a covariate of interest. Results: Across participants, a significant negative relationship between SRS-A scores and the functional connectivity of the pregenual anterior cingulate cortex with the anterior portion of the mid-insula was found. Specifically, low levels of autistic traits were observed when a substantial portion of the anterior mid-insula showed positive connectivity with the pregenual anterior cingulate cortex. In contrast, elevated levels of autistic traits were associated with negative connectivity between these two regions. Conclusions: Resting state functional connectivity of the pregenual anterior cingulate cortex-insula social network was related to autistic traits in neurotypical adults. Application of this approach in samples with autism spectrum disorders is needed to confirm whether this circuit is dimensionally related to the severity of autistic traits in clinical populations.
Appreciation of the likely dimensional nature of autistic symptoms (1 – 3) has led to the development of measures to evaluate autism-related social traits in the general population (2 – 4) . The Social Responsiveness Scale (SRS) quantifies social, communicative, and other characteristic impairments of autism spectrum disorders. In the present article, the term autism refers to autistic disorder, Asperger’s syndrome, and pervasive developmental disorders not otherwise specified (3) . The SRS, which has been extensively validated in both child and adult populations (3 , 5 , 6) , provides a single measure of autistic traits that, although heavily weighted on social-communicative impairment, represent a “singular, continuously distributed underlying factor” in the population (7) . As such, the SRS may allow investigators to study the neurobiology of autistic traits in both clinical and nonclinical samples.
In the present study, we developed a protocol for examining the neural correlates of autistic traits by measuring the relation between informant-provided SRS scores and resting state functional connectivity measures in neurotypical individuals. Resting state functional magnetic resonance imaging (fMRI) has emerged as a powerful means of mapping and characterizing functional connectivity without the constraints of task-based approaches (8 – 10) . Imaging and electrophysiological studies (11 , 12) examining intrinsic brain activity at rest in individuals with autism support a model of compromised long-range cortico-cortical connectivity (13) . Recently, two resting state fMRI studies (14 , 15) that compared high functioning adults with autism with neurotypical comparison subjects revealed reduced intrinsic connectivity of long-range circuits based in pregenual portions of the anterior cingulate cortex.
Involvement of the pregenual anterior cingulate cortex in autism is consistent with this region’s role in an individual’s capacity to reason about the thoughts and beliefs of others—also referred to as “theory of mind” (16 , 17) —which is qualitatively impaired in individuals with autism. Furthermore, hypofunction of this region in autism was revealed by a recent voxel-wise meta-analysis of functional imaging studies of social processing (18) . This accumulating evidence suggests that disruption of the pregenual anterior cingulate cortex-based network may underlie autistic traits. Other likely nodes within this network are the posterior cingulate cortex, which is associated with self-referential processing and mentalizing (19 , 20) , and the anterior insula, which is linked to representing and/or monitoring the salience of one’s own emotions and the emotions of others (21 – 23) . Hypoactivation of both these regions has also been found in individuals with autism relative to neurotypical comparison subjects (18) .
In the present study, we hypothesized that interindividual differences in autistic traits would be related to differences in the functional connectivity of the pregenual anterior cingulate cortex, even in a nonclinical population. Specifically, we predicted that higher SRS scores, indexing greater autistic traits, would be inversely related to the strength of functional connectivity of the pregenual anterior cingulate cortex with the 1) posterior cingulate cortex and 2) anterior insula.
Method
Design
We contacted 46 right-handed, native English speaking individuals (mean age: 28.5 [SD=6.9] years; 23 male subjects) who had participated in previously published studies (24 – 28) . We requested that each subject select someone who knew him or her well in natural social settings to complete the adult version of the SRS questionnaire (SRS-A) (5) . All participants had no past or current psychiatric or neurological illness based on an unstructured psychiatric assessment.
Of the 25 participants who provided questionnaires (mean age: 26.4 [SD=5.6] years; nine male subjects), 20 had completed three resting state scans as part of a test-retest study (28) . The first of these three scans was collected 5 to 16 months prior to the other two, which were performed 45 minutes apart in the same session. Four participants had two resting state scans (45 minutes apart), and a single subject was scanned only once. All available scans were used to derive a best single estimate of functional connectivity for each participant. The study was approved by the institutional review boards of New York University School of Medicine and New York University. Signed informed consent was obtained prior to participation, which was compensated.
