Using Neuroplasticity-Based Auditory Training to Improve Verbal Memory in Schizophrenia
Abstract
Objective: Impaired verbal memory in schizophrenia is a key rate-limiting factor for functional outcome, does not respond to currently available medications, and shows only modest improvement after conventional behavioral remediation. The authors investigated an innovative approach to the remediation of verbal memory in schizophrenia, based on principles derived from the basic neuroscience of learning-induced neuroplasticity. The authors report interim findings in this ongoing study. Method: Fifty-five clinically stable schizophrenia subjects were randomly assigned to either 50 hours of computerized auditory training or a control condition using computer games. Those receiving auditory training engaged in daily computerized exercises that placed implicit, increasing demands on auditory perception through progressively more difficult auditory-verbal working memory and verbal learning tasks. Results: Relative to the control group, subjects who received active training showed significant gains in global cognition, verbal working memory, and verbal learning and memory. They also showed reliable and significant improvement in auditory psychophysical performance; this improvement was significantly correlated with gains in verbal working memory and global cognition. Conclusions: Intensive training in early auditory processes and auditory-verbal learning results in substantial gains in verbal cognitive processes relevant to psychosocial functioning in schizophrenia. These gains may be due to a training method that addresses the early perceptual impairments in the illness, that exploits intact mechanisms of repetitive practice in schizophrenia, and that uses an intensive, adaptive training approach.
One of the greatest challenges for 21st-century biomedicine is to develop an effective treatment for the cognitive dysfunction of schizophrenia. Antipsychotic medications and adjunctive cognitive-enhancing agents show little benefit thus far (1 – 5) . Cognitive remediation trials demonstrate some efficacy (6) , but a recent meta-analysis revealed a “glass ceiling” of low to medium effect sizes across a large variety of methods (7) . Clearly, a fresh approach to the treatment of cognitive dysfunction in this illness is warranted.
Verbal learning and memory are among the most robustly abnormal cognitive functions in schizophrenia and are key targets for treatment (8) . Impaired verbal memory is associated with poor community functioning and poor response to psychosocial rehabilitation programs (9 – 11) ; it may be the principal reason why the gains provided by such programs are lost once the intervention ends (12 – 14) . We wondered whether it is possible to develop a novel approach to the remediation of verbal memory deficits in schizophrenia based on recent developments in clinical and basic neuroscience.
In schizophrenia, abnormalities are observed in frontotemporal cortical networks during verbal working memory, word encoding, and word recognition (15 , 16) . However, disturbances are also present at the earliest stages of auditory processing—for example, in the abnormally low amplitudes of the mismatch negativity response obtained during the preattentive detection of auditory stimuli (17) . In healthy individuals, a reduced mismatch negativity response is associated with lower verbal learning and memory performance and with poorer psychosocial functioning (18) ; in people with schizophrenia, reduced mismatch negativity responses are significantly associated with impaired verbal memory (19) , with the inability to decode semantic and emotional aspects of speech (20) , and with poor functional status (21 , 22) . These findings suggest that efficient auditory processing is crucial for the successful encoding and retrieval of verbal information and that disturbances in these elemental processes are related to higher-order cognitive dysfunction in schizophrenia (18 – 22) .
This body of evidence, combined with the past decade of animal experiments in the basic neuroscience of learning-induced neuroplasticity (23) , served as the rationale for the development of a cognitive training program that targets both early auditory processing and working memory operations, with the ultimate goal of improving verbal memory performance in schizophrenia. Specifically, we investigated the effects of an intensive 50-hour program of computerized training—delivered as a stand-alone treatment—that places implicit, increasing demands on auditory perception and accurate aural speech reception. The psychophysical training is embedded within a suite of increasingly complex auditory working memory and verbal learning exercises that are delivered with a frequent reward schedule and an individually adaptive, repetitive practice schedule. This approach capitalizes on the fact that the neural responses to repetitive practice appear to be normal in schizophrenia (24) and that implicit, or procedural, learning is engaged through the repetitive practice of continuously adjusting psychophysical exercises (25 – 28) . The basic notion is that by improving the speed and accuracy of information processing in the auditory system, higher-order functions such as verbal encoding and verbal memory retrieval have more reliable signals on which to operate (29) .
