Randomized, Double-Blind, Placebo-Controlled Trial of Vigabatrin for the Treatment of Cocaine Dependence in Mexican Parolees
Abstract
Objective: Cocaine dependence is associated with severe medical, psychiatric, and social morbidity, but no pharmacotherapy is approved for its treatment in the United States. The atypical antiepileptic vigabatrin (γ-vinyl gamma-aminobutyric acid [GABA]) has shown promise in animal studies and open-label trials. The purpose of the present study was to assess the efficacy of vigabatrin for short-term cocaine abstinence in cocaine-dependent individuals. Method: Participants were treatment seeking parolees who were actively using cocaine and had a history of cocaine dependence. Subjects were randomly assigned to a fixed titration of vigabatrin (N=50) or placebo (N=53) in a 9-week double-blind trial and 4-week follow-up assessment. Cocaine use was determined by directly observed urine toxicology testing twice weekly. The primary endpoint was full abstinence for the last 3 weeks of the trial. Results: Full end-of-trial abstinence was achieved in 14 vigabatrin-treated subjects (28.0%) versus four subjects in the placebo arm (7.5%). Twelve subjects in the vigabatrin group and two subjects in the placebo group maintained abstinence through the follow-up period. The retention rate was 62.0% in the vigabatrin arm versus 41.5% in the placebo arm. Among subjects who reported prestudy alcohol use, vigabatrin, relative to placebo, was associated with superior self-reported full end-of-trial abstinence from alcohol (43.5% versus 6.3%). There were no differences between the two groups in drug craving, depressed mood, anxiety, or Clinical Global Impression scores, and no group differences in adverse effects emerged. Conclusions: This first randomized, double-blind, placebo-controlled trial supports the safety and efficacy of short-term vigabatrin treatment of cocaine dependence.
Cocaine dependence is associated with severe social and economic consequences, medical and psychiatric morbidity, and mortality (1) . While identifying effective pharmacotherapy for cocaine addiction is a major public health priority (2) and some agents have shown promise in controlled trials (3 – 5) , limitations have impeded their regulatory approval and clinical use (6) . No pharmacotherapy is currently approved for treating cocaine dependence in the United States.
Cocaine’s addictive properties have been associated with mesotelencephalic dopamine reward pathways (7) , and it raises extracellular dopamine concentration in the striatum (8) , where elevated dopamine receptor occupancy is associated with behavioral manifestations of reward in animals (9) and self-reported pleasure in humans (10) . The neurotransmitter GABA suppresses striatal dopamine release and blunts cocaine-induced increases in extracellular dopamine in the striatum and nucleus accumbens in animals (11 , 12) . Vigabatrin (γ-vinyl GABA) is an antiepileptic in clinical use outside of the United States that irreversibly inhibits GABA-transaminase, the principal catabolist of synaptic GABA, and rapidly elevates human GABA concentrations (13 , 14) . In animals, vigabatrin blocks cocaine-induced dopamine release in the nucleus accumbens (15) and prevents behavioral manifestations of cocaine dependence (16 – 18) without tolerance, dependence, or withdrawal (17 , 19) .
Clinical data on vigabatrin for stimulant dependence come from two small open trials (20 , 21) of directly observed vigabatrin administration and weekly supportive group psychotherapy in outpatient drug treatment settings in Mexico. In the first study of 20 subjects with cocaine dependence, eight were abstinent for at least 4 successive weeks during 10 weeks of treatment (20) . Of 30 subjects with methamphetamine and/or cocaine dependence in the second study, 16 were abstinent for at least 3 successive weeks over 9 weeks of treatment (21) . The most common adverse effects were transient somnolence and headache, but no serious events occurred in either study.
In the present study, we provide data from the first randomized, double-blind, placebo-controlled clinical trial of vigabatrin for the treatment of cocaine dependence.