Assessment
The SRS-A is a 65-item questionnaire completed by an informant who knows the proband in naturalistic social settings. Most SRS-A items (82%) emphasize social-communicative skills, but the SRS-A also queries stereotypical restricted behaviors/interests often observed in autism. Together, these items form a single measure of autistic traits rather than distinct scores associated with specific symptom domains (5 – 7) . Total SRS-A raw scores range from 0, corresponding to high social competence, to 195, corresponding to significant social impairment observed in individuals with severe autism. Scores between 60 and 80 are associated with mild forms of autism (5) .
fMRI Data Acquisition
Data were collected on a Siemens Allegra 3.0 Tesla scanner equipped for echo-planar imaging. A resting state fMRI scan entailed 197 continuous functional volumes (TR=2000 msec, echo time=25 msec, flip angle=90°, 39 slices, matrix=64×64, field of view=192 mm, voxels=3×3×3 mm, duration=6.5 minutes). During functional scans, participants were instructed to rest with their eyes open while the word “relax” was centrally projected onto a screen. For spatial normalization and localization, a high-resolution T1-weighted anatomical image was also acquired using a magnetization prepared gradient-echo sequence (TR=2500 msec, echo time=4.35 msec, TI=900 msec, flip angle=8°, 176 slices, field of view=256 mm).
Image Preprocessing
As detailed in prior studies (24 , 25) , data were processed using both Analysis of Functional NeuroImages (version AFNI_2008_07_18_1710 [http://afni.nimh.nih.gov/afni]) and FMRIB Software Library (version 3.3 [www.fmrib.ox.ac.uk]). Preprocessing using Analysis of Functional NeuroImages consisted of 1) slice time correction for interleaved acquisitions using Fourier interpolation, 2) three-dimensional volume registration using least squares alignment of three translational and three rotational parameters for motion correction, and 3) despiking of extreme time series outliers using a continuous transformation function. Preprocessing using FMRIB Software Library consisted of 1) mean-based intensity normalization of all volumes by the same factor, 2) spatial smoothing (Gaussian kernel of full width at half maximum=6 mm), 3) temporal high-pass filtering (Gaussian-weighted least squares straight line fitting with sigma=100.0 seconds), 4) temporal low-pass filtering (Gaussian filter with half width at half maximum=2.8 seconds), and 5) correction for time series autocorrelation (prewhitening).
Seed Selection
We used a spherical seed (diameter=10 mm) centered at the same pregenual anterior cingulate cortex location revealed to be hypoactive in autism by a recent meta-analysis (18) . The Talairach coordinates of this pregenual anterior cingulate cortex seed generated from the meta-analysis were transformed into Montreal Neurological Institute space (29) , centered at x=0, y=47, z=9. To obtain the seed time series for each subject and each available scan, we 1) transformed each subject’s time series into Montreal Neurological Institute space using a 12 degrees-of-freedom linear affine transformation implemented in FMRIB Software Library (voxels=2×2×2 mm) and 2) calculated the mean time series by averaging across all voxels within the seed. Further, as previously described (24 , 25) , we extracted the time series of the following nine nuisance signals: global signal, white matter, CSF, and six motion parameters.
Participant-Level Analyses
For each participant and each scan, we performed a multiple regression analysis (using FMRIB Software Library General Linear Model) that included the seed time series of the pregenual anterior cingulate cortex and the nine nuisance covariates as predictors. To ensure that the time series represented regionally specific neural activity, the mean seed time series was orthogonalized with respect to the nine nuisance signals using FMRIB Software Library Improved Linear Model. This analysis produced individual subject-level maps of all positively and negatively predicted voxels for the predictor (i.e., the pregenual anterior cingulate cortex time series). The second level included a fixed-effects model for each participant, with multiple resting state scans (i.e., three scans for 20 participants and two scans for four participants). This step served to combine all functional connectivity maps available for a given participant into one, using an equal weighting.