We predicted that this approach to cognitive training would produce enhanced verbal memory and global cognition performance in participants with schizophrenia. Our secondary objective was to examine whether subjects’ training-based psychophysical gains on a basic auditory discrimination task would be associated with improvement in their higher-order neuropsychological functions.
Method
Participants
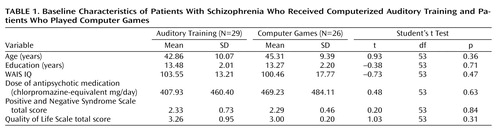
We present data from our first three cohorts of subjects (N=74). A final cohort (N=23) is participating in sequential imaging and is currently under study. Chronically ill volunteer schizophrenia subjects who were clinically stable were recruited from community mental health centers and outpatient clinics. Study announcements were posted at these sites, and subjects self-referred or were referred by their clinicians. After the procedures were explained to them, the participants gave written informed consent and underwent baseline assessments over 2–3 weeks. Fifteen subjects (20%) withdrew during the assessments. The subjects were stratified by age, IQ, gender, and symptom severity and were randomly assigned to either the auditory training condition or a control condition of commercial computer games.
The subjects engaged in the intervention for 1 hour per day, 5 days per week, for an average of 10 weeks. Four subjects (5%) withdrew from the study during the first 4 weeks (three in the training condition, one in the control condition) and declined further participation. Of the 55 remaining subjects, 29 received the active intervention (20 men and nine women) and 26 were in the control condition (20 men and six women). Thirty-nine subjects performed the exercises in the laboratory (training, N=19; control, N=20), while 16 performed them at home (training, N=10; control, N=6). Performance of the exercises was verified through the software, and home users were monitored by weekly calls from staff members. The subjects received $5 for each day of study participation, $20 for 5 consecutive days of participation, and $50 at the completion of 50 hours (10 weeks). Payment was contingent on study participation and not performance. The total mean training time was 47.9 hours (SD=7.5). The subjects continued to take stable doses of their medications during the study. Baseline characteristics of the subject groups are presented in Table 1 .
Auditory Training Exercises
The auditory training program consists of a set of computerized exercises designed to improve the speed and accuracy of auditory information processing while engaging neuromodulatory systems involved in attention and reward. The rationale is that, in order to understand and remember verbal information, the brain must first generate precise and reliable neurological responses that represent the frequency, the timing, and the complex sequential relationships between speech sounds. These exercises continuously adjust the difficulty level to user performance to maintain an approximately 85% rate of correct responses. Trials with correct responses are rewarded with points and animations. The exercises contain stimulus sets spanning the acoustic organization of speech. During the initial stages of training in all exercises, auditory stimuli are processed to exaggerate the rapid temporal transitions within the sound stimuli by increasing their amplitude and stretching them in time. The goal of the processing is to increase the effectiveness with which these stimuli engage and drive plastic changes in brain auditory systems in which individuals with schizophrenia exhibit relatively poor temporal responses (30) . This exaggeration is gradually removed so that by the end of training, all auditory stimuli have temporal characteristics representative of real-world rapid speech. In each training session, a participant works with four of six exercises for 15 minutes per exercise. Compliance is monitored by electronic data upload following each training session.
Exercise 1 requires users to make gradually more difficult distinctions between frequency modulation (FM) “sweeps” of auditory stimuli increasing or decreasing in frequency ( Figure 1 ), as the sweeps become progressively faster and are separated by shorter interstimulus intervals. Exercise 2 trains the subjects to distinguish, with increasing accuracy and speed, between two difficult-to-distinguish phonemes or open syllables (syllables ending in a long vowel), using synthesized speech. Exercises 3 and 4 focus on the accurate identification of increasingly long arrays of open and closed syllables in spatial and sequential contexts. These first four exercises train the user to become more efficient in the processing of basic auditory information, and they also heavily engage working memory. Exercise 5 engages both working memory and verbal learning as the user listens to a sequence of verbal instructions and carries them out. In exercise 6, brief conversational narrative is presented, and the user must remember increasingly more elusive details.

