Divided Doses for Methadone Maintenance
Opioid dependence, when untreated, typically progresses in a downhill course. Periods of remission are, more often than not, followed by a return to drug use accompanied by psychiatric and medical morbidity, incarceration, and frequently death. Opioid agonist therapy has proven to be a highly effective treatment for this otherwise highly morbid disorder (1) . Both methadone and buprenorphine dramatically attenuate the painful drug craving experienced during opioid withdrawal and abstinence, thus mitigating the perceived need for the drug. Key components of agonist therapies are their oral formulation and long half-life, allowing once-a-day dosing in a clinic or outpatient setting.
Nevertheless, a sizable number of patients who undergo agonist therapy persist in their illicit opioid use despite medication compliance. Continued, or a return to, opioid use during agonist therapy is often the result of breakthrough craving. When this occurs, increasing the agonist dose is typically the most appropriate intervention. For a small number of patients, breakthrough craving occurs because of 1) a genetic variant of the P450 3A4 or 2D6 enzymes, which increases methadone metabolism; 2) the concomitant use of other medications or alcohol that also induces P450; or 3) the hypermetabolism and increased volume of distribution during pregnancy. These patients may require twice-daily (split) dosing to produce more stable, steady-state methadone levels. However, the vast majority of patients with break-through craving are managed with an increase in their once-daily methadone dose.
An intriguing study in this issue of the Journal raises serious questions regarding this latter approach. Langleben et al. report that both cue-induced craving and its associated neural activation are heightened just prior to methadone dosing compared with the same measures obtained just after methadone dosing. In this thoughtfully designed study, heroin-dependent patients maintained on stable doses of methadone were assessed during two discrete periods: 90 minutes after (post) their morning dose of methadone and 90 minutes before (pre) their morning methadone dose. These two assessments roughly coincide with serum peak and trough methadone concentrations, although the two sessions were obtained 3 to 4 weeks apart (randomized and counterbalanced) to minimize the effect of the repeated measure. Craving was induced with visual heroin-associated stimuli, and brain activity was assessed with functional magnetic resonance imaging (fMRI). The investigators found that, whereas basal measures of craving before and after methadone dosing were not significantly different (p=0.2), cue-induced craving was markedly higher just prior to methadone dosing (p<0.002). Both sessions resulted in limbic neural activation, consistent with cue-reactivity studies in opioid-, cocaine-, alcohol-, and nicotine-dependent subjects. Importantly, however, cue-induced craving prior to methadone induced significantly greater activation in the amygdala, hippocampus, and insula compared with the session following methadone administration. Increases in amygdala and hippocampal signaling offer compelling evidence that activation of drug-related memories was more intense in the premethadone session. (Although an increase in insular activation may reveal interoceptive awareness of the drug-associated cues, this process is generally attributed to the anterior insula instead of the posterior insula [as seen in Figure 1 of the Langleben et al. article, bottom panel.]) Previous reports documenting an association between cue-induced neural activation in cocaine- or alcohol-dependent patients and subsequent relapse provide clinical relevance to these findings (2 , 3) .
There are shortfalls in the Langleben et al. study that may mitigate the findings. It is not known if other medications were prescribed that could have altered neural responsivity or methadone metabolism. At least some subjects reported relatively recent heroin use (4 days prior to the study). The lack of a placebo control did not allow a drug-expectancy effect to be assessed. The relationship between cue-induced craving and other withdrawal-related symptoms was not explored, and the relationship between craving and fMRI response with methadone levels was not presented. These potential weaknesses guide the way for future investigations. Nevertheless, the straightforward results of the study should alert the clinician that once-daily dosing may not suffice for a number of methadone-maintained patients. Particularly troubling is that basal measures of craving did not differ in the pre- and postsessions, suggesting that breakthrough craving may only occur in the presence of drug cues. This may obscure dosing troughs until cue-induced relapse has already occurred.
Although the appealing brain images focus our attention, do Langleben et al. really tell us anything new? When methadone maintenance was first developed as a treatment for heroin dependence, the once-daily regimen was instituted as a result of methadone’s long half-life and ease of administration in a clinic setting requiring on-site administration (4) . This dosing strategy has continued given its proven effectiveness. However, methadone dosing is thrice daily (or more) when used as an analgesic. The rationale for these divergent dosing approaches is presumably a result of differing pharmacodynamic requirements for opioid receptors involved in addiction relative to pain. Yet many investigators, including Dyer and White (5) and Kreek (6) , have noted that 30% to 40% of methadone-maintained patients endorse a progressive worsening of withdrawal symptoms that peak just prior to their methadone dose. Several decades of accumulated evidence may suggest that the once-daily dosing for methadone maintenance may not be sufficient for a large number of patients.
