Anorexia and Bulimia Nervosa in Same-Sex and Opposite-Sex Twins: Lack of Association With Twin Type in a Nationwide Study of Finnish Twins
Abstract
Objective: The authors tested the hypothesis that either prenatal feminization or masculinization hormone influences in utero or later socialization affects the risk for anorexia and bulimia nervosa and disordered eating in members of opposite-sex twin pairs. Method: Finnish twins (N=2,426 women, N=1,962 men with known zygosity) from birth cohorts born 1974–1979 were assessed at age 22 to 28 years with a questionnaire for eating disorder symptoms. Based on the questionnaire screen, women (N=292), men (N=53), and their cotwins were interviewed to assess diagnoses of anorexia nervosa and bulimia nervosa (per DSM-IV and broad criteria). Results: In women from opposite-sex twin pairs, the prevalence of DSM-IV or broad anorexia nervosa was not significantly different than that of women from monozygotic pairs or same-sex dizygotic pairs. Of the five male anorexia nervosa probands, only one was from an opposite-sex twin pair. Bulimia nervosa in men was too rare to be assessed by zygosity; the prevalence of DSM-IV or broad bulimia nervosa did not differ in women from opposite- versus same-sex twin pairs. In both sexes, the overall profile of indicators on eating disorders was rather similar between individuals from opposite- and same-sex pairs. Conclusions: The authors found little evidence that the risk for anorexia nervosa, bulimia nervosa, or disordered eating was associated with zygosity or sex composition of twin pairs, thus making it unlikely that in utero femininization or masculinization or socialization effects of growing up with an opposite-sex twin have a major influence on the later development of eating disorders.
The sex discrepancy in anorexia and bulimia nervosa remains significant despite the recent reports that anorexia nervosa and bulimia nervosa in males are more prevalent than traditionally thought (1 – 3) . One hypothesis is that in order to develop either disorder, men require a stronger genetic predisposition and/or more adverse environmental factors than women; and indeed, a large increase in relative risk of anorexia nervosa in female relatives of males with anorexia nervosa is reported (4) .
The observed sex difference in lifetime risk for eating disorders may also reflect hormonal factors: Klump and colleagues showed that in girls, higher circulating levels of estrogens increase the probability of disordered eating (5) and that, whereas environmental factors predominate prepubertally, genetic influences on disordered eating emerge after puberty in girls (6) . In women, decreases in estradiol, increases in progesterone, and higher serum levels of free testosterone seem to contribute to binge eating (7) and bulimia nervosa (8) . We are not aware of studies directly investigating hormonal factors in males with eating disorders.
There has recently been much attention in prenatal and perinatal factors influencing the onset of these disorders (9 – 12) . In a compelling study by Culbert and colleagues (11) , disordered eating exhibited significant linear trends, with females from same-sex pairs exhibiting the highest levels, followed by females from opposite-sex pairs, males from opposite-sex pairs, and males from same-sex pairs. These findings were unlikely to be accounted for socialization effects, since females from opposite-sex twin pairs reported significantly lower levels of disordered eating than nontwin females with at least one brother. The authors hypothesized that the critical influence of masculinizing prenatal hormonal environments to neurodevelopment in utero would explain their findings. Another study, a secondary analysis by Procopio and Marriott (13) conducted on a published table from the Swedish Twin Registry (2) , found that male cotwins from opposite-sex dizygotic twin pairs had rates of anorexia nervosa commonly found in women. Later on, the article was retracted from the scientific literature, when the published data were corrected in an erratum (12) . Thus, the data from Swedish twin cohorts (2 , 12) do not support the hypothesis of prenatal or postnatal feminizing, demasculinizing, or masculinizing influences on subsequent anorexia nervosa in offspring.
Using a nationwide Finnish twin cohort, we tested the hypothesis that being a member of an opposite-sex twin pair subsequently affects females’ risk of developing an eating disorder or disordered eating. Simultaneously, we tested whether prenatal feminizing gonadal hormone influences in utero or later feminizing socialization effects affect the neurodevelopment of the male co-twin of opposite-sex dizygotic twins, resulting in increased risk for eating disorders and disordered eating. We compared the prevalence of anorexia nervosa, bulimia nervosa, disordered eating behaviors, and other body size and shape-related symptoms in individuals from opposite-sex twin pairs compared with individuals from same-sex twin pairs.
