Plasma Melatonin Circadian Rhythm Disturbances During Pregnancy and Postpartum in Depressed Women and Women With Personal or Family Histories of Depression
Abstract
Objective: The purpose of this study was to test the hypothesis that disturbances in levels of plasma melatonin differentiate pregnant and postpartum women with major depression from matched pregnant and postpartum healthy comparison women. Method: Participants were 25 pregnant women (10 with major depression, 15 healthy) and 24 postpartum women (13 with major depression, 11 healthy). Healthy comparison women were matched on the number of weeks pregnant or postpartum. Plasma melatonin levels for each subject were measured every 30 minutes, in dim light (<30 lux), from 6:00 p.m. to 11:00 a.m. The values of plasma melatonin levels were log-transformed, and calculations were determined for the following measures: baseline and synthesis onset and offset times, duration, peak concentration, and area under the curve. Groups were compared by analyses of covariance, with age, number of weeks pregnant or postpartum, breast-feeding status, and body mass index as covariates. Results: Morning melatonin levels from 2:00 a.m. to 11:00 a.m. were significantly lower in pregnant women with major depression relative to healthy pregnant women. However, these levels were significantly higher in postpartum women with major depression across time intervals relative to postpartum healthy women. Pregnant but not postpartum women with a personal or family history of depression, regardless of their current diagnosis, had significantly earlier melatonin synthesis and baseline offset times relative to women without a family history of depression. In pregnant healthy women but not pregnant women with major depression, melatonin levels increased during the course of pregnancy. This association was not found among postpartum women with major depression or postpartum healthy women. Conclusions: Plasma nocturnal melatonin concentrations, particularly during morning hours, were lower in depressed pregnant women but elevated in depressed postpartum women relative to matched healthy comparison women. In addition, melatonin timing measures were advanced in pregnant women with a personal or family history of depression. These findings implicate disturbances in the regulation of the melatonin generating system in pregnancy and postpartum depression.
Major depression during pregnancy or the postpartum period can be a devastating illness to the mother, impair the neurocognitive and socio-emotional development of the child, and increase the risks of mental and medical disorders in the offspring later in life (1 – 5) . The causes of major depression during pregnancy or the postpartum period are unknown. Circadian rhythm dysregulation characterizes patients with mood disorders (6) . It has yet to be investigated systematically whether chronobiological disturbances also characterize women with pregnancy and postpartum depressive disorders. Since estrogen and progesterone are among the few known endogenous modulators of circadian rhythm amplitude and phase (7) , it is conceivable that the marked changes in gonadal steroids during pregnancy and the postpartum period might alter these biological rhythms, predisposing vulnerable women to develop mood disorders.
The best measure of circadian rhythmicity in humans is plasma melatonin (8) . The hypothalamic circadian pacemaker regulates the secretion of melatonin, which is synthesized from serotonin in the pineal gland under the rate limiting influence of norepinephrine. Melatonin generally is not secreted during the day, increases approximately 2 hours before sleep onset, and declines during early morning hours. Since even relatively dim light (200 lux) can suppress melatonin, it must be measured under controlled light conditions. Melatonin serves as an important regulator of other circadian rhythms and regulates cycles of reproduction and hibernation in seasonally breeding animals.
Melatonin circadian rhythms are of lower amplitude in some but not all depressed patients (9 , 10) . In previous studies, we observed blunted and phase-advanced (earlier shifted) melatonin rhythms in women with premenstrual dysphoric disorder (11 , 12) but higher melatonin levels in women with menopausal depression (13) . Melatonin studies of pregnant or postpartum women are few and do not include major depression (cf. 14 ).
The objective of the present study was to test the hypothesis that disturbances in melatonin circadian rhythms distinguish pregnant and postpartum women with major depression from matched healthy pregnant and postpartum women. Based on findings from one of our earlier studies (13) , we expected to find associations between altered melatonin rhythms and measures of depressed mood.
