Longitudinal Trajectory of the Link Between Ventral Striatum and Depression in Adolescence
Abstract
Objective:
Research in adolescent depression has found aberrant intrinsic functional connectivity (iFC) among the ventral striatum (VS) and several brain regions implicated in reward processing. The present study probes this question by taking advantage of the availability of data from a large youth cohort, the IMAGEN Consortium.
Methods:
iFC data from 303 adolescents (48% of them female) were used to examine associations of VS connectivity at baseline (at age 14) with depressive disorders at baseline and at 2-year (N=250) and 4-year (N=219) follow-ups. Eleven regions of interest, key nodes of the reward system, were used to probe the reward network and calculate the connectivity strength of the VS within this network (VS connectivityrw). The main analyses assessed associations of VS connectivityrw with depressive disorders, anhedonia, and low mood using logistic regression. Autoregressive models accounting for carryover effects over time were conducted to further evaluate these brain-behavior associations.
Results:
Higher right VS connectivityrw was associated with higher probability of depressive disorders at baseline (odds ratio=2.65, 95% CI=1.40, 5.05). This finding was confirmed in the autoregressive model, adjusting for carryover effects of the depressive disorders across the three time points. VS connectivityrw was not predictive of depressive disorders at follow-up assessments. Longitudinal associations between VS connectivityrw and anhedonia emerged in the structural equation model: left VS connectivityrw was associated with anhedonia at 2 years (odds ratio=2.20, 95% CI=1.54, 3.14), and right VS connectivityrw was linked to anhedonia at 4 years (odds ratio=1.87, 95% CI=1.09, 3.21). VS connectivityrw did not predict low mood at any time point in the structural equation model.
Conclusions:
The connectivity strength of the VS within the reward network showed distinct patterns of association with depressive disorders and anhedonia from mid to late adolescence, suggesting that the role of this circuitry in depression changes with age. This study replicates, in an independent sample, the association between the VS and depression previously reported in younger adolescents. The findings suggest a role of VS connectivityrw in anhedonia but not in low mood.
Multiple studies have reported dysfunction of the neural network underlying reward processing in depression (1–6). The most common study design is based on task-based functional MRI (fMRI), addressing regional activations (e.g., striatum) in response to reward-based tasks. Relatively few studies have examined intrinsic functional connectivity (iFC) in task-free resting-state fMRI approaches (7–10). Associations between ventral striatum (VS) activation and depression have been fairly consistent across task-based studies, showing blunted striatal responses to rewards in depressed individuals (4). However, VS-iFC associations with depression have been less consistent (7, 8, 11).
Findings of resting-state fMRI in adolescents show both increased and decreased frontostriatal iFC (7, 8, 11). These inconsistent findings may arise from differences between cross-sectional and longitudinal designs. The largest study to date exploring this relationship used cross-sectional data from the Adolescent Brain Cognitive Development cohort (9–10 years old) and found decreased iFC between the VS and the cingulo-opercular network in children with anhedonia, but not in children with low mood (8). However, few studies have explored the role of the centrality of the VS within the reward network in depression. Theoretically, this measure of centrality of a given node within a network, obtained via a graph theory approach, represents the level of functional tightness of a node with the rest of the network. Accordingly, network-based analyses are used to examine proxies of cross-regional integration, which informs paths of communication across critical brain regions and provides maps of the complex dynamic functioning of the brain (12). Here, we explore the centrality of the VS node within the reward network using a network measure called the node strength (VS connectivityrw). Broadly, the VS connectivityrw can be interpreted as the level of contribution of the VS to the function of the reward network, which encompasses reward-related regions such as the ventromedial prefrontal cortex, ventral tegmental area, and anterior cingulate cortex.
In this study, we examined the developmental pattern of brain-behavior (VS-depression) associations during the period that marks the lifetime peak onset of depression, namely, mid to late adolescence (13). The earlier developmental period—late childhood and early adolescence—was recently captured by Pan and colleagues’ longitudinal Brazilian study (7). Using logistic regressions, the study found a positive association between a centrality measure of the VS within the reward network collected in childhood (at ages 6–12) and depression scores collected 3 years later, in early adolescence (at ages 9–15). That study was remarkable for three reasons. It was based on a large pediatric community sample (N=637); it used a single computed measure of VS-iFC connectivity within the reward network; and it provided a unique perspective on a potential role of the VS in early adolescent depression. The striatal measure was the reward-centered connectivity estimate of the VS (VS connectivityrw) (3).
These recent findings provide the backdrop for the present work. They raise three questions. The first is whether to interpret the VS connectivityrw measure as a consequence of depression (following or concurrent with depression) or as a vulnerability factor for depression (preceding depression). Indeed, the VS’s role as a vulnerability factor rather than a consequence of depression was predicated by the absence of association with concurrent depression at baseline (ages 6–12). However, this question could not be reliably tested in the Brazilian study because of the low rate of depression at this early age (around 4%). The second question is whether the observed VS-depression association was specific to the relatively young age of the sample, particularly given the well-demonstrated and substantial nonlinear changes in the dopaminergic system across adolescence (14–17). The third question relates to the specificity of the role of the VS with respect to anhedonia and low mood, the two cardinal symptoms of depression. This last question is motivated by previous findings in adolescence that showed an association between blunted VS and anhedonia, but not low mood (18).