Method
Study Design
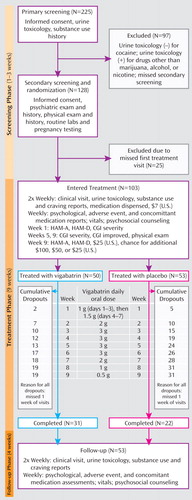
The three study phases, along with assessment timing, treatment dosing and timing, and numbers of subjects recruited and retained, are illustrated in Figure 1 and consisted of 1) a 1- to 3-week screening phase of two visits followed by 2) randomization to vigabatrin (N=50) or placebo (N=53) for a 9-week treatment phase, with biweekly visits, and 3) a 4-week follow-up phase, with weekly visits.
Subject Recruitment
Parolees of a Mexico City prison were recruited at parole centers via flyers and word of mouth. The study population was selected because endemic cocaine use has been reported in prisons in Mexico and the use of cocaine is considered a principal cause of recidivism among parolees (personal communication with Pedro Arellano, 2005). The study sample consisted of individuals who were poor and unemployed or underemployed, and none had permanent telephone numbers. Approximately 225 individuals reporting cocaine use and seeking treatment were screened. Screened participants were not told how the results of confidential urine toxicology testing would affect the study eligibility.
Setting
The setting was one of several urban, private, nonprofit, government-funded substance abuse clinics in Mexico City serving a population of 27 million in a 100-square-mile area. The clinic is located 12 miles from the parole office nearest the prison, and public transportation generally required more than 2 hours each way.
Inclusion Criteria
Parolees were individuals ages 18 to 55 years old who were capable of giving informed consent, had DSM-IV cocaine dependence, and were urine positive for cocaine and negative for heroin and methamphetamine at screening. Female participants consented to pregnancy testing and the use of birth control.
Exclusion Criteria
Screening participants were excluded for dependence on substances other than cocaine, alcohol, nicotine, or marijuana; alcohol dependence requiring detoxification; prior cocaine use treatment; significant cocaine abstinence within 6 months; current court-mandated cocaine use treatment; intravenous drug use within 2 months; recent medical study participation; or history of major medical, neurological, or psychiatric disorders. Because of the association between lifetime vigabatrin dose and visual field defects in youth with epilepsy, participants were excluded for visual field defects or predisposing factors, including glaucoma, severe myopia, retinal disorder, cataracts, diabetes, or uncontrolled hypertension. Participants were also excluded for a violent crime conviction or pending reincarceration or relocation.
Informed Consent
Medical risks and benefits of treatment and safety procedures were discussed as well as the procedures described in the present article. Participation in the study did not alter subjects’ parole or legal status or confer legal benefits, and drug testing results were confidential and not shared with parole personnel. Attending at least one of two weekly clinic visits satisfied the standard parole requirement of weekly meetings with an officer, which consists of a brief meeting in the regional parole office without drug testing or treatment. Parole officers called clinic staff weekly to confirm clinic attendance, and subjects reverted to the standard parole requirement after study completion or discontinuation, mandated by two consecutive missed visits. Participants were reimbursed $7 (U.S. dollars) per treatment visit plus an incentive payment of $25 (U.S. dollars) upon treatment phase completion and inclusion in a drawing for three monetary prizes ($100, $50, or $25 [U.S. dollars]). After complete description of the study was given, written informed consent was obtained.
Assessment
An addiction psychiatrist (Dr. Figueroa) screened participants with a substance use questionnaire, consisting of categorical DSM-IV symptom items, at the initial screening visit and clinically assessed subjects at the subsequent visit to establish DSM-IV diagnoses. Two psychiatrists and the study psychologist consensus rated global psychiatric illness using the Clinical Global Impression Scale (CGI) (22) and anxiety and depressive symptoms using the Hamilton Anxiety Rating Scale (HAM-A) (23) and Hamilton Depression Rating Scale (HAM-D) (24) at weeks 1, 5, 9, and 13. As a result of an error in administration, only the first 10 items of the HAM-D were consistently recorded and included in the analyses. Additionally, measures of cocaine craving (25) and self-reported interim substance use were recorded at all visits, and mental status, adverse events, and concomitant medication use were recorded weekly during treatment and follow-up.
The study physician conducted a medical history and physical examination, including funduscopy and visual field testing by confrontation, and obtained routine blood and urine tests at the second screening visit. Repeat physical exams were conducted at weeks 5 and 9, and vital signs were obtained at every visit.