Method
Virtually all twins from FinnTwin16 cohorts born between 1975 and 1979 and a few born in late 1974 were identified from the Finnish population register (14) and assessed at ages 16, 17, 18, and 22–28 years. Data collection and analysis were approved by the ethics committee of Helsinki University and the institutional review boards at Columbia University and Indiana University. At age 22–28 years (mean=24.4 years, SD=0.9), we screened all women and men by questionnaire for various eating disorder symptoms. We also assessed their current self-reported height and weight variables after reaching the adult height (minimum, maximum, current, and subjective ideal) from which we calculated their body mass index (BMI). In a subsample of 133 men not selected for eating disorders or eating behavior, the correlation between self-reported and later measured BMI was 0.91.
Among women, a response rate of 90% yielded a total of 2,426 female twins with known zygosity (opposite-sex dizygotic: N=793, same-sex dizygotic: N=765, monozygotic: N=868). In men, a response rate of 83% yielded a total of 1,962 male twins with known zygosity (opposite-sex dizygotic: N=717, same-sex dizygotic: N=705 monozygotic: N=540). Information on zygosity could not be obtained from 119 women and 164 men, who were excluded from this study.
Eating Disorder Symptoms
In the questionnaire, we addressed three subscales of the Eating Disorder Inventory-1 (EDI) (15) : body dissatisfaction (Cronbach’s alpha 0.92 for women, 0.86 for men), drive for thinness (0.87 for women, 0.75 for men), and bulimia (0.83 for women, 0.65 for men). Intentional weight loss (16) and purging (17) were also assessed. Leisure aerobic activity (work metabolic rate divided by resting metabolic rate) was calculated from the product of self-reported intensity by duration by days/year frequency and was expressed as Metabolic Equivalent (MET) values (18 , 19) . We used MET values 4 (light aerobic activity, walking), 6 (moderate aerobic activity, brisk walking), 10 (intense aerobic activity, jogging), 13 (very intense activity, running). In addition, to emphasize male-specific body dissatisfaction and to better address the eating disorder symptom spectrum in the male population (20) , we assessed muscle dissatisfaction and muscle building supplement and/or anabolic hormone use among men as described previously (21) .
Screening for Eating Disorders and Diagnostic Interviews
In women, screen-positivity was defined by a reported or suspected eating disorder and a composite measure of EDI cutoff-scores and low lifetime BMIs as described in detail elsewhere (17) . Of men, those who reported or suspected ever having had an eating disorder were considered screen positive. Subsequently, screen-positive women (N=292) and men (N=20), their same-sex cotwins (N=130 women, N=11 men), and those screen-negative men (N=33) who reported a minimum BMI ≤17.5 after reaching the adult height were invited to participate in diagnostic telephone interviews. In addition, a random sample of screen-negative female-female twin pairs (N=210 women) and a female co-twin of a male anorexia nervosa proband were invited. Experienced clinicians (five MDs and one RN) from the Eating Disorder Unit of Helsinki University Central Hospital conducted the short version of the Structured Clinical Interview for DSM-IV (SCID) (22) by telephone to obtain current and lifetime diagnoses of anorexia nervosa and bulimia nervosa. The overall interview participation rate for women was 85.2% (90.1% for the screen-positives, 76.2% for the screen-negative female cotwins, and 84.8% for the random sample of the screen-negatives). For men, the overall rate was 90.7% (90.0% for screen-positives, 90.9% for screen-negative male cotwins, and 91.3% for low BMI men).
Definition of Disorders
We used DSM-IV and broad definitions of both disorders. In women, broad anorexia nervosa included DSM-IV anorexia nervosa and ICD-10 atypical anorexia, here defined as without amenorrhea or weight loss of at least 15% that led to a BMI ≤19, coupled with undue influence of body weight on self-evaluation or intense fear of weight gain (23) . Broad bulimia nervosa included bulimia nervosa as defined in DSM-IV but with frequency criterion C relaxed to allow a symptom frequency of once a week (24) . Among men, we used only DSM-IV criteria for anorexia nervosa but excluded criterion D (amenorrhea).