In contrast to the earlier Pan et al. study (7), the present study is focused on mid to late adolescence, ages 14–18, the period most sensitive to the development of depression across the entire lifespan. Whereas in the earlier study, Pan et al. analyzed data from a large community sample of children in Brazil, we accessed data from a European community sample of adolescents from the IMAGEN Consortium (19), which provided resting-state fMRI measures at baseline (age 14) and clinical measures at three time points—at baseline and at the 2-year (age 16) and 4-year (age 18) follow-up assessments. In addition to replicating the same analytical approach (logistic regressions), which we adopted for optimal comparison between studies, we extended the scope of the present work in three ways: 1) measures of anhedonia and low mood were also examined with respect to their associations with the VS; 2) a structural equation modeling analysis was conducted to supplement the logistic regression analyses, allowing us to account for carryover effects across the first to the second to the third wave of clinical data; and 3) VS activation blood-oxygen-level-dependent (BOLD) responses to a reward task (monetary incentive delay task), from IMAGEN data that were previously analyzed (18), were explored in relation to the VS connectivityrw measure.
Based on the previous work reviewed above, two main hypotheses were tested. First, we predicted that increased VS connectivity at baseline (age 14) would be significantly associated with the concurrent presence of depressive disorders. This would support the notion that during youth, VS connectivity is a marker of the expression of depression. Second, we predicted that increased VS connectivity at baseline would also be associated with new depression 2 years and 4 years later, arguing for an additional role of the VS as a vulnerability factor for depression. Two secondary questions were examined. First, regarding anhedonia and low mood, we expected that anhedonia, but not low mood (18), would be associated with VS connectivityrw. Second, we explored the potential relationships between VS activation to reward and VS connectivityrw.
Methods
Sample
The IMAGEN study is a European multisite neuroimaging initiative whose aim is to investigate the neurobiological causes of reinforcement-related behavior and its relationship with psychopathology in adolescence (https://imagen-europe.com) (19). The study enrolled 13- to 15-year-old participants from middle schools across eight European sites. Exclusion criteria consisted of severe medical conditions, treatment for bipolar disorder or schizophrenia, major neurodevelopmental disorders, and birth weight <800 g. The study procedures have been described elsewhere in detail (19). Only five of the eight sites of the IMAGEN consortium collected resting-state fMRI, because this procedure was not funded in the initial European grant. These sites—Dresden, Dublin, London, Mannheim, and Paris—provided scanning data for a total of 381 participants. After image preprocessing and quality control, data from 304 subjects were analyzed, which is the same set from a previous report from the same group (20). Briefly, 77 participants were excluded: 72 for excessive head motion (see criteria below), two for poor spatial normalization, one for corrupted neuroimaging data, and two for missing important sociodemographic data. Data for one additional participant was excluded because of missing data in depression measures, yielding a final sample of 303. Follow-up clinical assessments occurred 2 years and 4 years after baseline, where data were available for 250 (82.5%) and 219 (72.3%) participants, respectively.
Institutional review boards and/or local ethics committees of involved universities and research institutions approved the research protocol. All 14- and 16-year-old participants provided written assent, and their parents provided written consent. Participants age 18 or older provided written informed consent.
Clinical Phenotyping
The IMAGEN Consortium evaluation centers assessed psychiatric symptoms and disorders using a computerized psychiatric interview, the Developmental and Well-Being Assessment (DAWBA) (21). The DAWBA is a self-administered questionnaire consisting of open- and closed-ended questions, which are completed by the participants and their parents.
All participants completed the DAWBA assessment of depression, providing diagnoses as well as rates of and severity scores for anhedonia and low mood. Resting-state fMRI scans were collected at baseline, and the behavioral assessments were collected at baseline and at the 2- and 4-year follow-ups.
The diagnostic status of depressive disorders (dichotomous variable: 1=yes, 0=no) was the primary focus of this study. This measure comprised depressive disorders (five depressive symptoms over the past 4 weeks, including at least one cardinal symptom) and subthreshold depressive disorders (three or four depressive symptoms within the past 4 weeks, including at least one cardinal symptom). This approach was adapted from previous reports on the IMAGEN sample (18).
In addition to our primary focus on depressive disorders, two more variables from the DAWBA were investigated in exploratory analyses: anhedonia and low mood. These variables were rated as present or absent (1 or 0, respectively) and corresponded to the following questions: for anhedonia, “In the last 4 weeks, have there been times when you have lost interest in everything, or nearly everything, that you normally enjoy doing?” and for low mood, “In the last 4 weeks, have there been times when you have been very sad, miserable, unhappy, or tearful?” These items have been shown to have good face validity (18), and they represent distinct facets of depression that may map to unique neural circuits (18, 22, 23). Moreover, for completeness, dimensional measures of anhedonia and low mood were computed based on questions from the DAWBA’s depression section regarding frequency and severity of the reported symptoms. Analyses of these dimensional variables will be mainly presented in the online supplement.
Neuroimaging
Imaging data were acquired on 3-T MRI scanners (Philips in Dublin, GE in London, and Siemens in Paris, Dresden, and Mannheim) (19, 20). Resting-state echo-planar images were acquired with the following sequence parameters: TR=2,200 ms; TE=30 ms; flip angle=75°; and acquisition matrix=64×64×40 with 2.4 mm slice thickness and 1 mm slice gap; therefore, acquisition voxel resolution was 3.4 mm isotropic. A total of 187 volumes were collected over 6.5 minutes of data acquisition. Structural anatomical image voxel size was 1.1×1.1×1.1 mm, following acquisition parameters from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol. Preprocessing followed a stepwise procedure. Data preprocessing was conducted using FreeSurfer, version 5.3, and the AFNI suite (24), using the same approach as that described by Ernst et al. (20). First, we discarded the first four volumes to account for T1 saturation effects. Functional volumes were then slice-time corrected, aligned, and coregistered to each participant’s T1-weighted structural scan. Normalization to the Colin 27 Average Brain standardized template was performed using a nonlinear transformation (AFNI program 3dQwarp). Spatially smoothing with a 6-mm full width at half maximum Gaussian kernel was applied. Finally, the following parameters were regressed from the time series: six head motion parameters and their derivatives, the average time series extracted from the ventricles, and the time series from local white matter within a 25-mm-radius sphere surrounding each voxel, using the ANATICOR approach (25). Functional volumes with a Euclidean norm motion derivative >0.25 mm were censored, and we excluded individuals with >30% of volumes censored from group-level analysis.