Urine samples for cocaine, opiate, amphetamine, marijuana, and benzodiazepine testing were obtained under direct observation at initial screening and all visits. Urine benzoylecgonine concentrations ≥300 ng/ml were considered cocaine positive.
Treatments
A research pharmacist dissolved each daily vigabatrin dose in 250 ml of orange juice according to a fixed titration ( Figure 1 ). Placebo consisted of identical bottles of juice. The pharmacist maintained subject treatment assignments in a locked file that was inaccessible to study personnel. Staff, blind to assignments, directly observed consumption of the dose for a particular day and distributed bottles for use until the next visit. The cumulative vigabatrin dose for individuals who completed the study was 131.5 g. During treatment and follow-up, subjects received weekly individual cognitive-behavioral therapy focused on supporting abstinence in accordance with routine clinic practice.
Outcome Measures
Primary cocaine use measure
The prespecified primary outcome measure was full end-of-trial abstinence, which was defined as twice-weekly urine toxicology tests negative for cocaine (clean) during the last 3 weeks of the trial. Missing urine toxicology tests were considered positive. The measure was selected for two principal reasons. First, cocaine dependence is currently conceptualized as a chronic relapsing disorder (26) in which abstinence, rather than reduced or episodic use, confers the most significant improvement in medical and functional status and most plausibly identifies treatment response. Second, sustained abstinence from cocaine for 3 weeks in one short-term trial for cocaine dependence (27) strongly predicted abstinence at the 6-month follow-up, while other short-term use measures have not been shown to predict longer-term abstinence. The abstinence period required included the last 3 weeks of the treatment phase because the investigators considered early abstinence followed by relapse during the final 3 weeks to be an ambiguous treatment response indicator.
Secondary cocaine use measures
The principal secondary cocaine use outcome measure was partial end-of-trial abstinence, which allowed no more than one relapse. Other secondary cocaine use outcome measures were the 1) number of weeks urine toxicology tests were clean, 2) total number of clean urine toxicology tests, 3) weekly self-reported cocaine dose and number of days used, and 4) time to full end-of-trial abstinence. Missing urine toxicology tests were considered cocaine positive for these analyses.
Other outcomes
Full end-of-trial abstinence from marijuana, determined by urine toxicology screening, and from alcohol, determined by self-report, were measured. Psychological outcomes were the change from baseline for cocaine craving; CGI severity scores; CGI improvement scores at weeks 5 and 9; HAM-A and HAM-D scores; and mood, anxiety, and somatic symptoms from a psychological review used routinely at the study clinic. Safety outcomes were reported adverse events and drug-attributable physical examination and vital sign abnormalities.
Statistical Methods
Significance level and power
Contrasts were made at a 0.05 significance level without correcting for multiple comparisons. Based on prior data (20 , 21) , we assumed that 40% of subjects taking vigabatrin and 10% of subjects taking placebo would achieve full end-of-trial abstinence and estimated that 50 subjects per arm were sufficient to detect this difference with 90% power. Analyses were conducted using SAS 9.1.3 (SAS Institute, Cary, N.C.).
Baseline characteristics
Group comparisons utilized continuity adjusted chi-square tests for categorical variables and t tests for continuous variables.
Full end-of-trial abstinence from cocaine
The proportion of subjects achieving full end-of-trial abstinence was calculated for each group and compared using logistic regression, with covariates of baseline self-reported daily cocaine consumption or duration of cocaine use. The comparison was considered significant if the coefficient of the treatment-group term differed significantly from 1 and was the principal test of the primary study outcome measure.
Kaplan-Meier survival distributions of time to onset of full end-of-trial abstinence were calculated and compared using the log-rank test.
To estimate the median time to abstinence onset (speed of onset) and the probability of abstinence (response rate), taking into account the censoring of observations, a nonparametric survival analytic “cure model” was used (28) . Onset was computed conditionally for subjects achieving abstinence, and group comparisons of response rates were conducted using the Laska-Meisner test (29) . Fitting efforts using parametric assumptions failed to converge.
Other abstinence outcomes
Logistic regression methods similar to those for group comparison of full end-of-trial abstinence from cocaine were used for partial end-of-trial abstinence from cocaine and full end-of-trial abstinence from alcohol and marijuana.