All subjects who met DSM-IV criteria were also part of the group that satisfied the broader definition of both disorders, but not necessarily vice versa. The distinction between DSM-IV and broad anorexia nervosa applied only for females because the amenorrhea criterion was not relevant in males, in whom the broad and DSM-IV groups coincided.
Analysis
We investigated associations between independent and dependent variables using cross-tabulations, Pearson’s chi-squared test of independence, Fisher’s exact test, linear regression, binary logistic regression, and ordinal logistic regression. Lifetime prevalence cases of DSM-IV anorexia nervosa and bulimia nervosa among individuals with known zygosity identified in this study were defined regardless of age at onset, age at interview, or recovery status. In males, we computed lifetime prevalence by zygosity groups using Poisson-based confidence intervals. For females in each zygosity group, binomial confidence intervals for lifetime prevalence were calculated. All analyses, excluding Fisher’s exact test for males with DSM-IV anorexia nervosa, were controlled for clustered sampling (25) using Stata Version 9.0 (26) .
Results
Anorexia Nervosa
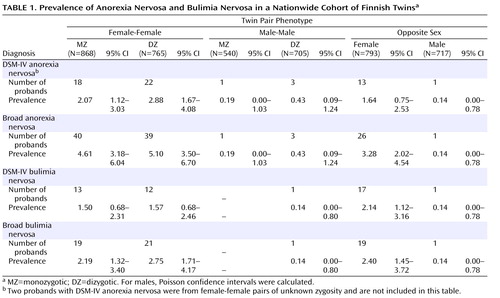
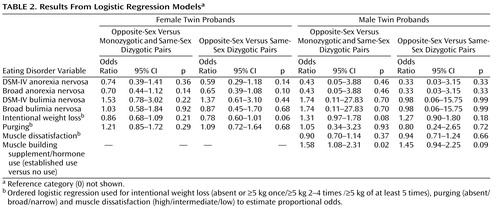
Table 1 presents the prevalence of anorexia nervosa for the different twin phenotypes using DSM-IV and broad diagnostic criteria. In women, the lifetime prevalence of DSM-IV or broad anorexia nervosa did not differ significantly across zygosity groups (p=0.24 and p=0.27, respectively). Of the five male DSM-IV anorexia nervosa probands in our sample, only one was from an opposite-sex twin pair (Fisher’s exact test, p=0.54). Furthermore, to examine the magnitude of the effect of zygosity on anorexia nervosa, we used a logistic regression model to analyze the effect on anorexia nervosa of zygosity dichotomized as opposite-sex versus all same-sex twins as well as opposite-sex versus same-sex dizygotic twins ( Table 2 ). Relative to women from same-sex dizygotic pairs, women from opposite-sex pairs did not differ in diagnosis of DSM-IV anorexia nervosa or broad anorexia nervosa. Likewise, relative to all women from same-sex pairs, women from opposite-sex pairs did not differ in diagnosis of DSM-IV anorexia nervosa or broad anorexia nervosa.
Bulimia Nervosa
Table 1 also presents the prevalence of bulimia nervosa for the different twin phenotypes using DSM-IV and broad diagnostic criteria. In women, the lifetime prevalence of DSM-IV or broad bulimia nervosa did not differ statistically significantly across zygosity groups (p=0.45 and p=0.45, respectively). Only two male participants had suffered from DSM-IV bulimia nervosa, one man being from a same-sex dyzygotic pair and the other from an opposite-sex dyzygotic pair ( Table 1 ). In logistic regression models, no significant differences in diagnosis of DSM-IV or broad bulimia nervosa were found for either sex between same-sex (either all or dyzygotic only) and opposite-sex twin pairs ( Table 2 ).