Group-Level Analyses
Group-level mixed-effects analyses were carried out using FMRIB Software Library Local Analysis of Mixed Effects. In addition to the group mean vector, demeaned informant-based SRS-A scores and three nuisance variables (sex, age, and the number of resting state scans per participant) were included in the model. Corrections for multiple comparisons were carried out at the cluster level. Thus, cluster size was determined using Gaussian random field theory (voxel-wise: minimum z score >2.3; cluster significance: p<0.05, corrected). This group-level analysis produced the following two types of thresholded z score maps: 1) maps of the significant positive and negative functional connectivity of the pregenual anterior cingulate cortex across the entire sample and 2) maps of positive and negative pregenual anterior cingulate cortex functional connectivity in relation to SRS-A informant results (i.e., regions in which connectivity with the seed was predicted by SRS-A scores). Finally, to examine the reliability of our connectivity measures over time, we computed short- and long-term voxel-wise intraclass correlation of the pregenual cingulate seed connectivity, with data from the 20 participants who provided three scans over two sessions (5 to 16 months intersession; 45 minutes apart intrasession), as described in Shehzad et al. (28) .
Results
The informants who provided SRS-A scores consisted of nine partners/spouses (36%), 14 close friends (56%), and two relatives (8%). As expected in a sample of unaffected screened volunteers, total SRS-A scores ranged between 5 and 59, which is below the minimum score associated with clinically significant impairment. The mean score (29.9 [SD=16.0]) was essentially identical to that of prior representative samples of adults (5) .
Pregenual Anterior Cingulate Cortex Connectivity
Positive connectivity
Consistent with its role in social processes, spontaneous pregenual anterior cingulate cortex activity was significantly correlated with that of brain regions implicated in social cognition. These regions were the 1) bilateral ventral and dorsal medial prefrontal areas of the anterior cingulate cortex, 2) posterior cingulate cortex, and 3) precuneus (16 – 19 , 30 , 31) . The superior frontal cortex, superior and middle temporal gyri, portions of the lateral occipital cortex, and angular gyrus were also positively correlated with the pregenual anterior cingulate cortex time series. Finally, ventral aspects of the anterior insula, implicated in evaluating one’s own emotions and the emotions of others (21 , 32) , were also positively correlated with the pregenual anterior cingulate cortex.
Negative connectivity
The pregenual anterior cingulate cortex was negatively correlated with primary sensory and motor cortices as well as regions involved in the dorsal attentional network (superior parietal cortices, dorsal precuneus, and lateral and medial occipital cortices) and in motor control (bilateral inferior frontal gyrus, cingulate motor area, and supplementary motor cortex). Negative correlations were also observed in posterior aspects of the mid-insula, adjacent but not extending to the posterior insula, which is commonly implicated in the primary experience of somatic and visceral sensations (33) . The group maps and peaks of significant positive and negative correlations are illustrated in Figure 1 as well as in the data supplement accompanying the online version of this article.

a Surface maps of the left and right hemispheres were generated using the Analysis of Functional NeuroImages Surface Mapper (AFNI SUMA) package. Significant positive (red) and negative (blue) connectivity is illustrated for the pregenual anterior cingulate cortex in neurotypical adults (z>2.3; cluster significance: p<0.05, corrected using Gaussian random field theory). The sagittal and axial images (bottom) illustrate the location of the pregenual anterior cingulate cortex seed of interest used to map functional connectivity (a 10-mm diameter sphere was centered at x=0, y=47, z=9 in Montreal Neurological Institute standard space).
Pregenual Anterior Cingulate Cortex Connectivity Predicted by SRS-A Score
Positive relationships
Analyses that included SRS-A scores as a covariate of interest revealed that functional connectivity of the pregenual anterior cingulate cortex with a left hemisphere cluster in the lateral occipital cortices, superior parietal cortex, and angular gyrus was positively related to SRS-A scores. Increased SRS-A scores predicted increased positive functional connectivity between the pregenual anterior cingulate cortex and these left hemisphere cortical regions ( Figure 2 ).