Measures over time
Weekly cocaine abstinence probability was compared between the two groups using mixed-model repeated-measures analysis of a logit transformation including terms for subject, treatment, and time-by-treatment interaction, and covariates of baseline reported daily cocaine consumption, use duration, and CGI severity scores. In this analysis, missing urine toxicology tests were imputed to be dirty. Treatments were considered different over time if the coefficient of the time-by-treatment interaction term differed from 0, and weekly differences were tested with simple main effects. The mixed-model repeated-measures analysis assumed a means model, unstructured covariance matrix, and estimation based on restricted maximum likelihood. Baseline values were adjusted by the overall mean. A similar analysis was performed for weekly self-reported cocaine use.
Daily use
Treatment differences in weekly reported days of use and dose of cocaine and the use of other substances were examined with mixed-model repeated-measures analysis. The total number of clean urine toxicology tests was compared utilizing a Wilcoxon signed-rank test.
Other outcomes
Changes from baseline for other outcomes were compared using a similar mixed-model repeated-measures analysis. An overall test of treatment differences and tests of differences at each week were performed for craving; weight change; HAM-A and HAM-D scores; CGI severity scores; and psychological review items. CGI improvement was dichotomized to improvement (score: 1–3) versus no improvement (score: 4–7) and analyzed using the Cochran-Mantel-Haenszel test. Dropout rates were compared between the two groups using the log-rank test.
Results
Baseline Characteristics
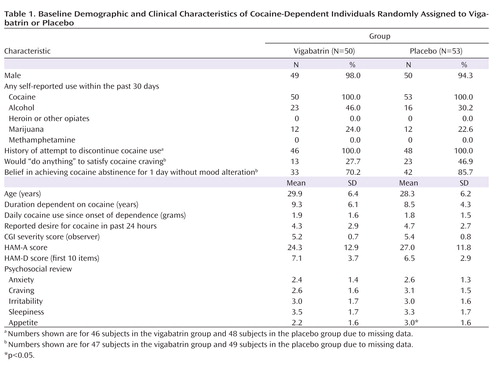
Baseline demographic and clinical characteristics of subjects in the vigabatrin and placebo treatment groups are presented in Table 1 . The groups differed only in that appetite was better in the placebo group relative to the vigabatrin group (3.0 versus 2.2, p≤0.03). Participants were approximately 30 years old, and all but four were men. Participants reported using 2 g of cocaine per day over 9 years. Most study participants reported a moderate desire to use cocaine, while a significant minority reported that they would “do anything” to satisfy their craving, and all previously attempted quitting. Forty-six percent of individuals in the vigabatrin group and 30% of individuals in the placebo group reported alcohol use in the last month, and approximately one-quarter in both groups reported marijuana use in the last month. CGI scores indicated marked illness severity, and HAM-A scores were high.
Cocaine Use Outcomes
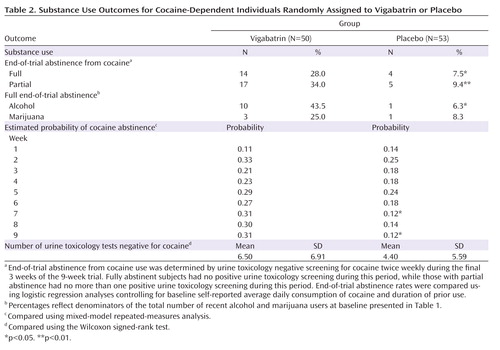
Full end-of-trial abstinence rates are presented in Table 2 , along with secondary measures. Nearly four times as many subjects taking vigabatrin relative to those taking placebo achieved full end-of-trial abstinence (28.0% versus 7.5%, p≤0.01). Similar results were obtained for partial end-of-trial abstinence (34.0% versus 9.4%, p≤0.005). Neither baseline self-reported average daily consumption nor duration of use was a significant covariate in the logistic models, and unadjusted group differences remained significant when covariates were removed.
Kaplan-Meier probability distributions favoring onset of full end-of-trial abstinence for the vigabatrin versus placebo groups (p≤0.01) are presented in Figure 2 .