Indicators of Disordered Eating and Body Dissatisfaction in Women
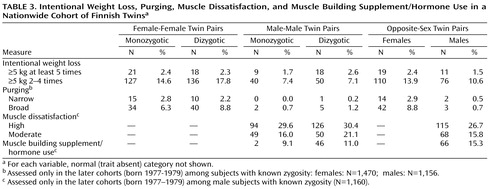
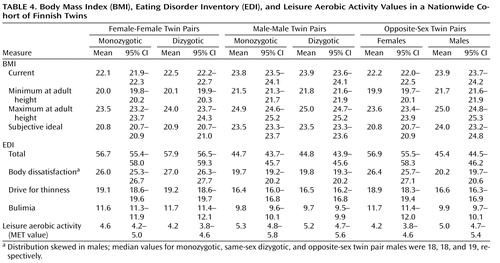
Among women, no statistically significant differences emerged across zygosity groups on any of the indicators of eating disorders ( Table 3 and Table 4 ). The only finding to approach statistical significance was the observation that women from the opposite-sex pairs were marginally less likely to have had engaged in intentional weight loss ( Table 2 ) than women from same-sex dyzygotic pairs. To specifically address the hypothesis presented by Culbert and colleagues (11) suggesting that prenatal hormonal influences manifest through zygosity and affect disordered eating behavior, we also analyzed the effect of being from an opposite-sex twin pair on total EDI scores using linear regression: in women, we did not observe any tendency between EDI scores and being from an opposite-sex twin pair relative to all (monozygotic and dizygotic) same-sex pairs (beta coefficient=–0.34, 95% CI=–2.09–1.42) or when compared to same-sex dizygotic pairs only (beta coefficient=–0.98, 95% CI=–3.05–1.09). Adjusting these analyses with current or lifetime minimum BMI did not significantly affect the results.
Indicators of Disordered Eating and Body Dissatisfaction in Men
Among men, of the potential indicators of eating disorders, only muscle building supplement use was significantly associated with being from an opposite-sex twin pair relative to men from all same-sex pairs ( Table 2 ). Relative to men from all same-sex pairs, men from opposite-sex pairs were also marginally more likely to have engaged in recurrent intentional weight loss ( Table 2 ). In addition, we specifically analyzed the effect among men of being from an opposite-sex twin pair on total EDI scores using linear regression. The total EDI scores of men from opposite-sex twin pairs did not significantly differ from those of all same-sex twin pairs (both monozygotic and dizygotic) (beta coefficient=0.61, 95% CI=–0.48–1.69) nor from those of only same-sex dizygotic pairs (beta coefficient=0.59, 95% CI=–0.61–1.80). Adjusting these analyses for current or lifetime minimum BMI did not affect the results. Results for the EDI subscales showed no differences.
Discussion
No association with opposite-sex zygosity and DSM-IV or broad anorexia nervosa, bulimia nervosa, or disordered eating could be established in our study in either sex. However, despite the overall negative results, we observed a marginally decreased risk of developing anorexia nervosa and of engaging in recurrent weight loss attempts among women from opposite-sex twin pairs. In men, the overall profile of indicators of disordered eating was very similar between men from opposite- and same-sex pairs: of the numerous tests done on indicators of disordered eating and body dissatisfaction, only muscle-building supplement use was significantly increased, and recurrent intentional weight loss was marginally increased, among men from opposite-sex pairs.
Of interest, our null finding for disordered eating behavior (measured by EDI) was at variance with the study of Culbert and colleagues (11) conducted with the twin sample of Michigan State University, in which the authors concluded that disordered eating exhibited significant linear trends by zygosity according to the hypothesis of prenatal hormonal influences. Even though their measure for disordered eating (Minnesota Eating Behavior Survey [MEBS]) differed from ours (the EDI), the items in both measures are relatively similar (15 , 27) and unlikely to fully explain the discrepancy in results. However, the MEBS includes the scale for binge eating, which our three-subscale EDI lacks. It is thus possible that the MEBS covers more obesity-related behaviors such as binging and overeating that are relatively common and presumably associated with serum-free testosterone levels (8 , 28) . Further, it is possible that in the population, manifestations of disordered eating such as binge eating, dieting, and body dissatisfaction mirror the prevalence of obesity. In the United States, the mean prevalence of obesity (BMI ≥30 kg/m 2 ) in young adults (18–29 years) was 13.5% at year 2000 (29) , whereas in our base sample (data collected in 2000–2002) it was 3.9%. In addition, the obesogenic environment in the United States is probably even more ubiquitous than in a contemporary Finnish society and thus more prone to trigger dieting and disordered eating in vulnerable individuals. Earlier studies show that the heritability of behavioral traits tend to be greater in permissive environments that provide a greater diversity of exposures compared to restrictive environments (30) . In this context this would imply that the underlying biological vulnerability for disordered eating in the population would manifest in the United States but not so in Finland. Moreover, whereas our sample was representative for the socioeconomically heterogeneous general population, the twins in Culbert and colleagues’ study were largely in the middle to upper levels of socioeconomic status, which is known to be associated with dieting and some disordered eating behaviors at least in females (31) . However, in line with the prenatal hypothesis, we did observe a marginal decrease of recurrent intentional weight loss attempts among women from the opposite-sex pairs in our study, and its simultaneous increase among men was equally marginally associated with being from an opposite-sex pair.
