Elucidating the Role of Brain-Derived Neurotrophic Factor in the Brain
Brain-derived neurotrophic factor (BDNF) is the most prevalent growth factor in the brain and regulates diverse aspects of neuronal function. However, the specific mechanisms whereby BDNF regulates complex behavior have been difficult to assess because of a lack of pharmacological tools selective for BDNF and the low viability of the BDNF knockout mouse. Partial (but viable) BDNF knockout animals, where gene expression is reduced at an early developmental stage, mix the effects of the BDNF depletion itself with the developmental abnormalities caused by low BDNF.
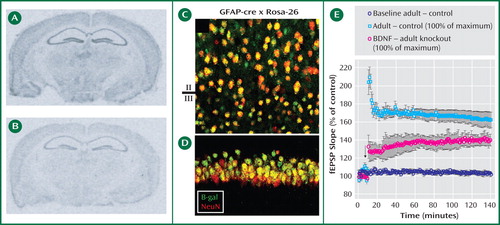
Figure 1. The images in A and B show an in situ hybridization analysis of BDNF mRNA levels in brain slices. “A” is from a normal (wild type) mouse and “B” from a transgenic mouse, each 6 months old, with the BDNF gene deleted at 12 weeks of age. Contrasting the figures shows lower levels of BDNF expression in the adult knockout animal (B) compared to the wild type (A) throughout the neocortex and the hippocampus. In images C and D, fluorescent immunohistochemistry analysis illustrates the tissue distribution of the BDNF transgene: neurons are red, cells expressing the BDNF transgene are green, and the combination (i.e., neurons that express the transgene) are yellow. Ninety-eight percent of neurons in the neocortex (C) and of the hippocampus (D) undergo recombination to express the transgene (appearing in yellow). The graph E shows the change in field excitatory postsynaptic potentials (fEPSP) in CA1 evoked by stimulation from CA3; the adult control mouse showed at least a 170% enhancement of fEPSP (i.e., full long-term potentiation), whereas the BDNF knockout mouse showed only a partial enhancement of long-term potentiation at the same stimulation, suggesting a modulatory effect of BDNF on long-term potentiation induction.
To study BDNF function in the adult brain, we developed an inducible mouse BDNF knockout, illustrated in the figure (A, B), where BDNF expression can be blocked at specific developmental times and in defined brain regions. Moreover, in this knockout animal, BDNF is not entirely deleted, but its levels are diminished. In the adult mouse BDNF knockout, BDNF reductions can be demonstrated in neurons of the neocortex and the hippocampus (C, D). Behavioral studies with this inducible knockout animal show that BDNF has distinguishable functions if depleted during early development (e.g., extreme impairments in learning and memory) compared with depletion only in the adult brain (e.g., measurable but less extensive impairments). The adult reduction in BDNF results in diminished long-term potentiation in the hippocampus, the process that is believed to be the cellular mechanism of learning and memory (E) and in alterations in memory performance in the knockout mice. Curiously, reductions in BDNF in adult animals show gender-specific differences in motor and depression behaviors. Specifically, adult male BDNF knockouts show hyperactivity without depression behavior, whereas the female knockout mice show no motor changes but they express depression behaviors. It may be the case that the loss of BDNF increases the vulnerability of cerebral systems to adverse environmental events and decreases overall neural resilience.