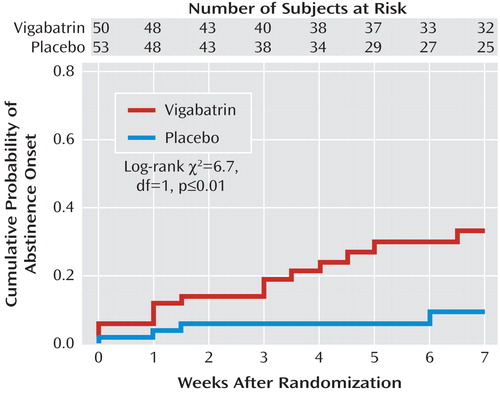
Using the cure model (29) , median time to full end-of-trial abstinence onset for those achieving abstinence was 3 weeks in the vigabatrin group and 2 weeks in the placebo group. Probability estimates of full end-of-trial abstinence onset were 0.33 for the vigabatrin group and 0.09 for the placebo group, and these differed significantly (p≤0.02).
Weekly abstinence did not differ over the entire treatment phase, but differences favoring vigabatrin were found in weeks 7 (p≤0.02) and 9 (p≤0.02), and a nonsignificant tendency favored vigabatrin at week 8 (p≤0.06). No group differences were found in self-reported days or dose used. The number of clean urine toxicology tests demonstrated a nonsignificant tendency favoring the vigabatrin group (mean=6.5 versus 4.4, p≤0.09).
Other Substance Use
The two groups did not differ in full end-of-trial abstinence from marijuana for subjects who reported recent marijuana use at baseline. However, for subjects who reported recent alcohol use at baseline, those taking vigabatrin were seven times more likely than those taking placebo to report full end-of-trial alcohol abstinence (43.5% versus 6.3%, p≤0.03). Among only those subjects who achieved full end-of-trial abstinence from cocaine, eight of the 14 vigabatrin subjects reported recent alcohol use at baseline, and six of these reported full end-of-trial alcohol abstinence, while none of the four placebo subjects reported alcohol use in either period.
Psychological Outcomes
Although subjects taking vigabatrin showed greater mean reduction in irritability (1.2 versus 0.5, p<0.001) and improvement in appetite (0.1 versus –0.6, p≤0.003) over baseline, there were no differences in cocaine craving, HAM-A and HAM-D scores, or CGI severity and CGI improvement scores between the two arms.
Subject Retention
Subject retention was 62.0% in the vigabatrin arm versus 41.5% in the placebo arm (p≤0.04). Weekly cumulative dropouts during the treatment phase are illustrated in Figure 1 . The median time to dropout was 7 weeks in the placebo group and exceeded the 9-week trial in the vigabatrin group.
Cocaine Abstinence During Follow-Up
Of the 14 subjects in the vigabatrin group with end-of-trial abstinence, 12 remained abstinent; one relapsed during the second week; and one missed a visit during the fourth week. Of the four subjects in the placebo group with end-of-trial abstinence, two remained abstinent; one relapsed during the second week; and one missed a visit during the fourth week.
Safety and Tolerability
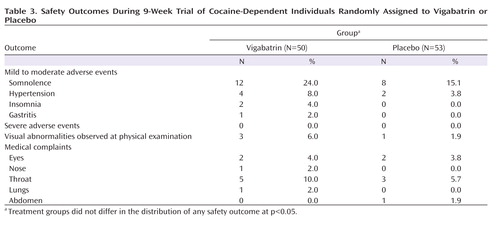
No serious adverse event occurred ( Table 3 ). Adverse event rates did not differ between the two groups, and early somnolence was the most common complaint among subjects taking vigabatrin and subjects taking placebo (24.0% and 15.1%). Transient hypertension was observed in two subjects taking placebo and four subjects taking vigabatrin. One mild to moderate episode was apparently associated with vigabatrin use, emerging in week 2, which required no treatment and remitted by week 9.