Among men from opposite-sex pairs, we observed an increase of muscle building supplement use. It has been suggested that in males, the pursuit toward muscularity shows parallels to the pursuit of thinness in females and is associated with disturbed eating behavior (20 , 21 , 32) . On the other hand, since men from the opposite-sex pairs did not exhibit higher muscle dissatisfaction, higher subjective ideal, or higher lifetime maximum BMI, and since the association was not particularly strong, our result may be just a chance finding.
In our limited data from males with an eating disorder, we did not observe a positive association with anorexia nervosa and opposite-sex zygosity, i.e., men from opposite-sex pairs were not overrepresented among males with DSM-IV anorexia nervosa. On the contrary, out of the five male anorexia nervosa probands in our cohort only one was from an opposite-sex twin pair. It is evident that in males, these findings cannot really oppose the hypothesis of intrauteral feminizing gonadal hormone influences on anorexia nervosa because of the lack of power, but our findings from other eating-related behaviors provide an overall profile of opposite-sex men not at increased risk for eating disorders or disturbed eating. Among women, we observed a nonsignificant tendency of decreased anorexia nervosa among those from opposite-sex pairs for both DSM-IV and broad diagnoses. This is in accordance with the prenatal hormone hypothesis and was equally present as a nonsignificant effect in the Swedish twin cohorts (2) .
Strengths and Limitations
The strengths of our study include good population coverage, excellent participation, and expert diagnostic assessment of eating disorders at the end of the peak age of risk for anorexia nervosa and bulimia nervosa (33) . However, the age range of our sample may as well operate as a limitation, since some forms of eating disorders (e.g., binge eating disorder) or other disordered eating behaviors may yet be unexpressed by the mid-20s. Limitations further include self-report bias, less than perfect sensitivity of the screening for eating disorders (particularly among men), and the lack of statistical power resulting from the small number of detected cases of anorexia nervosa and bulimia nervosa among males.
Conclusion
We found little support for the hypothesis that prenatal feminizing, masculinizing or, demasculinizing gonadal hormones in utero or later socialization influence the neurodevelopment of female or male cotwins in a way that increases or decreases their vulnerability to eating disorders or disordered eating in later life. It is worth noting that so far, the intriguing theory of prenatal hormonal effects in utero and later development of disordered eating is based on observed twin concordance rates only, and the studies showing directly measured association with postnatal sex hormones are conducted only among females (5 – 8) . Thus, since a whole line of research suggests the importance of gonadal hormones in eating disorders, appetite, and disordered eating, future studies should still explore the hypothesized prenatal effect directly in humans.
1. Hudson JI, Hiripi E, Pope HG, Kessler KC: The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 2007; 1:348–358Google Scholar
2. Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL: Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry 2006; 63:305–312Google Scholar
3. Woodside DB, Garfinkel PE, Lin E, Goering P, Kaplan AS, Goldbloom DS, Kennedy SH: Comparisons of men with full or partial eating disorders, men without eating disorders, and women with eating disorders in the community. Am J Psychiatry 2001; 158:570–574Google Scholar
4. Strober M, Freeman R, Lampert C, Diamond J, Kaye W: Males with anorexia nervosa: a controlled study of eating disorders in first-degree relatives. Int J Eat Disord 2001; 29:263–269Google Scholar
5. Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Marc Breedlove S: Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med 2006; 36:539–546Google Scholar
6. Klump KL, Perkins PS, Burt SA, McGue M, Iacono WG: Puberty moderates genetic influences on disordered eating. Psychol Med 2007; 37:627–634Google Scholar