Method
Subjects
Participant recruitment procedures have been described elsewhere (15) . Pregnant (approximately ≤34 weeks) and postpartum (≤1 year) women, ages 20–45 years, who resided in San Diego, Calif. were screened via telephone. Women were eligible to participate in the study if they did not 1) smoke; 2) use hormonal contraceptives (if postpartum); or 3) use medications, herbs, or over-the-counter preparations that would interfere with neuroendocrine measures. Participants agreed to multiple overnight hospital stays in the General Clinical Research Center, where they were permitted to bring their child if necessary. Eligible women underwent the following laboratory tests: a chemistry panel, thyroid indices, complete blood count, urinalysis, and urine toxicology screening. Eligible women could not have significant medical illness, and women who were receiving drug treatment were required to discontinue any medication that would interfere with melatonin measures. Women with major depression were required to have discontinued antidepressant treatment ≥2 weeks (≥4 weeks for fluoxetine treatment) before the start of the study. Both women with major depression and healthy comparison women could not have had an alcohol abuse problem within the past year. Women with bipolar or primary anxiety disorders were excluded from the study.
To establish DSM-IV (16) entrance and baseline criteria, the following assessments were administered to each participant by trained clinicians: the Structured Clinical Interview for DSM-IV (SCID) (17) and at least two baseline evaluation ratings, scheduled 1 week apart, using the 21-item Hamilton Depression Rating Scale (HAM-D) (18) . Baseline evaluation ratings were determined by an atypical depressive symptom inventory (19) , the Beck Depression Inventory (BDI) (20) , and the Edinburgh Postnatal Depression Scale (21) , which has been validated for use during pregnancy (22) .
For study inclusion, women with major depression were required to have a mean HAM-D score ≥14 and BDI and Edinburgh Postnatal Depression Scale scores ≥10 for 2 weeks. Women with postpartum major depression had experienced the onset of a major depressive episode ≤3 months postpartum. Healthy comparison subjects were required to have a mean HAM-D score ≤8 and a mean BDI score ≤5.
Procedure
Women who met entrance criteria were admitted to UC San Diego General Clinical Research Center at 4:00 p.m. Following one night of adaptation to the sleep room, participants received an intravenous catheter, administered by licensed nurses, at 5:00 p.m., and blood (3 cc) was drawn every 30 minutes from 6:00 p.m. to 11:00 a.m. in order to measure plasma melatonin levels. Serum for estradiol and progesterone was obtained at 6:00 p.m. and again at 6:00 a.m. Participants remained at bed rest in a single room with double doors, and the windows were covered with heavy drapery in order to block extraneous light from entering between the hours of 4:00 p.m. and 11:00 a.m. Light panels allowed daytime light exposure to remain relatively dim (<30 lux). We considered this light intensity to be too dim to substantially suppress melatonin in undilated pupils, disrupt sleep, or shift circadian rhythms but not dim enough to serve as a dark pulse (23) . Subjects slept in the dark with an eye mask. Nurses or sleep technicians entered the room only when necessary (recorded by infrared camera), using a pen-sized dim red flashlight. During sleep times, nurses threaded the intravenous catheter through a porthole in the wall and drew blood samples from an adjoining room in order to minimize sleep disturbances.
The UC San Diego Institutional Review Board approved the study protocol. All subjects gave written informed consent after study procedures were fully explained.
Assays
Blood samples for melatonin were 1) placed in plastic tubes containing ethylenediaminetetraacetic acid, 2) centrifuged, 3) frozen immediately, and 4) stored at –70°C until assayed. All blood samples for the same subject were run in the same assay. Initial assays for melatonin, estradiol, and progesterone have been described elsewhere (24 , 25) . We assayed plasma melatonin concentrations of the first 44 subjects by radioimmunoassay, using a kit manufactured by Immuno-Biological Laboratories (Hamburg, Germany). Since the manufacturer changed this kit, plasma for the remaining five subjects (three pregnant women, two postpartum women) were assayed using a Direct Melatonin radioimmunoassay kit, manufactured by Bühlmann Laboratories (ALPCO Diagnostics, Windham, N.H.). This widely used radioimmunoassay kit utilizes calibrators ranging from 1–81 pg/ml and reports intra- and interassay coefficients of variation of 6.7% and 10.4%, respectively. The standard range has an analytical sensitivity of 0.8 pg/ml. For melatonin statistical analyses, assay method type (Immuno-Biological Laboratories kit versus Bühlmann Laboratories kit) was included as a covariate in order to correct for differences between assays.