a The learner must perform a time-order judgment task and identify each of two successive frequency modulation sweeps as either “up” (sweep on left) or “down” (sweep on right). Sweep duration and interstimulus interval are modified parametrically as the learner’s performance improves.
Control Condition
The computer games condition was designed to control for the effects of computer exposure, contact with research personnel, and monetary payments. This “placebo” was also selected to control for the nonspecific engagement of attentional systems, executive functions, and motivation through the reinforcement of graphics-based computer games. The control subjects rotated through a series of 16 different enjoyable commercially available games (e.g., visuospatial puzzle games, clue-gathering mystery games, pinball-style games) for the same number of hours as the subjects who received the training program. They played four or five games on any given day and were monitored by staff in the same manner as the subjects in the training condition. The subjects rated the two conditions as equally enjoyable on the 7-item interest/enjoyment subscale of the Intrinsic Motivation Inventory (31 , 32) .
Assessment Materials
The Positive and Negative Syndrome Scale (PANSS) (33) , Quality of Life Scale—Abbreviated Version (34) , and all measures recommended by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) committee (35) , with the exception of the mazes subtest of the Neuropsychological Assessment Battery, were administered at baseline and after training. The Tower of London from the Brief Assessment of Cognition in Schizophrenia (36) was substituted for the mazes test to assess problem solving. At the time of study initiation, the MATRICS Consensus Cognitive Battery was not yet available, but the MATRICS-recommended instruments were available and served as our primary outcome measures. The instruments were obtained from test publishers, and the raw scores were converted to z-scores by using the normative data, stratified by age, published by the test authors. Because of software difficulties, data from the Continuous Performance Test—Identical Pairs were not interpretable. All primary outcome measures were distinct and independent from the tasks practiced during training, and the assessment personnel were blind to the subjects’ group assignments. Alternate forms of tests were administered and counterbalanced at baseline and posttraining for tests sensitive to practice effects, i.e., the Hopkins Verbal Learning Test—Revised, Brief Visuospatial Memory Test—Revised, Tower of London, and Brief Assessment of Cognition in Schizophrenia.
Monitoring Progress in Auditory Training
As a positive control for task learning, we monitored the subjects’ ability to progress in basic psychophysical performance, on the basis of their performance on exercise 1, which targets time and frequency resolution by using rapidly presented FM sweeps in a time-order judgment task. In this exercise, the subject identifies each of two successive sweeps (separated by an interstimulus interval) as either “up” or “down” ( Figure 1 ). The exercise contains 15 stimulus sets composed of combinations of base frequency (500 Hz, 1000 Hz, and 2000 Hz) and duration (from 80 to 35 msec); subjects must first complete stimulus sets with longer-duration stimuli by demonstrating sustained successful performance at short interstimulus intervals (i.e., 20 msec) before stimulus sets with shorter-duration stimuli are made available.
We monitored each subject’s auditory training gains in exercise 1 by recording the number of stimulus sets (measured as a percent of all available stimulus sets) that were completed in exercise 1 over the course of training. A higher progression score signifies that the subject reliably advanced through more of the stimuli content of the exercise (i.e., became proficient on trials with progressively shorter FM sweep durations); a lower progression score signifies that the subject remained at the easiest training levels (long stimulus durations) without advancing to more difficult levels. Progression by this metric is conceptually unrelated to hours of training in that two subjects could train for an identical number of hours and yet have very different progression scores if one subject was able to advance through the most challenging stimulus sets while the other reached a plateau at the easiest initial levels of training.