a Surface maps (left column) depict regions for which pregenual anterior cingulate cortex functional connectivity is related to SRS-A scores across all 25 participants (z>2.3; cluster significance: p<0.05, corrected using Gaussian random field theory). Functional connectivity between the pregenual anterior cingulate cortex and a cluster centered in the anterior mid-insula (red) was negatively related to SRS-A scores bilaterally, while a cluster centered in the left hemisphere parietal/occipital cortices (green) was positively related to SRS-A scores. Scatterplots depict relationships between individual SRS-A scores and the parameter estimates of pregenual anterior cingulate cortex functional connectivity for A) the left anterior mid-insula (r 2 =0.56, p<0.001), B) left parietal/occipital cortices (r 2 =0.51, p<0.001), and C) right anterior mid-insula (r 2 =0.44, p<0.001).
Negative relationships
We found a significant relationship between SRS-A scores and pregenual anterior cingulate cortex and anterior mid-insula connectivity (r 2 =0.44 and 0.56 for right and left insula regions, respectively). Specifically, lower SRS-A scores (i.e., greater social competence) were associated with higher positive connectivity between these two regions. Examination of the functional connectivity of this circuit and SRS-A ratings indicated that subjects with high SRS-A scores (i.e., lower social competence) were characterized by negative connectivity between the pregenual anterior cingulate cortex and anterior mid-insula, while those subjects with lower SRS-A scores were characterized by positive connectivity between these two regions. Although present in both hemispheres, this pattern was more robust in the left hemisphere ( Figure 2 , Figure 3 ).

a The images illustrate ventral anterior insula regions exhibiting positive pregenual anterior cingulate cortex functional connectivity and posterior mid-insula regions exhibiting negative pregenual anterior cingulate cortex functional connectivity. Also shown is the anterior mid-insula region (y approximately >1) where pregenual anterior cingulate cortex functional connectivity was found to be negatively related to SRS-A scores across all 25 participants. Areas of overlap between anterior mid-insula regions related to SRS-A scores and regions exhibiting negative functional connectivity are also depicted. Last, insula regions in which no relationship was found with the pregenual anterior cingulate cortex are shown, with insular cortex defined using the Harvard-Oxford Cortical Structural Atlas (minimum probability threshold=25%). Of note, the posterior insula (y approximately <–15) does not show any functional connectivity with the pregenual anterior cingulate cortex in either hemisphere, which is in agreement with recent findings (23). SRS-A=Social Responsiveness Scale—Adult Version.
Based on these findings, we examined among individual subjects the functional connectivity patterns between the pregenual anterior cingulate cortex seed and the anterior mid-insula in greater detail by trisecting the study group based on SRS-A scores. Individuals scoring in the lowest SRS-A tercile exhibited the highest degree of positive connectivity between these two regions, as indexed by the number of significant positively correlated voxels within the anterior mid-insula area revealed by the SRS-A covariate analysis ( Figure 4 ). In contrast, for individuals scoring in the highest tercile, the majority of significantly correlated voxels in the anterior mid-insula region were negatively correlated with the pregenual anterior cingulate cortex. We summarized these contrasts as the difference between the percentage of significant positively and negatively correlated voxels between the pregenual anterior cingulate cortex and anterior mid-insula for each hemisphere. To test for ordered differences in the three SRS-A subgroup medians, we used the Jonckheere-Terpstra Test (34) . There was a significant ordered difference among the three subgroup medians such that the more negative the difference between the percentage of positively and negatively correlated voxels, the higher the SRS-A scores (Jonckheere-Terpstra Test statistic: 35 and 50, z scores=–3.4 and –2.7, asymptotic significance: p=0.001 and p=0.007 for left and right hemispheres, respectively).