Discussion
Subjects taking vigabatrin achieved full end-of-trial abstinence from cocaine at a rate four times that of subjects taking placebo, and weekly abstinence probabilities for subjects in the vigabatrin group were almost three times that of subjects in the placebo group by week 9 of treatment. Median onset of full abstinence occurred after 3 weeks of vigabatrin treatment. We found only positive tendencies but no significant group differences in the weekly probability of abstinence over the full course of the treatment phase or in the total number of clean urine toxicology tests. We also observed no group differences in global illness severity or in most psychological outcome measures, including drug craving, although modest differences in irritability favored the vigabatrin group.
Properly interpreting our pattern of findings requires consideration of other successful controlled trials for cocaine dependence and of the relationship between clinical treatment goals in cocaine dependence and trial outcome measures. Differences were found in 3-week sustained abstinence rates between topiramate (59%) versus placebo (26%) and weekly abstinence during the final 4 weeks of one trial (5) but not the full 12-week treatment. The 3-week abstinence rate was significantly greater for modafinil (33%) relative to placebo (13%), as was the proportion of clean urine toxicology tests, for an 8-week trial (4) . In both these studies, placebo and treatment abstinence rates were higher than the rates observed in the present study, but the sustained abstinence outcomes were not restricted to the end of both previous trials. A multiarm trial (30) found significant reductions in the probability of dirty urine toxicology tests and self-reported cocaine use for disulfiram versus placebo over a 12-week treatment course but did not present data on abstinence. Our study retention of 51% (66% in the vigabatrin arm) was within the wide range experienced in similar trials (3 – 5) , despite minimal financial inducement and poor access to the study site, which suggests high motivation for treatment.
We chose abstinence during the final 3 weeks of the trial as the primary outcome measure because it is consistent with a clear, empirically supported (27 , 31) short-term goal of treatment for cocaine dependence. Our study and other trials (5) suggest that measuring response with sustained abstinence may also more powerfully differentiate between an effective treatment and placebo compared with changes in average use, which lose power if the effect of the active treatment among subjects who do not respond to treatment is limited.
While differences in study design and outcome measures complicate efficacy comparisons, our abstinence findings appear comparable with those of other successful trials of treatments for cocaine dependence. The failure of other agents to emerge as mainstays of community treatment suggests that adoption may depend on many factors, including tolerability and ease of administration.
Among subjects with recent alcohol use, those taking vigabatrin were seven times more likely than those taking placebo to report alcohol abstinence for the final 3 weeks of treatment. Preclinical and open-label data on treatment of methamphetamine dependence (21 , 32) may, in combination with our findings, prompt future research on vigabatrin for substance use commonly comorbid with cocaine dependence.
Vigabatrin was well tolerated, and we found no safety differences between the two study groups. Vigabatrin is used most commonly outside of the United States to treat infantile spasms, a severe pediatric epilepsy. The major serious adverse effect is a tardive peripheral visual field defect that is typically asymptomatic and neither progresses nor resolves upon treatment cessation (33) . Risk is believed to be dose dependent (34 – 37) , commencing at cumulative doses above 1,500 g (34) , although some studies have not found a dose relationship (38) . We detected no abnormalities on objective visual field perimetry and funduscopic examination in a prior open trial using an identical 131.5 g cumulative dose protocol (39) .
Our study has a number of limitations. We tested urine samples twice, rather than thrice, weekly because travel to the study site was onerous and precluded daytime employment for some subjects. Urine benzoylecgonine testing typically detects cocaine for 3 days after use, depending on the amount used, and thus use 4 days prior to testing would have gone undetected. Additionally, nicotine use was not assessed. Since subjects lacked telephones, we did not ascertain reasons for dropout. Finally, the trial was conducted in a nonacademic setting in a resource-poor nation. Preparatory staff training, weekly remote oversight, regular visits by the principal investigator, and review of all subject records by an independent auditor were conducted to assure trial integrity. Nevertheless, results from a foreign, community-based trial without independent, daily on-site monitoring must be viewed conservatively.