Analyses
From the melatonin secretory profile, we estimated measures of timing (synthesis and baseline onset time, synthesis and baseline offset time, and duration) and quantity (peak concentration and area under the curve) using methods previously described (13) .
In addition to our attempt to match depressed women with healthy comparison women on the number of weeks pregnant and the number of weeks postpartum, we included weeks pregnant/postpartum as a covariate in order to control statistically for differences in the number of weeks pregnant or postpartum. For the initial repeated-measures analyses (e.g., time-by-diagnosis-by-weeks pregnant/postpartum), a three-factor “mixed” analysis of variance (ANOVA) was used with Geisser-Greenhouse correction for the sphericity of repeated measures. Follow-up analyses were conducted for pregnant and postpartum women separately, using two-factor (time-by-diagnosis) analyses. Plasma melatonin timing and quantitative measures were analyzed with multivariate analyses of covariance (MANCOVAs), followed by univariate analyses of covariance (ANCOVAs) for individual variables of interest. For pregnant women, the number of weeks pregnant and assay method type were applied as covariates. For postpartum women, the number of weeks postpartum, log-transformed body mass index, and breast-feeding status were applied as covariates.
Results
Subject Characteristics
Among pregnant women who met screening criteria, one was dropped as an outlier because her delayed melatonin onset (12:30 p.m.) and advanced baseline offset (5:30 a.m.) differed from the group mean by more than three standard deviations. Thus, the remaining 25 pregnant women (10 with major depression, 15 healthy) were examined. The pregnant women were Caucasian (N=17), Hispanic (N=3), African American (N=3), Asian (N=1), and multiethnic (N=1). Two pregnant healthy comparison women reported a family history of depression, and two had no family history but reported one previous major depressive episode. Five pregnant women with major depression reported a history of 1–6 major depressive episodes (mean=1.1 episodes [SD=1.9]). Based on retrospective reports, depression onset occurred during pregnancy for all 10 pregnant women with major depression (mean onset=12.1 weeks pregnant [SD=3.8]). Their current depressive episodes ranged in duration from 3 to 28 weeks (mean=13.6 weeks [SD=3.4]).
Among postpartum women who met screening criteria, two were dropped from the study. One woman’s sleep onset time was later than the group mean by more than three standard deviations (11:34 a.m. versus 1:58 a.m.), and the second woman’s melatonin offset time was earlier than the group mean by more than three standard deviations (6:00 a.m. versus 9:56 a.m.). We analyzed data for the remaining 24 postpartum women who met screening criteria (13 with major depression, 11 healthy). The postpartum women were Caucasian (N=11), Hispanic (N=8), Asian (N=3), and multiethnic (N=2). Two postpartum healthy comparison women reported one prior major depressive episode as well as a family history of depression, and one comparison woman reported only a family history. Ten of the 13 postpartum women with major depression reported a history of 1–8 major depressive episodes (mean=2.0 episodes [SD=2.2]). Based on retrospective reports, depression onset occurred during pregnancy for six of these women (mean onset=18.2 weeks pregnant [SD=4.7]) and during the postpartum period for seven of these women (mean onset=5.7 weeks postpartum [SD=3.0]). Their current depressive episodes ranged in duration from 3 to 40 weeks (mean=17.0 weeks [SD=3.9]).
Data pertaining to age, parity, personal and family history of depression, and mood ratings are detailed in Table 1 .