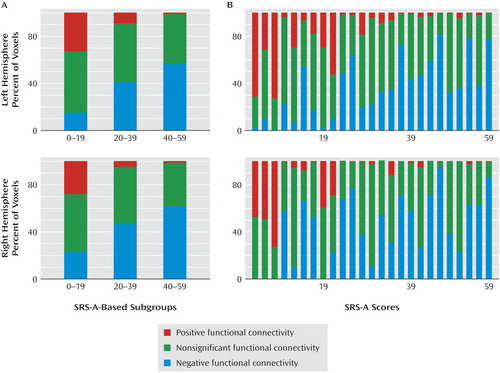
a For each subject, the percentage of voxels within the clusters (for each hemisphere) showing significant positive or negative pregenual cingulate functional connectivity was calculated (panel A). For each of three subgroups of participants determined by SRS-A terciles (lowest SRS-A tercile: N=8; intermediate SRS-A tercile: N=9; highest SRS-A tercile: N=8), tricolor plots illustrate the mean percentage of voxels within the anterior mid-insula mask exhibiting significantly positive (red), negative (blue), or nonsignificant (green) pregenual anterior cingulate cortex functional connectivity. Panel B illustrates the percentage of voxels with positive, negative, or nonsignificant functional connectivity represented for each subject, sorted by SRS-A scores. Overall, our findings indicate that subjects with lower SRS-A scores show greater positive connectivity between the pregenual anterior cingulate cortex and anterior mid-insula. In contrast, those with higher SRS-A scores show a greater pattern of negative connectivity between the two regions. This pattern was confirmed by the Jonckheere-Terpstra Test of the differences between the percentage of voxels positively connected minus the percentage of voxels negatively connected to the pregenual anterior cingulate cortex in the anterior mid-insula masks for each of the three SRS-A terciles (Jonckheere-Terpstra Test-based z scores=–3.4 and –2.7, for left and right hemispheres, respectively; p<0.01).
To confirm these results, we conducted further secondary analyses, treating individuals in the highest and lowest terciles as separate groups. Between-group (highest versus lowest tercile) analyses revealed significant voxel-wise differences (see the data supplement accompanying the online version of this article). Specifically, the lowest SRS-A tercile exhibited significantly greater positive functional connectivity between the pregenual anterior cingulate cortex seed region and a cluster centered in the left anterior mid-insula. In contrast, the highest SRS-A tercile showed significantly greater positive pregenual anterior cingulate cortex functional connectivity with a left hemisphere region consisting of the lateral occipital cortex, superior parietal gyrus, and angular gyrus. (See the data supplement accompanying the online version of this article for within-group statistical maps with a voxel-wise threshold of z=1.64, showing that the pattern of positive functional connectivity extends to the anterior mid-insula only in the lowest SRS-A subgroup. By contrast, in the highest SRS-A subgroup, the anterior mid-insula was characterized by negative functional connectivity with the pregenual anterior cingulate cortex.)
Test-Retest Reliability
Using the analytical approach detailed in Shehzad et al. (28) , we computed intraclass correlation values of pregenual anterior cingulate cortex connections, over both the long- and short-term, for the 20 subjects who had completed three resting state scans as part of a test-retest study. As illustrated in Figure 5 , key components of the examined network, including the posterior cingulate cortex, insular cortex, anterior cingulate cortex, and inferior frontal gyrus, showed moderate to high intraclass correlation values (>0.5). Of note, the anterior mid-insula region, in which functional connectivity with the pregenual anterior cingulate cortex was found to be directly related to SRS-A scores, exhibited high reliability over both the long- and short-term (intraclass correlation values: 0.68 and 0.77, respectively).

a The top row (left) illustrates the sagittal and axial images of the pregenual anterior cingulate cortex seed of interest (centered at x=0, y=47, z=9 in Montreal Neurological Institute standard space) and (right) sagittal and axial images of the left anterior mid-insula mask (corresponding to the cluster revealed in the analysis using SRS-A as covariate of interest; peak x=34, y=0, z=–2 in Montreal Neurological Institute standard space). The upper and lower scatter plots (right) show the parameter estimates of functional connectivity between the pregenual anterior cingulate cortex and anterior mid-insula for the long- and short-term (r 2 =0.45 and 0.60; p<0.01 and p<0.001, respectively). The upper and lower images (left) illustrate surface maps of voxel-wise intraclass correlation values for pregenual anterior cingulate cortex functional connectivity over the long-term (intersession: scans 1 and 2 separated by 5 to 16 months) and short-term (intrasession: scans 2 and 3 separated by 45 minutes) in the 20 participants who completed three scans. The upper image illustrates a map of the intersession reliability of parameter estimates for pregenual anterior cingulate cortex/left anterior mid-insula functional connectivity across scans 1 and 2. The lower image illustrates a map of the intrasession reliability across scans 2 and 3.