After excluding these subjects, the mean number of included volumes was 166.78 (SD=16.68, range=121–183). The mean number of included volumes was compared between outcome groups using independent t tests. Participants with depressive disorders (t=2.87, df=248, p=0.004) and those with anhedonia (t=2.67, df=248, p=0.008) at 2-year follow-up showed a statistically significantly lower number of included scans than the control group. There was no significant association between the mean number of included volumes and low mood at any time point. Therefore, the number of included scans was used in logistic regression models testing depression disorders and anhedonia at 2-year follow-up.
Connectivity network.
The same analytic approach as that used by Pan et al. (7), namely, computation of VS connectivityrw and logistic regressions, was employed here. The overall strategy consisted of three steps: 1) identify the reward network, 2) compute the node connectivity strengths for VS, and 3) conduct correlation analyses between the VS connectivity and measures of depressive disorders. Symptoms of anhedonia and low mood were also examined.
A total of 11 nodes were selected to define the reward network, based on a meta-analysis of reward-based fMRI studies (26). Nodes were defined as 5-mm-radius spheres centered at the regions’ of interest coordinates (Figure 1; see also Table S1 in the online supplement). Descriptive values for all 55 edges from the reward network are reported in Table S2 in the online supplement.
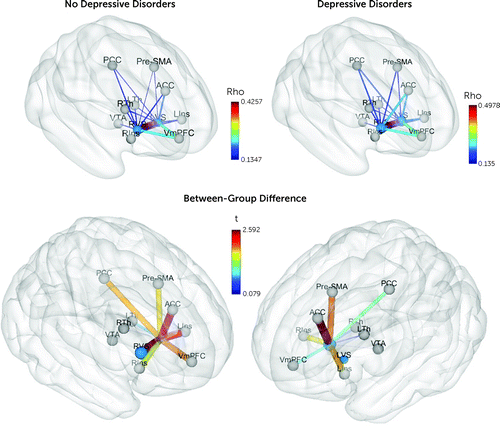
FIGURE 1. Schematic representation of reward network edges and ventral striatum nodes at baselinea
aBrain coordinates for reward network nodes are described in Table S1 in the online supplement. Values of the reward network edges (node-to-node resting-state fMRI time-series Spearman correlation values [rho]) are described in Table S2 in the online supplement. The thickness of the edges and the diameter of the nodes are proportional to their actual values. Colors represent rho values (upper panel) and group difference t values (bottom panel) for depressive disorders compared with no depressive disorders at study baseline, when participants were 14 years old. The t values for each edge are listed in Table S10 in the online supplement. LVS=left ventral striatum; RVS=right ventral striatum; VmPFC=ventromedial prefrontal cortex; LIns=left anterior insula; RIns=right anterior insula; PCC=posterior cingulate cortex; VTA=brainstem-ventral tegmental area; ACC=anterior cingulate cortex; Pre-SMA=pre-supplementary motor area; LTh=left thalamus; RTh=right thalamus.
The VS connectivityrw informs how the VS node contributes to the reward network. All 11 nodes, as part of the reward network, were expected to be significantly connected to one another, yielding a matrix of 55 pairwise connections. In computer networking, these one-to-one connections are called “edges.” The strength of each edge is typically indexed as a Fisher-z-transformed Spearman correlation coefficient, whose statistical significance level is adjusted for multiple comparisons (for a total of 55 edges, Bonferroni-corrected p<0.05/55=0.00091). The node connectivity strength is calculated as the mean of all significant edges (fMRI time-series correlations) of the node of interest (e.g., VS) to all other nodes of the given network (e.g., reward network). It is a weighted measure, since the edge values are continuous variables corresponding to the Spearman correlation coefficients. For instance, the VS connectivityrw equals the sum of the VS edges connecting the left VS node to the other 10 nodes of the reward network that survived to multiple-comparison thresholding. In the present sample, from the total 55 edges of the 11 nodes of the reward network, 47 were found to be significantly different from 0 after Bonferroni adjustment. Furthermore, among edges connected with the VS, the left VS–ventral tegmental area edge did not survive correction for multiple comparisons and therefore was excluded. An analysis was conducted that included this edge to ensure that excluding it was not critical to the results (see the online supplement). The final connectivity measure, VS connectivityrw, was computed as the sum of the absolute value of significant edges (correlation values) (see Table S3 in the online supplement).
The network measure (node strength) uses absolute values, so that positive and negative iFC do not cancel each other when they are summed. Therefore, this measure evaluates the importance of a given node within the entire network—that is, the correlations or anticorrelations between a specific node and all other nodes of the network are considered equally relevant to inform the centrality of that node within the network.
Striatal activation to reward.
Striatal responses to reward were collected using a gradient-echo echo-planar imaging sequence and a modified version of the monetary incentive delay task (27). A total of 300 volumes were acquired per subject, which included 40 slices each (2.4-mm slice thickness, 1-mm gap). (For a detailed description of the brain imaging procedures for task-based fMRI and task parameters, see reference 18.) The monetary incentive delay task included three conditions: receipt of positive outcomes, receipt of negative outcomes, and reward anticipation. We used the reward anticipation condition for the present analyses, since it is a condition with well-replicated findings in youth depressive disorders (4).