Plasma Melatonin
A repeated-measures ANOVA for log-transformed melatonin concentrations across 35 successive half-hour intervals yielded a highly significant time-by-diagnosis-by-weeks pregnant/postpartum status interaction (F=11.8, df=1, 44, p=0.001). Subsequent ANCOVAs conducted for pregnant women, with weeks pregnant and assay method type as covariates, showed a significant diagnosis effect (F=8.02, df=1, 21, p=0.010) as well as a significant time-by-diagnosis interaction (F=3.19, df=34, 714, p=0.028). Analysis of the time-by-diagnosis interaction showed that melatonin concentrations were lower in depressed pregnant women relative to healthy pregnant women from 2:00 a.m. to 11:00 a.m. (all p values <0.05 [ Figure 1 ]). In contrast, melatonin levels were significantly higher across time points in postpartum women with depression relative to healthy postpartum women (F=6.13, df=1, 20, p=0.022 [ Figure 1 ]) when the number of weeks postpartum and breast-feeding status were applied as covariates. Although the time-by-diagnosis interaction was not significant (p=0.73), since depressed pregnant women and healthy pregnant women showed differences in morning melatonin levels, we calculated individual paired comparisons for postpartum women, which showed that the most reliable differences in melatonin secretion between depressed and healthy postpartum women occurred from 6:30 a.m. to 9:30 a.m. (all p values <0.05).

*p≤0.05.
Baseline and Synthesis Melatonin Measures
Among pregnant women, the omnibus MANCOVA was significant for diagnosis (p=0.037), with the number of weeks pregnant and melatonin assay method type as covariates. The univariate ANCOVA showed that the area under the curve (F=7.66, df=1, 21, p=0.012) and synthesis area under the curve (F=6.27, df=1, 21, p=0.021) were lower in pregnant depressed women relative to pregnant healthy comparison women ( Table 2 ). Melatonin onset, synthesis offset, and baseline offset did not differ between the two groups of pregnant women. Among postpartum women, the omnibus MANCOVA was marginally significant for diagnosis (p=0.055) for the quantitative melatonin measures when breast-feeding status and log–transformed body mass index were included as covariates. In contrast to pregnant women, the univariate ANCOVA showed that the area under the curve (F=9.02, df=1, 20, p=0.007), synthesis area under the curve (F=8.17, df=1, 20, p=0.010), and log transformed peak melatonin concentration (F=7.89, df=1, 20, p=0.011) were greater in postpartum women with major depression relative to healthy postpartum women ( Table 2 ). Melatonin onset, synthesis offset, and baseline offset did not differ between the two groups of postpartum women.
We compared melatonin concentration levels in pregnant depressed and healthy women with the levels in postpartum depressed and healthy women. ANCOVAs revealed that healthy pregnant women had higher melatonin concentration levels than healthy postpartum women across time intervals (F=7.84, df=1, 20, p=0.011). In contrast, pregnant women with major depression did not differ significantly from postpartum women with major depression.
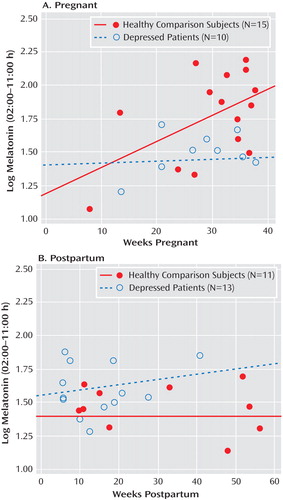
Since the number of weeks pregnant was a significant covariate for several melatonin level analyses (p≤0.05), we determined the partial correlation between the number of weeks pregnant and log-transformed melatonin concentrations from 2:00 a.m. to 11:00 a.m., with age controlled. For healthy pregnant women, this correlation was highly significant (r=0.688, p=0.007), but the correlation showed no significance for pregnant women with major depression ( Figure 2 ). Thus, melatonin levels increased significantly across the number of weeks during pregnancy in healthy comparison women but not in women with major depression. Similar analyses for postpartum women showed that melatonin concentrations were unrelated to the number of weeks postpartum in healthy (r=0.044, p=0.898) and depressed (r=0.202, p=0.485) women ( Figure 2 ).