Discussion
The present study represents an initial application of a protocol developed to examine the neural correlates of autistic traits, as indexed by the SRS-A, in neurotypical individuals using resting state fMRI. As hypothesized, we found that individual differences in autistic traits in a nonclinical population were related to pregenual anterior cingulate cortex functional connectivity. Several studies have linked individual behavioral performance to patterns of functional connectivity observed during rest (e.g., 35 ). The present study extends this finding by relating an informant-based measure of social skills related to autistic traits (3 , 5 – 7) directly to pregenual anterior cingulate cortex connectivity.
Consistent with one of our two predictions, autistic traits were related to pregenual anterior cingulate cortex connectivity with the insula, although this relationship was specifically limited to the anterior mid-insula rather than anterior insula per se. Discussing the implications of this finding requires considering the pattern of pregenual anterior cingulate cortex connectivity with various insula subregions. The insula can be subdivided broadly into the anterior, middle, and posterior, with the anterior insula containing the ventral and dorsal regions (36) . The posterior insula, commonly involved in primary representations of visceral and somatic sensation (33) , showed no connectivity with the pregenual anterior cingulate cortex, which is consistent with a recent report (23) . In contrast, the anterior and mid-insula subregions, implicated in maintaining higher order representations of sensation and emotion (21 , 33 , 37) , exhibited a significant pattern of pregenual anterior cingulate cortex functional connectivity along an anterior/posterior gradient, extending from positive to negative connectivity. At the anterior-most extent of the insula, the ventral anterior insula was positively connected with the pregenual anterior cingulate cortex along with a broader network of structures implicated in social cognition, such as the retrosplenial complex. By contrast, at the posterior extent of this gradient, the posterior mid-insula was negatively connected with the cingulate region of interest along with a network of structures implicated in somatic and self-focused attention (primary somatosensory cortices, superior parietal cortices, and primary motor cortices). Between these, an intervening anterior mid-insula region exhibited variable patterns of functional connectivity across subjects. This pattern of differential pregenual anterior cingulate cortex connectivity in anterior and middle insula subregions is consistent with the notion that the ventral anterior and posterior insula underlie social cognition and somatic attention, respectively (21 , 33 , 37) .
These findings of differentiable mid-insula subregions are noteworthy when considered in the context of current models of insula organization, which tend to treat the mid-insula as a single subregion (38) . Specifically, we found that the anterior mid-insula appears to function as a transition zone between the ventral anterior insula, which is related to social cognition (21) , and the posterior mid-insula, which is likely involved in somatic attention (33 , 37) . As indicated by secondary analyses, in the subgroup with low levels of autistic traits, a substantial portion of the anterior mid-insula showed a positive connectivity pattern similar to that observed in the ventral anterior-insula. However, in the subgroup with elevated levels of autistic traits, the valence of functional connectivity in the anterior mid-insula resembled that of the posterior mid-insula (i.e., negative correlation with the pregenual anterior cingulate cortex). These results have broad implications for future efforts to relate interindividual differences in behavior to functional connectivity. Specifically, they highlight the need to interrogate connections exhibiting a high degree of variability across subjects as opposed to limiting analyses to a priori connections of interest or to only those analyses showing consistent patterns of connectivity across subjects.
We did not detect a significant relationship between SRS-A scores and pregenual anterior cingulate cortex functional connectivity with the posterior cingulate cortex, a region implicated in the development of theory of mind (20) . A meta-analysis (18) of functional imaging studies of autism found the posterior cingulate cortex to be hypoactive in autism during social tasks. Further, initial studies of functional connectivity in autism (14 , 15) reported decreased connectivity between the posterior cingulate cortex and pregenual anterior cingulate cortex. Beyond potential power concerns related to our sample size, we used a nonclinical screened sample that may not recapitulate brain-behavior relationships encountered in clinically diagnosed individuals with higher SRS-A scores. Future studies with affected individuals will test whether greater severity of autistic traits is related to functional connectivity between anterior and posterior cingulate regions implicated in social cognition (18) .