7. Edler C, Lipson SF, Keel PK: Ovarian hormones and binge eating in bulimia nervosa. Psychol Med 2007; 37:131–141Google Scholar
8. Sundblad C, Bergman L, Eriksson E: High levels of free testosterone in women with bulimia nervosa. Acta Psychiatr Scand 1994; 90:397–398Google Scholar
9. Montgomery SM, Ehlin A, Ekbom A: Smoking during pregnancy and bulimia nervosa in offspring. J Perinat Med 2005; 33:206–211Google Scholar
10. Favaro A, Tenconi E, Santonastaso P: Perinatal factors and the risk of developing anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry 2006; 63:82–88Google Scholar
11. Culbert KM, Breedlove SM, Burt SA, Klump KL Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry 2008; 65:329–336Google Scholar
12. Coyle JT: Erratum: “Prevalence, heritability, and prospective risk factors for anorexia nervosa” (2006; 63:305–312). Arch Gen Psychiatry 2008; 65: 994Google Scholar
13. Procopio M, Marriott P: Intrauterine hormonal environment and risk of developing anorexia nervosa. Arch Gen Psychiatry 2007; 64:1402–1407Google Scholar
14. Kaprio J, Pulkkinen L, Rose RJ: Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res 2002; 5:366–371Google Scholar
15. Garner DM: Eating Disorder Inventory 2, Professional Manual. Odessa, Fla, Psychological Assessment Resources, 1991Google Scholar
16. Keski-Rahkonen A, Neale BM, Bulik CM, Pietiläinen KH, Rose RJ, Kaprio J, Rissanen A: Intentional weight loss in young adults: sex-specific genetic and environmental effects. Obes Res 2005; 13:745–753Google Scholar
17. Keski-Rahkonen A, Sihvola E, Raevuori A, Kaukoranta J, Bulik CM, Hoek HW, Rissanen A, Kaprio J: Reliability of self-reported eating disorders: optimizing population screening. Int J Eat Disord 2006; 39:754–762Google Scholar
18. Wilson PWF, Paffenbarger RS, Morris JN, Havlik RJ: Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J 1986; 111:1177–1192Google Scholar
19. Kujala UM, Kaprio J, Sarna S, Koskenvuo M: Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA 1998; 279:440–444Google Scholar
20. Olivardia R, Pope HG Jr, Hudson JI: Muscle dysmorphia in male weightlifters: a case-control study. Am J Psychiatry 2000; 157:1291–1296Google Scholar
21. Raevuori A, Keski-Rahkonen A, Bulik CM, Rose RJ, Rissanen A, Kaprio J: Muscle dissatisfaction in young adult men. Clin Pract Epidemol Ment Health 2006; 2:6Google Scholar
22. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, Biometrics Research, NY, State Psychiatric Institute, 2002Google Scholar
23. Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A: Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 2007; 164:1259–1265Google Scholar
24. Keski-Rahkonen A, Hoek HW, Linna MS, Raevuori A, Sihvola E, Bulik CM, Rissanen A, Kaprio J. Incidence and outcomes of bulimia nervosa: a nationwide population-based study. Psychol Med 2008; 8:1–9Google Scholar
25. Williams RL: A note on robust variance estimation for cluster correlated data. Biometrics 2000; 56:645–646Google Scholar
26. Stata, Version 9.0. College Station, Tex. http://www.stata.comGoogle Scholar
27. von Ranson KM, Klump KL, Iacono WG, McGue M: The Minnesota Eating Behavior Survey: a brief measure of disordered eating attitudes and behaviors. Eat Behav 2005; 6:373–392Google Scholar
28. Madrid JA, Lopez-Bote C, Martin E: Effect of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiol Behav 1993; 53:329–335Google Scholar
29. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP: The continuing epidemics of obesity and diabetes in the United States. JAMA 2001; 286:1195–1200Google Scholar
30. Kendler KS: Twin studies of psychiatric illness: an update. Arch Gen Psychiatry 2001; 58:1005–1014Google Scholar
31. Rogers L, Resnick MD, Mitchell JE, Blum RW: The relationship between socioeconomic status and eating-disordered behaviors in a community sample of adolescent girls. Int J Eat Disord 1997; 22:15–23Google Scholar
32. Pope HG Jr, Katz DL, Hudson JI: Anorexia nervosa and “reverse anorexia” among 108 male bodybuilders. Compr Psychiatry 1993; 34:406–409Google Scholar
33. Currin L, Schmidt U, Treasure J, Jick H: Time trends in eating disorder incidence. Br J Psychiatry 2005; 186:132–135Google Scholar