Correlations of Melatonin Measures With Mood and Depressive History
Overall, partial correlations between mood and melatonin measures (with age and number of weeks pregnant or postpartum controlled) were small and not significant among both pregnant (N=25) and postpartum women (N=24). In contrast, a personal or family history of depression was positively correlated with HAM-D scores among both pregnant (r=0.466, p=0.025) and postpartum (r=0.500, p=0.018) women, as was the number of prior episodes of depression among pregnant women (r=0.543, p=0.007). Among pregnant but not postpartum women, partial correlation (with age and number of weeks pregnant controlled) showed that the number of prior episodes of depression was negatively correlated with melatonin synthesis offset time (r=–0.677, p=0.0004) and baseline offset time (r=–0.629, p=0.001) in depressed plus healthy comparison women combined. Thus, earlier synthesis and baseline offset times were associated with a greater number of previous depressive episodes among pregnant women, for whom melatonin quantity measures were reduced in depressed relative to healthy subjects.
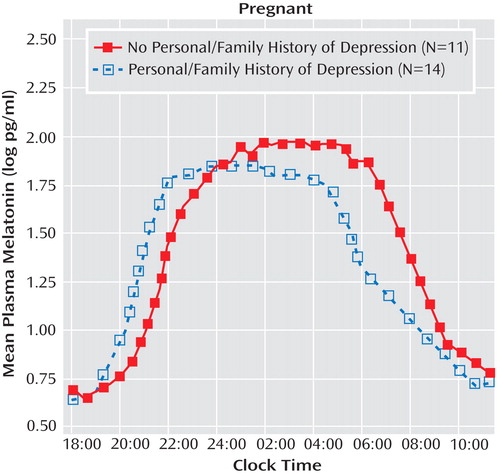
For melatonin timing measures, the omnibus MANCOVA revealed a significant main effect of personal or family history of depression (p=0.023) but no significant main effect or interaction for diagnosis in pregnant women (depressed plus healthy comparison combined), when the number of weeks pregnant and melatonin assay method type were included as covariates. The univariate ANCOVA suggested that melatonin timing parameters were temporally advanced in women with a personal or family history of depression relative to women without a personal or family history ( Figure 3 ). Pregnant women with a positive personal or family history of depression had, on average, marginally earlier melatonin onset times (7:08 p.m. versus 8:07 p.m.; F=3.62, df=1, 19, p=0.073), significantly earlier synthesis offset times (4:08 a.m. versus 6:04 a.m.; F=12.33, df=1, 19, p=0.002), and significantly earlier baseline offset times (9:06 a.m. versus 11:04 a.m.; F=9.17, df=1, 19, p=0.007). In contrast, melatonin timing measures in postpartum women were not significantly affected by a personal or family history of depression. Thus, depression history affected melatonin timing measures for pregnant but not postpartum women.
Menstruation, Breast-Feeding Status, and Child Presence in Examination Room
None of the pregnant women brought their children to the General Clinical Research Center during test nights. Among postpartum women, nine healthy mothers (81.8%) either 1) brought their children to and nursed their children in the center (N=7) or 2) pumped their breasts but did not nurse their children during test nights (N=2). Five postpartum women (38.5%) with major depression brought and breast-fed their children (significance of difference between healthy comparison and depressed women: p=0.03). Among postpartum women, two healthy women and two women with major depression reported resumption of menstruation prior to testing. One healthy comparison woman was examined during the follicular phase, and one was examined during the luteal phase. Similarly, one woman with major depression was examined during the follicular phase, and one was examined during the luteal phase. Total sleep time, sleep latency, sleep efficiency, and wake-after-sleep onset, ascertained via polysomnography recording, were available for all 25 of the pregnant women in the study and 23 of the 24 postpartum women in the study. Analyses of the polysomnography data showed no significant differences in sleep quality variables as a function of breast-feeding status, child presence in the room, or the interaction of breast-feeding status and child presence in the room. Furthermore, breast-feeding status, child presence in the room, and the interaction of breast-feeding status and child presence in the room were without significant effect during morning melatonin secretion.