So how might the physician determine if split dosing is required? Absolute measures of serum methadone concentrations have not proven to be particularly helpful in guiding dosing (5) , although peak methadone levels should be above 400 ng/ml, and the ratio of peak-to-trough methadone levels should not differ by more than a factor of 2 (7) . Brain opioid receptor binding displacement by methadone, as measured by [ 11 C]diprenorphine binding with positron emission tomography, reveals that relatively few opioid receptors are displaced during peak methadone dosing, indicating that even sophisticated neuroimaging assessments of receptor displacement would also not assist in dosing strategies (8) . It appears that only patient endorsement of craving, withdrawal symptoms, or a return to heroin use—particularly just prior to the expected methadone dose—can accurately advise the clinician. Future research may offer better alternatives.
As a practical matter, it may be difficult to use split dosing for patients in methadone maintenance, particularly during the early stages of maintenance. Since federal regulations (42 CFR Part 8) require that take-home methadone be “limited to a single dose each week” during the first 90 days of treatment, twice-daily visits for split dosing would be impractical for most patients and nonviable because most clinics only offer morning dispensing. Federal regulations continue to limit take-home to “doses” rather than daily dosing for the first 9 months of treatment. These regulations can also be superseded by more restrictive state or program regulations. Although exemptions for split dosing can be requested, the study by Langleben et al. emphasizes the nontherapeutic rigidity of the federal guidelines. That being said, split dosing should not be taken lightly. Compliance typically drops sharply with twice-daily dosing, diversion worsens, and costs increase. An alternative approach, of course, is to switch these patients to the even longer acting partial agonist buprenorphine, although empirical (9) and anecdotal evidence suggests that this medication may also require split dosing in some patients.
Langleben et al. highlight the usefulness of well-designed neuroimaging studies. Although perhaps only confirming clinical observations described over decades of study, there is something particularly convincing about seeing our clinical insights confirmed in the visual images of neurobiological activation.
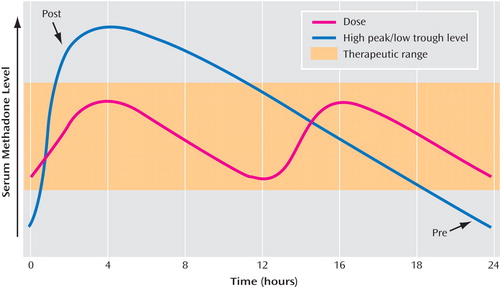
a Splitting the dose (red line) keeps serum methadone level within the therapeutic range (orange zone), avoiding high peak and low trough levels (blue line) (8). In Langleben et al., the approximate times of the sessions are noted by “post” (postmethadone) and “pre” (premethadone). Figure adapted from Leavitt SB: “Methadone Dosing and Safety in the Treatment of Opioid Addiction,” Addiction Treat Forum 2003; (Sept):1–8. Copyright © Addiction Treatment Forum. Reprinted with permission.
1. Lowinson JH, Marion I, Joseph H, Langrod J, Salsitz EA, Payte JT, Dole VP: Methadone maintenance, in Substance Abuse: A Comprehensive Textbook. Edited by Lowinson JH, Ruiz P, Millman RB, Langrod JG. Philadelphia, Lippincott Williams and Wilkins, 2005, pp 616–633Google Scholar
2. Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE: Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005; 183:171–180Google Scholar
3. Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A: Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004; 175:296–302Google Scholar
4. Dole VP, Nyswander ME, Kreek MJ: Narcotic blockade. Arch Intern Med 1966; 118:304–309Google Scholar
5. Dyer KR, White JM: Patterns of symptom complaints in methadone maintenance patients. Addiction 1997; 92:1445–1455Google Scholar
6. Kreek MJ: Medical safety and side effects of methadone in tolerant individuals. JAMA 1973; 223:665–668Google Scholar
7. Leavitt SB: Methadone dosing and safety in the treatment of opioid addiction (special report). Addiction Treatment Forum 2003; (Sept):1–8Google Scholar
8. Melichar JK, Hume SP, Williams TM, Daglish MR, Taylor LG, Ahmad R, Malizia AL, Brooks DJ, Myles JS, Lingford-Hughes A, Nutt DJ: Using [ 11 C]diprenorphine to image opioid receptor occupancy by methadone in opioid addiction: clinical and preclinical studies. J Pharmacol Exp Ther 2005; 312:309–315 Google Scholar
9. Rosado J, Walsh SL, Bigelow GE, Strain EC: Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100 mg of daily methadone. Drug Alcohol Depend 2007; 90:261–269Google Scholar