The present trial provides evidence of the efficacy and safety of vigabatrin in promoting short-term cocaine abstinence in subjects with chronic, severe cocaine dependence. Vigabatrin’s short-term tolerability, ease of administration, long duration of action, renal excretion without metabolism, and likely absence of abuse potential further contribute to its promise as a treatment for cocaine dependence in community settings. However, a number of important questions remain unanswered. Acute treatment of cocaine dependence characterized by binge rather than stable chronic use has not been evaluated, and vigabatrin’s associated risk of visual field defects in long-term treatment remains unclear. Additional trials are needed to determine an optimal long-term treatment paradigm, including maintenance dosing and duration, and to evaluate long-term efficacy and safety outcomes of vigabatrin treatment for cocaine dependence.

1. NIDA: Cocaine: Abuse and Addiction. Washington, DC, HHS, 2004Google Scholar
2. Leiderman DB, Shoptaw S, Montgomery A, Bloch DA, Elkashef A, LoCastro J, Vocci F: Cocaine Rapid Efficacy Screening Trial (CREST): a paradigm for the controlled evaluation of candidate medications for cocaine dependence. Addiction 2005; 100(suppl 1):1–11Google Scholar
3. Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ: Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry 2004; 61:264–272Google Scholar
4. Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP: A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005; 30:205–211Google Scholar
5. Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP: A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend 2004; 75:233–240Google Scholar
6. Kampman KM: The search for medications to treat stimulant dependence. Addict Sci Clin Pract 2008; 4:28–35Google Scholar
7. Gardner EL: Brain Reward Mechanisms in Substance Abuse: A Comprehensive Textbook. Baltimore, Williams and Wilkins, 1997Google Scholar
8. Dewey SL, Morgan AE, Ashby CR Jr, Horan B, Kushner SA, Logan J, Volkow ND, Fowler JS, Gardner EL, Brodie JD: A novel strategy for the treatment of cocaine addiction. Synapse 1998; 30:119–129Google Scholar
9. Gerasimov MR, Schiffer WK, Gardner EL, Marsteller DA, Lennon IC, Taylor SJ, Brodie JD, Ashby CR Jr, Dewey SL: GABAergic blockade of cocaine-associated cue-induced increases in nucleus accumbens dopamine. Eur J Pharmacol 2001; 414:205–209Google Scholar
10. Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE: Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 1997; 386:827–830Google Scholar
11. Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP, Volkow ND, Fowler JS, Meller E: GABAergic inhibition of endogenous dopamine release measured in vivo with [ 11 C]-raclopride and positron emission tomography. J Neurosci 1992; 12:3773–3780 Google Scholar
12. Molina PE, Ahmed N, Ajmal M, Dewey S, Volkow N, Fowler J, Abumrad N: Co-administration of gamma-vinyl GABA and cocaine: preclinical assessment of safety. Life Sci 1999; 65:1175–1182Google Scholar
13. Petroff OA, Rothman DL, Behar KL, Collins TL, Mattson RH: Human brain GABA levels rise rapidly after initiation of vigabatrin therapy. Neurology 1996; 47:1567–1571Google Scholar
14. Mattson RH, Petroff OA, Rothman D, Behar K: Vigabatrin: effect on brain GABA levels measured by nuclear magnetic resonance spectroscopy. Acta Neurol Scand Suppl 1995; 162:27–30Google Scholar
15. Schiffer WK, Marsteller D, Dewey SL: Sub-chronic low dose gamma-vinyl GABA (vigabatrin) inhibits cocaine-induced increases in nucleus accumbens dopamine. Psychopharmacology (Berl) 2003; 168:339–343Google Scholar
16. Kushner SA, Dewey SL, Kornetsky C: Gamma-vinyl GABA attenuates cocaine-induced lowering of brain stimulation reward thresholds. Psychopharmacology (Berl) 1997; 133:383–388Google Scholar
17. Kushner SA, Dewey SL, Kornetsky C: The irreversible gamma-aminobutyric acid (GABA) transaminase inhibitor gamma-vinyl-GABA blocks cocaine self-administration in rats. J Pharmacol Exp Ther 1999; 290:797–802Google Scholar
18. Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP, Dewey SL: The effect of gamma-vinyl-GABA on the consumption of concurrently available oral cocaine and ethanol in the rat. Pharmacol Biochem Behav 2001; 68:291–299Google Scholar
19. Takada K, Yanagita T: Drug dependence study on vigabatrin in rhesus monkeys and rats. Arzneimittelforschung 1997; 47:1087–1092Google Scholar
20. Brodie JD, Figueroa E, Dewey SL: Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse 2003; 50:261–265Google Scholar
21. Brodie JD, Figueroa E, Laska EM, Dewey SL: Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse 2005; 55:122–125Google Scholar
22. Guy W: Assessment Manual for Psychopharmacology: Publication ADM 76–338. Washington, DC, HHS, 1976Google Scholar
23. Bruss GS, Gruenberg AM, Goldstein RD, Barber JP: Hamilton Anxiety Rating Scale Interview Guide: joint interview and test-retest methods for interrater reliability. Psychiatry Res 1994; 53:191–202Google Scholar
24. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Google Scholar
25. Muñoz M, Cepeda-Benito A, Vila J, Fernandez MC: Development of two cocaine craving questionnaires, in Proceedings of the First International Research Collaboration Pre-conference to the Fourth National Conference: A Roadmap for Hispanic Drug Abuse Research. San Antonio, Tex, National Hispanic Science Network on Drug Abuse, 2004Google Scholar
26. McLellan AT, Lewis DC, O’Brien CP, Kleber HD: Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 2000; 284:1689–1695Google Scholar
27. Kosten TR, Gawin FH, Kosten TA, Morgan C, Rounsaville BJ, Schottenfeld R, Kleber HD: Six-month follow-up of short-term pharmacotherapy for cocaine dependence. Am J Addict 1992; 1:40–49Google Scholar
28. Berkson J, Gage RP: Survival curves for cancer patients following treatment. J Am Stat Assoc 1952; 47:501–515Google Scholar
29. Laska EM, Meisner MJ: Nonparametric estimation and testing in a cure model. Biometrics 1992; 48:1223–1234Google Scholar
30. Carroll KM, Rounsaville BJ, Nich C, Gordon L, Gawin F: Integrating psychotherapy and pharmacotherapy for cocaine dependence: results from a randomized clinical trial. NIDA Res Monogr 1995; 150:19–35Google Scholar
31. Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F: One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: delayed emergence of psychotherapy effects. Arch Gen Psychiatry 1994; 51:989–997Google Scholar
32. Gerasimov MR, Ashby CR Jr, Gardner EL, Mills MJ, Brodie JD, Dewey SL: Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse 1999; 34:11–19Google Scholar
33. Gillis MC: CPS: Compendium of Pharmaceutical and Specialties. Ottawa, Canadian Pharmaceutical Association, 2002Google Scholar
34. Manuchehri K, Goodman S, Siviter L, Nightingale S: A controlled study of vigabatrin and visual abnormalities. Br J Ophthalmol 2000; 84:499–505Google Scholar
35. Toggweiler S, Wieser HG: Concentric visual field restriction under vigabatrin therapy: extent depends on the duration of drug intake. Seizure 2001; 10:420–423Google Scholar
36. Vanhatalo S, Nousiainen I, Eriksson K, Rantala H, Vainionpaa L, Mustonen K, Aarimaa T, Alen R, Aine MR, Byring R, Hirvasniemi A, Nuutila A, Walden T, Ritanen-Mohammed UM, Karttunen-Lewandowski P, Pohjola LM, Kaksonen S, Jurvelin P, Granstrom ML: Visual field constriction in 91 Finnish children treated with vigabatrin. Epilepsia 2002; 43:748–756Google Scholar
37. Hardus P, Verduin WM, Engelsman M, Edelbroek PM, Segers JP, Berendschot TT, Stilma JS: Visual field loss associated with vigabatrin: quantification and relation to dosage. Epilepsia 2001; 42:262–267Google Scholar
38. Newman WD, Tocher K, Acheson JF: Vigabatrin associated visual field loss: a clinical audit to study prevalence, drug history and effects of drug withdrawal. Eye 2002; 16:567–571Google Scholar
39. Fechtner RD, Khouri AS, Figueroa E, Ramirez M, Federico M, Dewey SL, Brodie JD: Short-term treatment of cocaine and/or methamphetamine abuse with vigabatrin: ocular safety pilot results. Arch Ophthalmol 2006; 124:1257–1262Google Scholar