Reproductive Hormones
MANCOVA, with the number of weeks pregnant or postpartum (and postpartum breastfeeding status), showed no significant main effect of diagnosis, personal or family history of depression, or their interaction on log-transformed estradiol or progesterone levels in pregnant or postpartum women. Partial correlation, for which age, number of weeks pregnant or postpartum, and breast-feeding status were controlled (when appropriate), revealed no significant correlations between mood measures (HAM-D, atypical ratings, BDI, Edinburgh Postnatal Depression Scale) and reproductive hormones (estradiol, progesterone) in pregnant (N=25) or postpartum (N=24) women when women with major depression and healthy comparison women were combined. Similar tests revealed no significant partial correlations between melatonin measures and reproductive hormones in pregnant or postpartum women.
Discussion
The main findings of the present study are 1) that pregnant women with major depression had decreased levels of nocturnal plasma melatonin, particularly during early morning hours, relative to matched healthy comparison women, whereas 2) postpartum women with major depression had increased levels of nocturnal plasma melatonin, especially during early morning hours, relative to matched healthy comparison women. We also found that pregnant women who had a personal or family history of depression also had earlier melatonin offset times, and melatonin secretion increased during pregnancy in healthy comparison women but not women with major depression.
Our finding of decreased levels of plasma melatonin in pregnant women with major depression parallels findings from studies of women with premenstrual dysphoric disorder (11 , 12) . In contrast, our finding of increased levels of plasma melatonin in postpartum women with major depression parallels findings from a study of menopausal women with major depression (13) . Taken together, our findings as well as those from previous studies suggest that depressive disorders that are related to the female reproductive cycle (premenstrual, pregnancy, postpartum, and menopause) may each represent a specific disorder, with its own characteristic pathophysiological mechanisms, rather than a shared common pathophysiology. As O’Hara (26) observed, social support and life events may contribute differently to pre- and postpartum depression.
One explanation for decreased melatonin secretion in pregnant women with major depression and increased secretion in postpartum women with major depression, relative to healthy comparison women, is that major depression might be less sensitive to the effects of estradiol or progesterone levels on melatonin receptors. As a result, the increase in gonadal hormones during pregnancy would increase melatonin secretion in healthy pregnant women but not in women with major depression, as found in the present study. In postpartum women, declining levels of estradiol and progesterone would decrease melatonin levels in healthy comparison women but not in women with major depression, resulting in higher melatonin levels in women with postpartum depression relative to healthy postpartum women, which we also observed. The effect of estradiol and progesterone levels on melatonin production may occur through rate limiting catecholamine pathways (27 – 30) .
An altered sensitivity to gonadal steroids in women with major depression might occur in other depressive disorders related to the reproductive cycle. Schmidt et al. (31) reported differential behavioral effects of gonadal steroids related to the menstrual cycle in women with and without depressive symptoms. Bloch et al. (32) found that simulated hormonal excursions of pregnancy and the postpartum period induced depressive relapses in women with a history of perinatal mood disorder but not in women without a history of perinatal mood disorder. Although we did not find significantly different levels of estradiol or progesterone among the subjects in the present study, individual variability in sensitivity to these hormones may have affected our results. For example, as a function of individual variability, estradiol treatment in postmenopausal women produces either an increase or a decrease in melatonin in different patients (33) .
Our finding that a depressive history and the number of prior depressive episodes were associated with earlier melatonin offset times in pregnant women suggests that abnormalities in melatonin timing parameters may be markers of vulnerability to depressive illness during pregnancy. The mechanism for this vulnerability might be that women with a depressive history have a lower strength or output of the hypothalamic circadian pacemaker that regulates melatonin secretion. Alternatively, since early morning light suppresses melatonin secretion, which causes an earlier offset, these women may have an increased sensitivity to early morning light, as do some other patients at risk for depression (34) , or they may experience more light in the morning as a result of early morning awakening that occurs in major depression.
Prepartum psychiatric illness is a risk factor for the development of postpartum mood disorders. Andersson et al. (35) reported that depression and/or anxiety was prevalent in 29.2% of pregnant women versus 16.5% of postpartum women. The variables most predictive of postpartum depression include antenatal depression and a history of postpartum depression (36) . Mothers who have a psychiatric history are four times more likely to develop depression postpartum, six times more likely to experience recurrent depressive symptoms, and more likely to experience physical and mental illness relative to women without a psychiatric history (37) . Mood symptoms may manifest particularly during pregnancy in women with a psychiatric history. Effects of depression on newborn physiology depend more on prepartum than postpartum maternal depression and on the duration of depressive symptoms (38) . Thus, melatonin timing parameters, as manifested particularly in measures of melatonin offset, appear to be sensitive physiological markers for vulnerable women whose history of depressive illness may predispose them to future episodes.