Statistical Analysis
The statistical analyses presented below are organized as follows: 1) test of the main hypothesis of VS connectivityrw and depressive disorders, 2) test of secondary hypotheses related to VS connectivityrw association with anhedonia and low mood, 3) exploratory analyses of the contribution and interaction of VS activationrw to the monetary incentive delay task (18) and VS connectivityrw, as a function of depressive disorders, 4) test of the clinical specificity of VS connectivityrw to depression by analyzing anxiety disorders instead of depressive disorders, and 5) test of the neural specificity of the VS centrality of the reward network by analyzing the thalamus as the core node of the reward network.
Associations of right and left VS connectivityrw strength with the three depressive measures (depressive disorders, anhedonia, and low mood) were tested using individual logistic regressions (as did Pan et al. in the earlier study [7]), employing R, version 3.6.1, as well as SPSS, version 22 for Windows. A structural equation modeling analysis was also conducted using Mplus, version 8.7 (28). The Brain Connectivity Toolbox codebase was used to create Figure 1 (12).
Polychoric correlations between depressive measures at each time point determined measurement stability (R package hector). Correlations were corrected for multiple comparisons using the false discovery rate.
VS Connectivityrw Associations With Depressive Disorders, Anhedonia, and Low Mood
Logistic regression models.
Regarding mood disorders, three sets of logistic regression analyses examined separately the associations of the right and left VS connectivityrw (independent variables) with depressive disorders. Hence, each set of analyses tested associations of brain measures collected at age 14, with depression-related scores collected concurrently with the scan and again 2 years and 4 years later. All models were adjusted for gender, age, and study site; these adjustments were considered in Pan et al. in the earlier study (7) and for replicability were also considered here. Models including follow-up outcome data also controlled for baseline depressive disorders. The Bonferroni-corrected p value for this set of regressions was 0.008 (six models: left and right VS connectivityrw at three time points).
Regarding anhedonia and low mood, univariate logistic regression models similar to those for depressive disorders were conducted. Models included gender, age, and site as covariates. Follow-up models also included the homotypic symptom at baseline to adjust for possible spurious associations between brain measures and future psychopathology, which may be modulated by the concurrent occurrence of baseline symptoms and brain network alterations. Baseline models were also adjusted for the dimensional measure of anxiety described below.
Logistic regression analyses were two-tailed, with the significance threshold set at 5%. Bootstrapping for 1,000 samples computed bias-corrected 95% confidence intervals (BC 95% CIs) for all regression models and are reported for significant associations. To adjust for spurious associations related to the high co-occurrence of depressive and anxiety symptoms, we added a dimensional measure of anxiety to the baseline model. This anxiety measure was derived from the DAWBA’s computerized probability level (“DAWBA bands”) that corresponds to symptoms meeting criteria for separation anxiety, specific phobia, social anxiety, panic disorder, agoraphobia, generalized anxiety, and other anxiety (i.e., failing to meet symptomatic or duration criteria, but meeting impairment criteria due to anxiety symptoms) (21).
Structural equation modeling: longitudinal model.
Structural equation modeling analyses were conducted to supplement the univariate logistic regressions described above. Specifically, the “perfect” simplex model (also known as an autoregressive model) was selected (29). This model assumes that the state at one point in time determines the state at the next point in time. Each dichotomous outcome (i.e., depressive disorders, anhedonia, and low mood) was run with a separate simplex model, using the link LOGIT. We adopted the full information maximum likelihood strategy, under an assumption of a missing at random mechanism, which permits use of the full sample (N=303) without imputing the missing values. Gender, head motion, and anxiety symptoms at baseline were included in the models. Finally, the effect of site (i.e., multilevel design) was treated using a cluster strategy, which adjusts the standard errors for the sites via the robust maximum likelihood estimator (30, 31). Three separate models were run, one for each outcome (depressive disorders, anhedonia, low mood). Predictors (VS connectivityrw) were regressed on baseline, 2-year, and 4-year follow-up assessments concomitantly. Effect sizes are reported as odds ratios estimated via a maximum likelihood robust estimator, logit link. A diagram of the depressive disorders structural equation model is depicted in Figure S1 in the online supplement.
Exploratory Analysis: VS Connectivityrw Associations With Striatal Activation to Reward Anticipation
In an exploratory analysis, we took advantage of the richness of the IMAGEN study, which includes data on brain responses to reward (task-based fMRI). Mean striatal response to the monetary incentive delay task (reward anticipation condition) was available for 264 participants (87% of the present sample). We explored a potential interaction of VS connectivityrw and VS activationrw on depressive disorders, using univariate logistic regression models for each time point.
Specificity Analysis: VS Connectivityrw Associations With Any Anxiety Disorder
To further evaluate the clinical specificity to depression, we tested whether the VS connectivity measures were associated with anxiety disorders (outcome; yes/no). This dichotomous variable encompassed the DAWBA’s clinician rating of separation anxiety, specific phobia, social anxiety, panic disorder, agoraphobia, generalized anxiety, and other anxiety (i.e., failing to meet symptomatic or duration criteria, but meeting impairment criteria due to anxiety symptoms). Logistic regression models of anxiety included the same covariates as the depressive disorder models, except that this analysis controlled for depressive disorders at baseline, while anxiety disorders at baseline were controlled for in the depressive disorder analyses.