Planned Analyses
To determine whether training site had a differential effect on treatment response, a repeated-measures analysis of variance (ANOVA) was conducted with the composite global cognition score at baseline and after training entered as the repeated measure and condition (training or control) and site (laboratory or home) entered as between-subjects factors. Repeated-measures ANOVAs were used to compare the subject groups on the change in the PANSS symptom rating, change in Quality of Life Scale rating, and change in cognitive measures. The planned analyses focused on the following cognitive domains: 1) speed of processing (symbol coding, category fluency—animal naming, Trail Making test part A), 2) verbal working memory (letter-number span), 3) verbal learning (trials 1–3 of the Hopkins Verbal Learning Test—Revised), 4) verbal memory (delayed recall, Hopkins Verbal Learning Test—Revised), 5) nonverbal working memory (spatial span), 6) visual learning (trials 1–3 of the Brief Visuospatial Memory Test—Revised), 7) visual memory (delayed recall, Brief Visuospatial Memory Test—Revised), 8) problem solving (Tower of London, Brief Assessment of Cognition in Schizophrenia), and 9) social cognition (managing emotions, Mayer-Salovey-Caruso Emotional Intelligence Test). The global cognition score was calculated as the average z-score across all measures.
A one-sample t test was used to examine whether the schizophrenia subjects made significant gains in psychophysical performance on exercise 1, which was determined by using their progression score. Pearson’s bivariate correlations (two-tailed tests of significance) were used to test the secondary hypothesis that the subjects’ ability to make training-induced psychophysical gains in exercise 1 (the progression score) would be associated with improvement in MATRICS outcome measures.
Results
Clinical and Cognitive Outcomes
The effect of training site (laboratory or home) was nonsignificant (F=1.90, df=1, 51, p=0.17), as was the interaction of site and condition (F=0.29, df=1, 51, p=0.59).
At study entry, there were no significant differences between the two subject groups on the PANSS and Quality of Life Scale global ratings ( Table 1 ) or on subscale ratings. Repeated-measures ANOVA for these scores revealed no significant interaction of condition (training or control) and time (baseline or posttraining) and no significant main effect of condition or time.
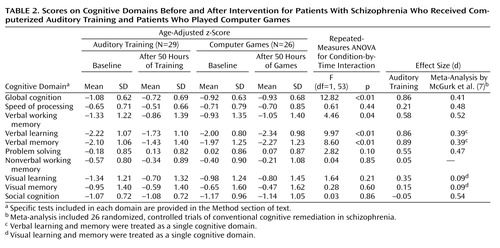
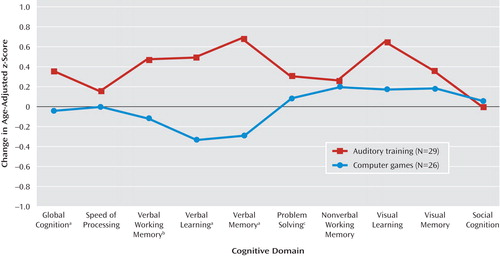
At study entry, there were no significant differences between the two subject groups on baseline measures of cognitive performance ( Table 2 and online data supplement). Repeated-measures ANOVA revealed a significant condition-by-time interaction: when compared to the control subjects, those who received training showed significant improvements from baseline to posttraining in verbal working memory, verbal learning, verbal memory, and global cognition, with nonsignificant improvement in problem solving ( Table 2 , Figure 2 ). There was no significant condition-by-time interaction on the measures of speed of processing, nonverbal working memory, visual learning, visual memory, or social cognition. The main effect of condition was nonsignificant, while the main effect of time was significant for nonverbal working memory (F=4.85, df=1, 53, p=0.03), visual learning (F=5.00, df=1, 53, p=0.03), problem solving (F=5.35, df=1, 53, p=0.03), and global cognition (F=12.01, df=1, 53, p=0.001).
a Significant difference between groups (p<0.01, repeated-measures ANOVA).
b Significant difference between groups (p<0.05, repeated-measures ANOVA).
c Nonsignificant difference between groups (p=0.10, repeated-measures ANOVA).
Relation of Auditory Training Progress to Cognitive Improvement
The subjects who received auditory training engaged in exercise 1 for an average of 11.2 hours (SD=2.4) on 6,200 trials. By the end of training, they had completed an average of 60% of the stimuli content (SD=30%, range=5%–100%) (t=10.4, df=28, p<0.001). These data indicate that the subjects with schizophrenia were able, on average, to make significant gains in this time-order auditory discrimination task, progressing from a performance level where FM sweeps were initially presented for 80 msec at a 600-msec interstimulus interval to successful performance on trials with FM sweeps of 40-msec duration separated by a 70-msec interstimulus interval. However, the large range observed in the progression score indicates that, on an individual basis, schizophrenia subjects showed a wide variability in their ability to make reliable gains in this basic psychophysical auditory training exercise.