We also found that increased SRS-A ratings were related to increased functional connectivity between the pregenual anterior cingulate cortex and higher order sensory processing regions (superior parietal gyrus, lateral occipital cortex, and angular gyrus). These regions are often abnormally hyperactive in individuals with autism (18) . As such, although not hypothesized, our findings suggest the need to consider abnormal patterns of connectivity beyond hypoconnectivity.
In considering the imaging approach used in the present study, we note that although SRS-A scores may also be related to evoked brain activation using task-based approaches, examining the relationship between SRS-A scores and measures of resting state functional connectivity has several advantages. First, practice effects, floor/ceiling effects, and the need for compliance with task demands are minimized (9) . This latter point is crucial for studies with behaviorally and intellectually challenged populations. Second, resting state analyses can be used to simultaneously delineate entire multiple networks, which are usually only partially observable in task-based studies, depending on the selected contrasts (8) . Third, despite concerns about the unconstrained nature of the resting state, patterns of intrinsic connectivity during rest are remarkably replicable across individuals and across research labs (39) . Further, our examination of the voxel-wise reliability of measures of functional connectivity within the pregenual anterior cingulate cortex network demonstrated moderate (intraclass correlation values >0.5) to high (intraclass correlation values between 0.7 and 0.95) test-retest reliability, a finding consistent with the stability of other resting state networks (28) . Nevertheless, we cannot exclude the possibility that interindividual differences in responding to the scanner environment (e.g., scanner noise, experience of remaining still in the scanner) may be related to both patterns of functional connectivity and autistic traits assessed by the SRS-A. Future studies of the resting state using a combination of objective (e.g., physiological) and subjective (e.g., self-report) indices will be needed in order to clarify this issue.
Our findings should be interpreted in light of several limitations. Seed-based approaches to mapping functional connectivity require a priori selection of a region of interest. We selected the pregenual anterior cingulate cortex because of its prominence in models of autism-related abnormalities and its emergence in our recent meta-analysis of autism (18) . Future studies will entail investigating the intrinsic connectivity of other regions of interest that may be implicated in the social impairments associated with autism (18) . On a related note, although the SRS-A primarily emphasizes social and communication impairments in characterizing autistic traits, specifically focusing on other facets of autistic traits such as restricted/repetitive behaviors or interests may be helpful in future studies. We maximized the representativeness of individual parameter estimates by combining data from all available scans. Since only 80% of the study sample provided three scans, we considered the possibility that differences in the number of scans might have influenced our results. Accordingly, we accounted for potential differences at the group level by including the number of scans as a covariate. In addition, we conducted confirmatory analyses limited to 1) a single scan per subject and 2) the 20 participants who provided three resting state scans, and neither produced results that differed substantially from our main findings (see the data supplement accompanying the online version of this article). Finally, significant controversy continues to surround the interpretation of negative relationships in functional connectivity (40) . Thus, although patterns of positive and negative connectivity differentiate insula subregions, caution should be taken when interpreting the meaning of negative relationships.
As has recently been noted (41) , when brain regions are identified by virtue of their correlation with behavioral measures, the corresponding correlation coefficients between the identified regions and the behavioral variables are generally somewhat inflated and should not be interpreted as unbiased. However, they do serve an illustrative purpose and are meaningful.
In summary, the present study demonstrates the utility of resting state fMRI for mapping functional connectivity in relation to the SRS-A, a continuous measure of autistic traits in the general population. This approach led us to identify the pregenual anterior cingulate cortex connectivity with the anterior mid-insula as a candidate marker of social competence related to autistic traits in a neurotypical sample. Application of this method in future studies examining patients with autism should allow confirmation of whether this circuit is a locus of dysfunction in autism that is dimensionally related to the severity of autistic traits.