Specificity Analysis: Thalamic Connectivityrw Associations With Depressive Disorders
We computed a thalamic connectivityrw score to serve as a control for the neural specificity of our finding, that is, VS connectivityrw associations with depressive disorders specific to the ventral striatum and not the result of a nonspecific subcortico-cortical coupling. The strategy to calculate the node strength measure of the left and right thalamic nodes within the reward network (thalamic connectivityrw) followed the same steps taken for VS connectivityrw. Logistic regression models tested whether thalamic connectivityrw was associated with depressive disorders at all time points, using the same covariates as in the VS connectivityrw models.
Results
Sample
Table 1 summarizes the sample’s demographic and clinical characteristics. The IMAGEN sample included 303 participants, of whom 146 (48.2%) were female. Overall, 82.5% (N=250) completed the 2-year follow-up assessment, and 72.3% (N=219) completed the 4-year follow-up assessment. The rate of depressive disorders was 7.9% at baseline, 13.2% at 2-year follow-up, and 9.6% at 4-year follow-up. Baseline estimated mean IQ was 110.25 (SD=13.8).
| Characteristic | Baseline (N=303) | 2-Year Follow-Up (N=250) | 4-Year Follow-Up (N=219) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 14.5 | 0.41 | 16.35 | 0.41 | 18.77 | 0.53 |
| N | % | N | % | N | % | |
| Female | 146 | 48.2 | 124 | 49.6 | 115 | 52.5 |
| Depressive disorders | 24 | 7.9 | 33 | 13.2 | 21 | 9.6 |
| Anhedonia | 39 | 12.9 | 33 | 13.2 | 27 | 12.3 |
| Low mood | 136 | 44.9 | 96 | 38.4 | 78 | 35.6 |
TABLE 1. Demographic and clinical characteristics of participants in the IMAGEN cohorta
Stability of Measures of Depression Across Time
As expected, concurrent rates of anhedonia, low mood, and depressive disorders tended to be correlated with one another across the whole sample (see Tables S4 and S5 in the online supplement). Most of the three measures of depression (depressive disorders, anhedonia, and low mood) exhibited significant correlations between baseline and 2-year follow-up (age 16) scores, and between 2-year follow-up and 4-year follow-up (age 18) scores, respectively. However, only low mood showed a significant correlation between baseline and 4-year follow-up, indicating a distinct stability of this measure over time.
VS Connectivityrw Associations With Depressive Disorders, Anhedonia, and Low Mood
Logistic regression models.
VS connectivityrw was positively associated with baseline depressive disorders for both the left VS (odds ratio=2.13, 95% CI=1.09, 4.15; p=0.027) and the right VS (odds ratio=2.65, 95% CI=1.40, 5.05; p=0.003) (Table 2, Figure 1). Both left (b=0.755, BC 95% CI=0.095, 1.542) and right (b=0.977, BC 95% CI=0.306, 1.967) VS connectivityrw associations with baseline depression were confirmed using 1,000-sample bootstrapping. Right VS connectivityrw remained significant after Bonferroni correction for multiple testing (p<0.008). Depressive disorders at the 2-year and 4-year follow-ups were not significantly associated with baseline VS connectivityrw (Table 2).
| Baseline | 2-Year Follow-Up | 4-Year Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p |
| Depressive disorders–VS connectivityrw associations | |||||||||
| Left VS | 2.13 | 1.09, 4.15 | 0.027 | 1.07 | 0.56, 2.02 | 0.842 | n.s. | ||
| Right VS | 2.65 | 1.40, 5.05 | 0.003 | 1.11 | 0.63, 1.95 | 0.715 | n.s. | ||
| Anhedonia–VS connectivityrw associations | |||||||||
| Left VS | 1.78 | 1.06, 3.01 | 0.030 | 1.56 | 0.87, 2.81 | 0.140 | n.s. | ||
| Right VS | 1.55 | 0.95, 2.54 | 0.080 | 1.12 | 0.65, 1.93 | 0.685 | n.s. | ||
| Low mood–VS connectivityrw associations | |||||||||
| Left VS | 1.05 | 0.70, 1.56 | 0.826 | 1.02 | 0.63, 1.64 | 0.947 | 0.55 | 0.31, 0.96 | 0.035 |
| Right VS | 1.42 | 0.98, 2.06 | 0.062 | 1.07 | 0.71, 1.62 | 0.751 | 0.70 | 0.44, 1.12 | 0.140 |
TABLE 2. Ventral striatum (VS) intrinsic functional connectivity within the reward network and depressive disorders in the IMAGEN cohorta
Left VS connectivityrw was associated with anhedonia at baseline (odds ratio=1.78, 95% CI=1.06, 3.01; p=0.030). This association was confirmed using the bootstrapping validation technique (b=0.578, BC 95% CI=0.013, 1.211). No other longitudinal significant associations emerged with left or right VS connectivityrw scores (Table 2). In other words, VS centrality within the reward system was associated with concurrent anhedonia but did not predict later anhedonia. Baseline low mood was not associated with left or right VS connectivityrw. In longitudinal analyses, left VS connectivityrw at age 14 was inversely associated with low mood at age 18 (odds ratio=0.55, 95% CI=0.31, 0.96; p=0.035) (b=−0.604, BC 95% CI=−1.175, −0.181). No other significant associations emerged with left or right VS connectivityrw (Table 1). Therefore, a stronger VS connectivityrw predicted lower probability of low mood 4 years later but was not associated with this symptom concurrently or 2 years later.
Structural equation modeling analyses: longitudinal model.