We examined the association between progression through exercise content and neurocognitive improvement within this group (N=29) and found that the progression score showed a significant positive correlation with the z-score changes in verbal working memory (r=0.46, p<0.03) and global cognition (r=0.39, p<0.04). This finding indicates that the subjects who were able to make the most progress through the basic psychophysical auditory exercise also showed the most improvement in higher-order measures of verbal working memory and global cognition.
Effect Sizes
As shown in Table 2 , strong positive effects on verbal cognition measures were found for the training condition, but with no difference between conditions in effect on visual cognition, indicating the targeted nature of the training approach. For comparison purposes only, effect sizes from a recent meta-analysis of existing cognitive remediation approaches (7) are also shown in Table 2 .
Discussion
In this study, we used a carefully controlled experimental design to test an innovative approach to cognitive training in schizophrenia, delivered as a stand-alone computer-based treatment for 50 hours over 10 weeks. We obtained significant treatment effects in MATRICS-based measures of verbal working memory, verbal learning, and verbal memory, with an overall large effect size of 0.86 in global cognition. In the comprehensive meta-analysis of cognitive remediation trials in schizophrenia by McGurk et al. (7) , only six out of 29 studies showed effect sizes comparable to ours (>0.75), but four of them used 10 or fewer subjects per treatment group, the fifth studied inpatients with a “treatment as usual” comparison condition, and the sixth used a one-on-one therapist-guided approach. Because of these methodological differences, our results are not directly comparable to those in prior studies.
Several factors may have contributed to the enhanced response we obtained using this method. First, prior cognitive remediation approaches have not specifically targeted impaired perceptual processes, although a growing body of research has identified a number of early sensory deficits in schizophrenia and has related them to higher-order cognitive impairments (17 , 19 – 22 , 37) . Second, as would be expected from a program that aims to improve psychophysical responses, the exercises harness the mechanisms of repetitive practice and procedural learning, which appear to be relatively intact in schizophrenia (24 – 28) . Third, the exercises are delivered in an attentionally engaging and continuously adaptive manner. This preserves an 85% reward schedule while providing a sufficient number of error trials to ensure the neurological reliability of the desired response (23 , 29) . Finally, each exercise is practiced for many thousands of trials.
Indeed, consistent with our study, the meta-analysis by McGurk et al. demonstrated that a higher number of hours of training and drill-and-practice methods show a distinct positive relationship to the remediation of verbal learning and memory in schizophrenia (7) . The large effect sizes obtained in the present study—not only in verbal learning and memory, but also in global cognition—may be due in part to the number of hours of the intervention. It is interesting that only two of the six exercises (or approximately 16 hours) were devoted to tasks that involved word stimuli or had an explicit learning/memory component; the majority (over 30 hours) focused on psychophysical processing of speech elements and on working memory load. At this point, we do not know whether the psychophysical components of the training program are critical or whether similar cognitive improvement could be obtained with sufficiently heavy dosing of simple computerized verbal working memory and verbal learning exercises.
Pursuant to this question, it is important to note that on the most basic exercise, requiring time and frequency resolution of rapidly presented FM sweeps, there was a wide range of progression at the individual level, from subjects who made essentially no performance gains on this exercise to those who became proficient with highly challenging stimuli. This finding indicates that although there was significant improvement on this exercise at a group level, individuals with schizophrenia vary widely in their ability to reliably increase their basic psychophysical auditory processing efficiency in response to training. Consistent with the “neuroplasticity-based” rationale of our approach, subjects who showed the largest training-induced gains in psychophysical performance showed the most improvement in verbal working memory and global cognition. We tentatively speculate that there was a significant relationship specifically between psychophysical gains and working memory because as the auditory cortex responds to the psychophysical training, a more salient verbal signal is “fed forward” into working memory operations; this then permits more efficient and accurate encoding of the verbal information, secondarily leading to improvement in long-term memory. Indeed, the basic neuroscience suggests that increased efficiency in lower-order auditory processes will generalize to higher-order cognitions owing to a more effective engagement of attentional and memory processes (29 , 38) . However, the hypothesized neural mechanisms underlying these behavioral observations require direct study, as is currently under way in our laboratory.