These findings have important treatment implications. The low melatonin levels in depressed pregnant women may compromise their ability to utilize melatonin as a regulator of other circadian rhythms. As a result, desynchronization of circadian rhythms may predispose them to further depressive mood changes (6) . Since melatonin treatment can alter reproductive function (39) , light therapy would be a better strategy to synchronize circadian rhythms and thereby mitigate depression. Bright light at critical times of the day is one of the most potent synchronizers of circadian rhythms and has been used in the treatment of mood disorders, including those disorders related to the reproductive cycle (40) . Since light has the ability to not only entrain circadian rhythms but also suppress melatonin, the elevated melatonin levels in postpartum depressed women also might be amenable to critically timed light administration. Further research is needed to define more precisely the abnormalities of melatonin secretion in pregnant and postpartum women with major depression as a basis for designing light, sleep, or pharmacological therapies that affect melatonin regulation.
1. Murray L, Woolgar M, Cooper P, Hipwell A: Cognitive vulnerability to depression in 5-year-old children of depressed mothers. J Child Psychol Psychiatry 2001; 42:891–899Google Scholar
2. Murray L, Halligan SL, Adams G, Patterson P, Goodyer IM: Socioemotional development in adolescents at risk for depression: the role of maternal depression and attachment style. Dev Psychopathol 2006; 18:489–516Google Scholar
3. Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G: Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002; 159:1889–1895Google Scholar
4. Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H: Offspring of depressed parents: 20 years later. Am J Psychiatry 2006; 163:1001–1008Google Scholar
5. Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, Wisniewski SR, Fava M, Hughes CW, Garber J, Malloy E, King CA, Cerda G, Sood AB, Alpert JE, Trivedi MH, Rush AJ: Remissions in maternal depression and child psychopathology: a STAR*D-Child report. JAMA 2006; 295:1389–1398Google Scholar
6. Wehr TA, Wirz-Justice A: Internal coincidence model for sleep deprivation and depression, in Sleep 1980. Edited by Koella WP. Basel, Switzerland, Karger, 1981, pp 26–33Google Scholar
7. Leibenluft E: Do gonadal steroids regulate circadian rhythms in humans? J Affect Disord 1993; 29:175–181Google Scholar
8. Arendt J: Melatonin and human rhythms. Chronobiol Int 2006; 23:21–37Google Scholar
9. Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, Parry B, Cardinali DP: Melatonin in mood disorders. World J Biol Psychiatry 2006; 7:138–151Google Scholar
10. Parry BL, Meliska CJ, Martinez LF, Lopez AM, Sorenson DL, Nowakowski S, Loving RT: Melatonin circadian rhythms in women with depression: relationship to the reproductive cycle, in Melatonin: From Molecules to Therapy. Edited by Pandi-Perumal SR, Cardinali DP. New York, Nova Publishers, 2007, pp 571–580Google Scholar
11. Parry BL, Berga SL, Kripke DF, Klauber MR, Laughlin GA, Yen SS, Gillin JC: Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry 1990; 47:1139–1146Google Scholar
12. Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A: Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms 1997; 12:47–64Google Scholar
13. Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Hauger RL, Elliott JA: Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J Clin Endocrinol Metab 2008; 93:54–60Google Scholar
14. Parry BL, Martinez LF, Maurer EL, Lopez AM, Sorenson DL, Meliska CJ: Sleep rhythms and women’s mood, Part I: menstrual cycle, pregnancy and postpartum. Sleep Med Rev 2006; 10:129–144Google Scholar
15. Parry BL, Sorenson DL, Meliska CJ, Basavaraj N, Zirpoli GG, Gamst A, Hauger R: Hormonal basis of mood and postpartum disorders. Curr Womens Health Rep 2003; 3:230–235Google Scholar
16. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Publishing, 1994Google Scholar
17. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition. New York, Biometerics Research Department, New York State Psychiatric Institute, 1995Google Scholar
18. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Google Scholar
19. Rosenthal NE, Heffernan MM: Bulimia, carbohydrate craving and depression: a central connection? in Nutrition and the Brain, vol 7. Edited by Wurtman RJ, Wurtman JJ. New York, Raven Press, 1986Google Scholar
20. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Google Scholar
21. Cox JL, Holden JM, Sagovsky R: Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987; 150:782–786Google Scholar
22. Green J, Murray D: The use of the Edinburgh Postnatal Depression Scale in research to explore the relationship between antenatal and postnatal dysphoria, in Perinatal Psychiatry: Use and Misuse of the Edinburgh Postnatal Depression Scale. Edited by Cox J. London, Gaskell, 1994, pp 180–198Google Scholar
23. Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL: Measuring melatonin in humans. J Clin Sleep Med 2008; 4:66–69Google Scholar
24. Brzezinski A, Lynch HJ, Seibel MM, Deng MH, Nader TM, Wurtman RJ: The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. J Clin Endocrinol Metab 1988; 66:891–895Google Scholar
25. Anderson DC, Hopper BR, Lasley BL, Yen SS: A simple method for the assay of eight steroids in small volumes of plasma. Steroids 1976; 28:179–196Google Scholar
26. O’Hara MW: Social support, life events, and depression during pregnancy and the puerperium. Arch Gen Psychiatry 1986; 43:569–573Google Scholar
27. Masana MI, Soares JM Jr, Dubocovich ML: 17Beta-estradiol modulates hMT1 melatonin receptor function. Neuroendocrinology 2005; 81:87–95Google Scholar
28. Wilkinson M, Arendt J: Effects of oestrogen and progesterone on rat pineal N-acetyl transferase activity and melatonin production. Experientia 1978; 34:667–669Google Scholar
29. Cagnacci A, Zanni AL, Veneri MG, Menozzi R, Volpe A, Rio GD: Influence of exogenous melatonin on catecholamine levels in postmenopausal women prior and during oestradiol replacement. Clin Endocrinol (Oxf) 2000; 53:367–372Google Scholar
30. Kostoglou-Athanassiou I, Athanassiou P, Treacher DF, Wheeler MJ, Forsling ML: Neurohypophysial hormone and melatonin secretion over the natural and suppressed menstrual cycle in premenopausal women. Clin Endocrinol (Oxf) 1998; 49:209–216Google Scholar
31. Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR: Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998; 338:209–216Google Scholar
32. Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR: Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000; 157:924–930Google Scholar
33. Bartsch C, Seeger H, Muck AO, Lippert TH: The effect of estradiol on the production of melatonin in postmenopausal women. Int J Clin Pharmacol Ther 1995; 33:401–403Google Scholar
34. Nurnberger JI Jr, Berrettini W, Tamarkin L, Hamovit J, Norton J, Gershon E: Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder: a preliminary report. Neuropsychopharmacology 1988; 1:217–223Google Scholar
35. Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M: Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstet Gynecol Scand 2006; 85:937–944Google Scholar
36. Dennis CL, Ross LE: The clinical utility of maternal self-reported personal and familial psychiatric history in identifying women at risk for postpartum depression. Acta Obstet Gynecol Scand 2006; 85:1179–1185Google Scholar
37. Josefsson A, Sydsjo G: A follow-up study of postpartum depressed women: recurrent maternal depressive symptoms and child behavior after four years. Arch Womens Ment Health 2007; 10:141–145Google Scholar
38. Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C: Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry 2004; 67:63–80Google Scholar
39. Diaz BL, Llaneza PC: Endocrine regulation of the course of menopause by oral melatonin: first case report. Menopause 2008; 15:388–392Google Scholar
40. Parry BL, Maurer EL: Light treatment of mood disorders. Dialog Clin Neurosci 2003; 5:353–365Google Scholar