Longitudinal structural equation models for depressive disorders provided the same results as those obtained using logistic regression models, showing that presence of depressive disorders at baseline was significantly associated with right VS connectivityrw (odds ratio=2.36, 95% CI=2.14, 2.61; p<0.001) (Figure 2). Interestingly, significant longitudinal associations between VS connectivityrw and anhedonia emerged in the structural equation modeling analysis. Anhedonia at 2-year follow-up was associated with left VS connectivityrw (odds ratio=2.20, 95% CI=1.54, 3.14; p<0.001), and anhedonia at 4-year follow-up was associated with right VS connectivityrw (odds ratio=1.87, 95% CI=1.09, 3.21; p=0.023). There were no significant associations between VS connectivityrw and low mood in the structural equation modeling analysis (see Figure S2 in the online supplement).
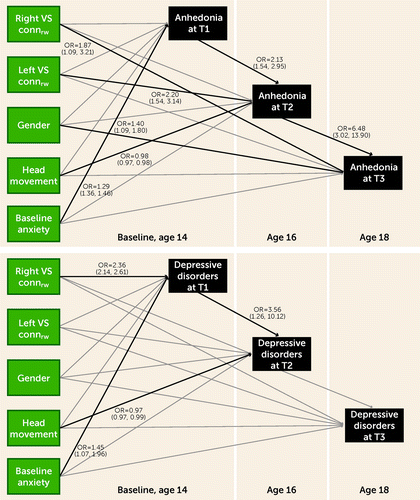
FIGURE 2. Ventral striatum (VS) connectivity associations with depressive disorders and anhedonia using structural equation modelinga
aBlack lines are significant at the p<0.05 level, and odds ratios and 95% confidence intervals are shown. VS connrw=ventral striatum connectivityrw (i.e., level of contribution of the VS to the function of the reward network).
Exploratory Analysis With Striatal Activation to Reward Anticipation
We exploited previously published data from this sample on reward-related striatal activation (VS activationrw) using the monetary incentive delay task (18). We explored whether an interaction between VS connectivityrw and VS activationrw predicted depressive disorders at baseline and 2- and 4-year follow-ups. The VS activationrw variable and the interaction were added to the logistic regression models conducted to test our main hypotheses (see the Methods section).
A significant left VS connectivityrw-by-VS activationrw interaction predicted depressive disorders at 2-year follow-up (p=0.03) (see Figures S3 and S4 in the online supplement). This interaction as a function of depressive disorders at age 16 indicated that VS activationrw was positively associated with VS connectivityrw in depressed youths, but this association was negative in the nondepressed.
Specificity and Exploratory Analyses: Anxiety Disorders, Thalamic Connectivityrw, Dimensional Measures of Anhedonia, and Low Mood
We used presence of anxiety disorders (yes/no) as the outcome of the univariate logistic regression approach to investigate the specificity of our findings to depressive disorders. The results revealed that neither left nor right VS connectivityrw was associated with any anxiety disorder at any time point (see Table S6 in the online supplement).
We used the thalamus node of the reward network to assess the specificity of the association of the VS node with depressive disorders. Using the logistic regression approach, in which depressive disorders was the outcome, we found that thalamus connectivityrw was not significantly linked to depressive disorders (see Table S7 in the online supplement).
The stability of dimensional measures of anhedonia and low mood was similar to results from categorical measures of these outcomes: only low mood at baseline showed a statistically significant association with low mood at 4-year follow-up (see Table S8 in the online supplement). In longitudinal brain-behavior analyses with continuous outcome variables, there was a positive correlation between baseline left VS connectivityrw and the dimensional measure of anhedonia at age 16 (see Table S9 in the online supplement).
Discussion
Multiple studies report a link between depression and striatal function, particularly in adolescence (4), and a recent longitudinal neuroimaging study, conducted in a large pediatric community sample in Brazil (7, 32), provided support for a striatal-depression link in early adolescence. That study showed that VS function, which was characterized in 9-year-old children, was linked to depressive disorders when these children reached 12 years of age. The same clinical assessment and neuroimaging design used in the Brazilian study was employed in the present European IMAGEN study. Notably, we implemented the same measure of striatal connectivity of the reward network (VS connectivityrw). However, a critical difference between samples was the age of the cohorts at baseline—9 years old for the Brazilian cohort and 14 years old for the IMAGEN cohort. This age difference was seen as a unique opportunity to complement and extend findings in children to adolescents and young adults. In addition, we extended previous analyses by modeling three time points in a structural equation simplex model. Structural equation modeling has several advantages over logistic regressions: it accounts for carryover effects between the repeated measures, allows processing of the full sample by full information maximum likelihood for missingness, and integrates all covariates in the same model. With this latter feature, running different and multiple models can be avoided, optimizing robustness and minimizing bias in the estimates and their respective standard errors.
Following in the footsteps of the Brazilian study, three main questions were addressed. The first was whether associations between VS connectivityrw and depressive symptoms continue to be present in 14-year-old adolescents. The question of a concurrent brain-behavior link could not be validly assessed in the original younger Brazilian sample because of the low prevalence of depressive disorders at their first assessment (4.2%). The second question was whether VS connectivityrw would be related to the emergence of depressive disorders 2 and 4 years later, when adolescents reached 16 and 18 years of age, respectively. And the third question was whether the associations of VS connectivityrw extended to more specific measures of depression, including the two cardinal symptoms of depression, anhedonia and low mood. The discussion will first integrate the present findings with our previous Brazilian work (7).