We observed no effect of the auditory training exercises on symptoms, but the subjects in this study were clinically stable with mild average PANSS symptom ratings. We also found no benefits for quality of life immediately after training, but patients who have been, on average, ill for 20 years may not show improvements in community functioning as a result of 10 weeks of computerized cognitive training. In a follow-up study of 22 subjects from this study, some of whom received an additional 50 hours of training, we found that many of the neurocognitive gains induced by the training endured 6 months after the intervention; we also found that neurocognitive gains showed a significant positive correlation with improved quality of life at the 6-month assessment point (39) .
The limitations of this study include our modest number of subjects, their relatively high level of education, and their recruitment through self-referral or clinician referral, which limit the generalizability of our results and likely inflate our effect sizes. Further, we did not conduct an intent-to-treat analysis, although our attrition rate was low, probably because of our subject payment schedule (which may also have produced outcomes different from those in previous studies). Because of these factors, we do not know whether this intervention can be successfully adapted to real-world treatment settings and whether participants can adhere to the demanding training schedule required by this approach outside of a controlled laboratory environment.
We are encouraged by these promising initial results using a “restorative” neuroplasticity-based cognitive training method in schizophrenia, although further research is required to replicate these findings in larger, more clinically representative samples of patients and to investigate the neural processes that underlie the response to training. It will also be important to investigate the utility of this cognitive enhancement approach in patients who are in the earliest phases of schizophrenia, in addition to those who are chronically ill.
1. Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA: Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry 2007; 64:633–647Google Scholar
2. Goff DC, Lamberti JS, Leon AC, Green MF, Miller AL, Patel J, Manschreck T, Freudenreich O, Johnson SA: A placebo-controlled add-on trial of the ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacology 2008; 33:465–472Google Scholar
3. Keefe RS, Malhotra AK, Meltzer HY, Kane JM, Buchanan RW, Murthy A, Sovel M, Li C, Goldman R: Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 2008; 33:1217–1228Google Scholar
4. Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, McMahon RP: Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry 2008; 165:82–89Google Scholar
5. Chouinard S, Stip E, Poulin J, Melun JP, Godbout R, Guillem F, Cohen H: Rivastigmine treatment as an add-on to antipsychotics in patients with schizophrenia and cognitive deficits. Curr Med Res Opin 2007; 23:575–583Google Scholar
6. Twamley EW, Jeste DV, Bellack AS: A review of cognitive training in schizophrenia. Schizophr Bull 2003; 29:359–382Google Scholar
7. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT: A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry 2007; 164:1791–1802Google Scholar
8. Heinrichs RW, Zakzanis KK: Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Google Scholar
9. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
10. Green MF, Kern RS, Braff DL, Mintz J: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000; 26:119–136Google Scholar
11. Evans JD, Bond GR, Meyer PS, Kim HW, Lysaker PH, Gibson PJ, Tunis S: Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophr Res 2004; 70:331–342Google Scholar
12. Brekke JS, Long JD, Nesbitt N, Sobel E: The impact of service characteristics on functional outcomes from community support programs for persons with schizophrenia: a growth curve analysis. J Consult Clin Psychol 1997; 65:464–475Google Scholar
13. Bell MD, Bryson G: Work rehabilitation in schizophrenia: does cognitive impairment limit improvement? Schizophr Bull 2001; 27:269–279Google Scholar