1. Folstein S, Rutter M: Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry 1977; 18:297–321Google Scholar
2. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I: The Reading the Mind in the Eyes Test—Revised Version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 2001; 42:241–251Google Scholar
3. Constantino JN, Todd RD: Autistic traits in the general population: a twin study. Arch Gen Psychiatry 2003; 60:524–530Google Scholar
4. Hurley RS, Losh M, Parlier M, Reznick JS, Piven J: The Broad Autism Phenotype Questionnaire. J Autism Dev Disord 2007; 37:1679–1690Google Scholar
5. Constantino JN, Todd RD: Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry 2005; 57:655–660Google Scholar
6. Constantino JN, Przybeck T, Friesen D, Todd RD: Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr 2000; 21:2–11Google Scholar
7. Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T: The factor structure of autistic traits. J Child Psychol Psychiatry 2004; 45:719–726Google Scholar
8. Fox MD, Raichle ME: Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8:700–711Google Scholar
9. Buckner RL, Vincent JL: Unrest at rest: default activity and spontaneous network correlations. Neuroimage 2007; 37:1091–1096Google Scholar
10. Biswal B, Yetkin FZ, Haughton VM, Hyde JS: Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34:537–541Google Scholar
11. Horwitz B, Rumsey JM, Grady CL, Rapoport SI: The cerebral metabolic landscape in autism: intercorrelations of regional glucose utilization. Arch Neurol 1988; 45:749–755Google Scholar
12. Murias M, Webb SJ, Greenson J, Dawson G: Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry 2007; 62:270–273Google Scholar
13. Minshew NJ, Williams DL: The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol 2007; 64:945–950Google Scholar
14. Cherkassky VL, Kana RK, Keller TA, Just MA: Functional connectivity in a baseline resting-state network in autism. Neuroreport 2006; 17:1687–1690Google Scholar
15. Kennedy DP, Courchesne E: The intrinsic functional organization of the brain is altered in autism. Neuroimage 2008; 39:1877–1885Google Scholar
16. Amodio DM, Frith CD: Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 2006; 7:268–277Google Scholar
17. Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW: Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci 2006; 18:932–948Google Scholar
18. Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP: Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry 2009; 65:63–74Google Scholar
19. Cavanna AE, Trimble MR: The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129:564–583Google Scholar
20. Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV: Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci 2007; 19:1803–1814Google Scholar
21. Singer T: The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev 2006; 30:855–863Google Scholar
22. Sridharan D, Levitin DJ, Menon V: A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 2008; 105:12569–12574Google Scholar
23. Taylor KS, Seminowicz DA, Davis KD: Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 2008; Dec 15 [Epub ahead of print]Google Scholar
24. Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP: Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex 2008; 18:2735–2747Google Scholar
25. Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP: Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 2007; 37:579–588Google Scholar
26. Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP: Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 2009; 45:614–626Google Scholar
27. Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP: Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex 2009; 19:640–657Google Scholar
28. Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP: The resting brain: unconstrained yet reliable. Cereb Cortex 2009; Feb 16 [Epub ahead of print]Google Scholar
29. Brett M: The MNI Brain and the Talairach Atlas. Cambridge, UK, Cambridge Imaging, 1999, pp 12–19Google Scholar
30. Northoff G, Bermpohl F: Cortical midline structures and the self. Trends Cogn Sci 2004; 8:102–107Google Scholar
31. Gallagher HL, Frith CD: Functional imaging of “theory of mind.” Trends Cogn Sci 2003; 7:77–83Google Scholar
32. Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD: The neural basis of economic decision-making in the ultimatum game. Science 2003; 300:1755–1758Google Scholar
33. Craig AD: How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666Google Scholar
34. Bewick V, Cheek L, Ball J: Statistics Review 10: further nonparametric methods. Crit Care 2004; 8:196–199Google Scholar
35. Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL: Disruption of large-scale brain systems in advanced aging. Neuron 2007; 56:924–935Google Scholar
36. Augustine JR: The insular lobe in primates including humans. Neurol Res 1985; 7:2–10Google Scholar
37. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ: Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Google Scholar
38. Mesulam MM, Mufson EJ: Insula of the old world monkey, I: architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol 1982; 212:1–22Google Scholar
39. Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF: Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 2006; 103:13848–13853Google Scholar
40. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA: The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage 2009; 44:893–905Google Scholar
41. Lieberman MD, Berkman ET, Wager TD: Correlations in social neuroscience aren"t voodoo. Perspect Psychol Sci 2009; 4:299–307Google Scholar