The present analyses revealed significant associations between VS connectivityrw and concurrent rate of depressive disorders at age 14. The earlier study showed a link between VS connectivityrw at age 9 with later depressive disorders at age 12. Taken together, these findings suggest that greater functional connectivity of the VS within the reward network might represent both a vulnerability factor (associated with later depression) and a marker of depression (associated with concurrent depression at age 14) in youths. The absence of an association between the VS measure at age 9 with concurrent depression could have been expected given the low rate of depressive disorders at this young age. However, the presence of a VS-depression association when children reached age 12 suggests that the VS already exhibited an activity pattern, at rest, that could predispose youths to depression. The present data add the possibility that the same pattern of VS connectivity continues to confer vulnerability for depression later, at age 14. Of note, in the IMAGEN cohort, only the right VS connectivityrw showed this association with depressive disorders after correction for multiple comparisons, whereas in the Brazilian cohort, only the left VS connectivityrw was significantly associated with depressive disorders. It is too early to speculate on a laterality effect across development, but this could become an important question for future work. In our data, the mean Spearman correlation (rho) between right and left VS node BOLD signal was 0.43 (SD=0.220), revealing a large amount of unshared covariance, suggesting functional differences as a function of laterality.
Of the two cardinal symptoms of depression, only anhedonia showed the same pattern of association with left VS connectivity as that shown with depressive disorders. This result is in line with recent cross-sectional work denoting specificity between anhedonia and aberrant ventral striatal intrinsic functional connectivity (8). Associations between VS connectivityrw and low mood were only significant with the clinical measures collected at age 18, and they were in the opposite direction: higher VS connectivityrw was associated with lower rates of low mood. This finding was not confirmed in the structural equation longitudinal model. Therefore, it is difficult to reconcile these different findings, except for proposing a speculative change in the nature of depression with age, or a change in striatal function with age, or, more likely, both. A recent study (33) reported distinct depressive symptom profiles in adolescents as compared to adults, where adolescents reported more energy loss and insomnia, and adults reported increased rates of anhedonia and concentration problems. As a note of caution, the present findings are only exploratory. Together with the paucity of the literature on these variables, particularly low mood, the present data call for developmental work, at both the behavioral and neural levels, on the role of low mood and anhedonia in the development and maintenance of depressive disorders. In sum, the present data suggest that the pattern of connectivity of the VS within the reward network could precede low mood in adolescents.
The most parsimonious interpretation of our findings is that striatal connectivity preferentially modulates anhedonia—a motivation deficit—in depressive disorders. For anhedonia, logistic regression models and structural equation longitudinal models exhibited complementary results. In logistic regression models, baseline anhedonia was associated with left VS connectivityrw, whereas structural equation longitudinal models revealed significant associations with anhedonia at the 2- and 4-year follow-ups. Therefore, anhedonia structural equation modeling showed longitudinal associations that were not found following the same procedure in previous work with younger subjects from the earlier Pan et al. study (7). As suggested above, the nature of this relationship may change with age. This scenario is in line with the reported age-related changes in the characteristics of motivated behaviors in adolescents. Indeed, these changes are understood to reflect, in part, ontogenetic modifications within the dopamine system (17), in interaction with prefrontal cortical maturation and changes in environment. Broadly, a peak in risk-taking behavior, mapping to a rise in impulsivity and motivational drive, has been described around 15 years of age in adolescents (34, 35).
Naturally, more specific functional interpretations of the present and previous findings (7) depend on the functional significance of the brain measure, that is, VS connectivityrw strength. This measure of VS connectivity is thought to reflect the degree of integration of the information flow across the reward network. It does not inform on the sensitivity of this network to reward signals per se, since it reflects the state of the network at rest. However, given that this measure encapsulates the cohesiveness of spontaneous BOLD signal across the reward network, it might inform on the degree of stability or flexibility of neural activity. A stronger level of coherence (connectivity) might suggest reduced flexibility of the network in responding to external rewarding stimuli. In our exploratory analyses, VS connectivityrw and VS activationrw to a reward task (data analyzed in a previous study by Stringaris et al. [18]) were not significantly correlated, but the interaction between these two measures was associated with depressive disorders at 2-year follow-up. Arguably, this result may add to the argument that both measures—resting-state connectivity and task-based activation—inform distinct but related neurobiological mechanisms of the VS’s role in youth depression.
Further testing of this hypothesis would require a dynamic analysis of the resting-state fMRI data and the acquisition of task-based reward-related fMRI data to examine the relationship between dynamic fluctuations of spontaneous activity and response flexibility of the reward network in larger samples. Future studies could also benefit from exploring distinct neuroimaging modalities or methodological designs, such as including drug challenges. For instance, Hamilton et al. (36) found that striatal dopamine deficits correlate with decreased, rather than increased, cortico-striatal functional connectivity in adult depression. Therefore, the interpretation of VS connectivityrw strength as an index of flexibility of the reward system is still speculative. However, if confirmed by future work, inflexibility could explain the inability of the reward system to properly respond to positive reward stimuli, leading to anhedonia and depression.
As a last point, we wish to highlight the replicability of the reward network as tested in the earlier Pan et al. study (7). We used the 11 nodes identified in this earlier study and replicated the significant internode connectivity that successfully passed the discovery and replication procedure applied in the Brazilian data set. We also replicated the selective role of the left VS connectivityrw (one of the 11 nodes) in depressive disorders.