14. McGurk SR, Mueser KT: Cognitive functioning, symptoms, and work in supported employment: a review and heuristic model. Schizophr Res 2004; 70:147–173Google Scholar
15. Weiss AP, Heckers S: Neuroimaging of declarative memory in schizophrenia. Scand J Psychol 2001; 42:239–250Google Scholar
16. Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE: Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 2004; 161:1004–1015Google Scholar
17. Kasai K, Nakagome K, Itoh K, Koshida I, Hata A, Iwanami A, Fukuda M, Kato N: Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: a high-resolution event-related potential study. Am J Psychiatry 2002; 159:546–553Google Scholar
18. Light GA, Swerdlow NR, Braff DL: Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci 2007; 19:1624–1632Google Scholar
19. Kawakubo Y, Kasai K, Kudo N, Rogers MA, Nakagome K, Itoh K, Kato N: Phonetic mismatch negativity predicts verbal memory deficits in schizophrenia. Neuroreport 2006; 17:1043–1046Google Scholar
20. Leitman DI, Hoptman MJ, Foxe JJ, Saccente E, Wylie GR, Nierenberg J, Jalbrzikowski M, Lim KO, Javitt DC: The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. Am J Psychiatry 2007; 164:474–482Google Scholar
21. Javitt DC, Shelley A, Ritter W: Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol 2000; 111:1733–1737Google Scholar
22. Light GA, Braff DL: Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry 2005; 62:127–136Google Scholar
23. Merzenich MM: Cortical plasticity: from synapse to maps. Annual Rev Neurosci 1998; 21:149–186Google Scholar
24. Koch K, Wagner G, Nenadic I, Schachtzabel C, Roebel M, Schultz C, Axer M, Reichenbach JR, Sauer H, Schlosser RG: Temporal modeling demonstrates preserved overlearning processes in schizophrenia: an fMRI study. Neuroscience 2007; 146:1474–1483Google Scholar
25. Kern RS, Green MF, Wallace CJ: Declarative and procedural learning in schizophrenia: a test of the integrity of divergent memory systems. Cogn Neuropsychiatry 1997; 2:39–50Google Scholar
26. Wexler BE, Hawkins KA, Rounsaville B, Anderson M, Sernyak MJ, Green MF: Normal neurocognitive performance after extended practice in patients with schizophrenia. Schizophr Res 1997; 26:173–180Google Scholar
27. Wexler BE, Anderson M, Fulbright RK, Gore JC: Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry 2000; 157:1694–1697Google Scholar
28. Danion J-M, Meulemans T, Kauffmann-Muller F, Vermaat H: Intact implicit learning in schizophrenia. Am J Psychiatry 2001; 158:944–948Google Scholar
29. Mahncke HW, Bronstone A, Merzenich MM: Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res 2006; 157:81–109Google Scholar
30. Kallstrand J, Montnemery P, Nielzen S, Olsson O: Auditory masking experiments in schizophrenia. Psychiatry Res 2002; 113:115–125Google Scholar
31. Deci EL, Eghrari H, Patrick BC, Leone DR: Facilitating internalization: the self-determination theory perspective. J Pers 1994; 62:119–142Google Scholar
32. Ryan RM, Koestner R, Deci EL: Ego-involved persistence: when free-choice behavior is not intrinsically motivated. Motiv Emot 1991; 15:185–205Google Scholar
33. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Google Scholar
34. Bilker WB, Brensinger C, Kurtz MM, Kohler C, Gur RC, Siegel SJ, Gur RE: Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology 2003; 28:773–777Google Scholar
35. Nuechterlein KH, Green MF: MATRICS Consensus Cognitive Battery Manual. Los Angeles, MATRICS Assessment, 2006Google Scholar
36. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L: The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004; 68:283–297Google Scholar
37. Wible CG, Kubicki M, Yoo S-S, Kacher DF, Salisbury DF, Anderson MC, Shenton ME, Hirayasu Y, Kikinis R, Jolesz FA, McCarley RW: A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. Am J Psychiatry 2001; 158:938–943Google Scholar
38. Merzenich MM: Cortical plasticity contributing to child development, in Mechanisms in Cognitive Development. Edited by McClelland J, Siegler R. Rahway, NJ, Lawrence Erlbaum, 2001, pp 67–96Google Scholar
39. Fisher M, Holland C, Subramaniam K, Vinogradov S: Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull, March 5, 2009, Epub ahead of printGoogle Scholar