This work is not without limitations. First, the integration of the present findings with previous work from the Brazilian sample is only tentative given the different time frames and ages. Second, we included subthreshold depression in the depressive disorders group. Previous reports from the same sample have shown structural abnormalities in subthreshold depressive disorder, which were highly consistent with findings from youth major depressive disorder. Even so, future studies should investigate differences and similarities in reward-network connectivity between these two groups. Third, anhedonia assessment relied on a single self-report item, which may have limited our ability to find significant brain-behavior associations at follow-up. We found somewhat distinct results from categorical anhedonia in our exploratory analyses using a dimensional measure of anhedonia, derived from the DAWBA interview, as the outcome (see the Supplemental Results section in the online supplement). Thus, future studies should explore associations between VS node strength within the reward network and further dimensional measures of anhedonia, provided by instruments specifically probing anhedonia, such as the Snaith-Hamilton Pleasure Scale (37). Fourth, follow-up data included only the clinical assessment; follow-up resting-state fMRI data were not available for analysis. This limits any inferences regarding the stability of VS connectivityrw strength measures over time and precludes the testing of brain-symptom associations over time or covariation of changes between these measures. Therefore, we are not able to conclude whether VS connectivity represents a trait-like marker or a state-like related variable. Fifth, the clinical assessment did not provide enough data (in addition to the younger ages of the participants) to reliably distinguish depressive disorders from depressive episodes. Finally, there was a substantial proportion of exclusions due to excessive head motion, which reflected the stringency of our criteria to avoid spurious associations.
In conclusion, this work underscores the importance of the reward network in the development of depression in childhood through adolescence, suggesting an evolution of its role in the core symptoms of anhedonia and low mood across development.
1 : Reward processing dysfunction in major depression, bipolar disorder, and schizophrenia. Curr Opin Psychiatry 2015; 28:7–12Crossref, Medline, Google Scholar
2 : Research review: altered reward function in adolescent depression: what, when, and how? J Child Psychol Psychiatry Allied Discip 2012; 53:3–15Crossref, Medline, Google Scholar
3 : Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology 2015; 40:2258–2268Crossref, Medline, Google Scholar
4 : Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry 2018; 175:1111–1120Link, Google Scholar
5 : Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry 2019; 9:293Crossref, Medline, Google Scholar
6 : The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 2013; 151:531–539Crossref, Medline, Google Scholar
7 : Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. Am J Psychiatry 2017; 174:1112–1119Link, Google Scholar
8 : Association between childhood anhedonia and alterations in large-scale resting-state networks and task-evoked activation. JAMA Psychiatry 2019; 76:624–633Crossref, Medline, Google Scholar
9 : Age-normative pathways of striatal connectivity related to clinical symptoms in the general population. Biol Psychiatry 2019; 85:966–976Crossref, Medline, Google Scholar
10 : Research review: brain network connectivity and the heterogeneity of depression in adolescence: a precision mental health perspective. J Child Psychol Psychiatry Allied Discip 2020; 61:1282–1298Crossref, Medline, Google Scholar
11 : Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry 2013; 52:628–641.e13Crossref, Medline, Google Scholar
12 : Complex network measures of brain connectivity: uses and interpretations. NeuroImage 2010; 52:1059–1069Crossref, Medline, Google Scholar
13 : Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 2015; 54:37–44.e2Crossref, Medline, Google Scholar
14 : Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav 2009; 93:199–211Crossref, Medline, Google Scholar
15 : Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology 2002; 27:683–691Crossref, Medline, Google Scholar
16 : Age-related changes in the intrinsic functional connectivity of the human ventral vs dorsal striatum from childhood to middle age. Dev Cogn Neurosci 2015; 11:83–95Crossref, Medline, Google Scholar
17 : Adolescent maturation of cortical dopamine. Neurotox Res 2010; 18:306–312Crossref, Medline, Google Scholar
18 : The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry 2015; 172:1215–1223Link, Google Scholar
19 : The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry 2010; 15:1128–1139Crossref, Medline, Google Scholar
20 : Pubertal maturation and sex effects on the default-mode network connectivity implicated in mood dysregulation. Transl Psychiatry 2019; 9:103Crossref, Medline, Google Scholar
21 : The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41:645–655Crossref, Medline, Google Scholar
22 : Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry 2016; 6:e810Crossref, Medline, Google Scholar
23 : Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev 2016; 65:21–35Crossref, Medline, Google Scholar
24 : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
25 : Mapping sources of correlation in resting state fMRI, with artifact detection and removal. NeuroImage 2010; 52:571–582Crossref, Medline, Google Scholar
26 : The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage 2013; 76:412–427Crossref, Medline, Google Scholar
27 : Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001; 21:RC159Crossref, Medline, Google Scholar
28 : Mplus User’s Guide, 8th ed. Los Angeles, Muthén & Muthén, 2021Google Scholar
29 : A new approach to factor analysis: the radex, in Mathematical Thinking in the Social Sciences. Edited by Lazarsfeld PF. Glencoe, Ill, Free Press, 1954, pp 258–348Google Scholar
30 : Sampling weights in latent variable modeling. Struct Equ Model 2005; 12:411–434Crossref, Google Scholar
31 : General multi-level modeling with sampling weights. Commun Stat Theory Methods 2006; 35:439–460Crossref, Google Scholar
32 : High risk cohort study for psychiatric disorders in childhood: rationale, design, methods, and preliminary results. J Methods Psychiatr Res 2015; 24:58–73Crossref, Medline, Google Scholar
33 : Adolescent and adult differences in major depression symptom profiles. J Affect Disord 2019; 243:175–181Crossref, Medline, Google Scholar
34 : Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J Neurosci 2015; 35:7226–7238Crossref, Medline, Google Scholar
35 : Developmental changes in cognitive control through adolescence. Adv Child Dev Behav 2009; 37:233–278Crossref, Medline, Google Scholar
36 : Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: a concurrent (11)C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl Psychiatry 2018; 8:264Crossref, Medline, Google Scholar
37 : A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995; 167:99–103Crossref, Medline, Google Scholar



