Frontotemporal Dementia and Mania
Case Presentation
“Ms. V,” a 60-year-old college-educated woman, was brought by her daughter to the emergency department at a teaching hospital for the evaluation of heart palpitations. Ms. V had personality changes and mood swings with aggressive verbal and physical behaviors that had progressively worsened over the past year. A psychiatric consultation was requested to evaluate these emotional outbursts. On the day of admission, Ms. V had had a court hearing for assaulting her brother, who is disabled due to mental retardation. One month before presentation, she had held a pillow over his face in front of the brother’s social worker, who then called the police. After this event, her brother, who has been dependent on others for self-care since childhood, disappeared for several days. She was unconcerned about her legal situation and appeared cold and indifferent. She did not report that she did anything more than throw a pillow at her brother’s face, and she laughed when she heard that he was missing. Ms. V’s daughter reported that her mother had become increasingly irritable, with episodes of unprovoked shouting of profanities at strangers and family members. These behaviors were uncharacteristic of the patient’s personality at baseline. She had had an increase in goal-directed activities, cleaning the house constantly, checking the locks, and checking the stove. Two years before, Ms. V was fired from her job as a school administrative assistant because of difficulties managing her relationships with the students’ parents. Since then, she had not made any attempts to seek other employment. There was no history of head trauma, loss of consciousness, seizures, or previous contact with mental health providers.
Ms. V reported no new stressors but did report some “moodiness” over the last 6 months. She reported no angry outbursts or violence but endorsed a decreased need for sleep (now 6 hours, down from 8, per night). Although she did not report feeling euphoric, she reported increased irritability, daytime energy, task-oriented behavior, impulsivity, distractibility, racing thoughts, and pressured speech. Ms. V did not report feeling depressed or experiencing anhedonia. She reported no anxiety or psychotic symptoms, substance use, or recent changes in medications. Ms. V did not report any specific memory complaints, word-finding difficulties, misplacing or losing objects, or problems using transportation, following directions, or navigating. Her family history was notable for a brother with mental retardation and a sister with unipolar depression. Ms. V’s vital signs were within normal limits, and complete physical and neurologic examinations were unremarkable. A CBC, a Chem 10 (basic chemistries), liver function tests, tests of thyroid-stimulating hormone, free thyroxine, rapid plasma reagin, B 12 , and folate, an ECG, a chest X-ray, and a head computerized tomography with and without contrast showed no abnormalities.
Mental Status Examination
Ms. V appeared a well-dressed and well-groomed woman with mild psychomotor agitation. She made good eye contact, although she rolled her eyes frequently when hearing her daughter discuss her emotional outbursts. She breached personal boundaries by touching the interviewer’s name tag without asking. Her speech was fluent, rapid, and pressured. She reported that her mood was “good.” Her affect was expansive, silly at times, and bordering on childish, and she often laughed inappropriately. Her thought process was rambling and circumstantial. Her thought content was without any overt auditory or visual hallucinations, paranoid ideations, and suicidal or homicidal thoughts. Her insight and judgment were poor. On the Mini-Mental State Examination, Ms. V scored 30/30.
Differential Diagnosis
The insidious onset of behavioral symptoms in a woman of this age suggested a neurological process such as neurodegeneration, a silent cerebral infarct, or, less likely, an intracranial mass. Temporal lobe epilepsy as a cause of intermittent aggressive outbursts was also considered. Finally, a primary psychiatric disorder such as late-onset bipolar disorder or unipolar depression was also in the differential diagnosis.
Follow-Up Course
The psychiatric team determined that Ms. V was not at immediate risk of self-harm or violence, so she was discharged with close outpatient follow-up. Because of a strong suspicion for a neurodegenerative process, in particular frontotemporal dementia, she was referred to a neurology clinic that specializes in the treatment of neurodegenerative disease with a particular expertise in frontotemporal dementia. There it was noted that on direct questioning Ms. V had difficulty with object naming and short- and long-term memory.
Formal cognitive testing revealed deficits in visual and verbal memory, confrontation naming, word recognition, and executive function. An electromyograph showed no evidence of motor neuron disease, and homocysteine levels were within normal limits. Brain magnetic resonance imaging (MRI) showed moderate to severe symmetric atrophy of the bilateral frontal lobes and insula with relative sparing of the dorsal frontal regions. There was asymmetric anterior temporal lobe atrophy, left greater than right, though the amygdalae were relatively spared and the hippocampi were moderately affected ( Figure 1 , top). Using an unbiased, quantitative, image analysis technique (voxel-based morphometry), we compared her scan to those of 94 age-matched (mean=67, SD=10) female comparison subjects ( Figure 1 , bottom). This analysis revealed significant atrophy in the subgenual cingulate gyrus in the ventromedial orbital frontal cortex. Other areas that showed less severe atrophy included the lateral orbitofrontal cortex, anterior cingulate, and hippocampi bilaterally. Ms. V was given escitalopram, 10 mg/day, to which she had a transient favorable response, with a decrease in aggressive behavior. However, over several weeks, this effect diminished, and her disinhibition escalated. For example, during this time, she found her way into a private funeral, allegedly hit the hostess, whom she felt had insulted her, and in response was physically assaulted by other attendees of the service. The violence stopped when her daughter shouted to the crowd that her mother had Alzheimer’s disease. The escitalopram was increased to 20 mg/day, and she was given quetiapine, titrating to 75 mg b.i.d., with improvement of her aggressive behavior.
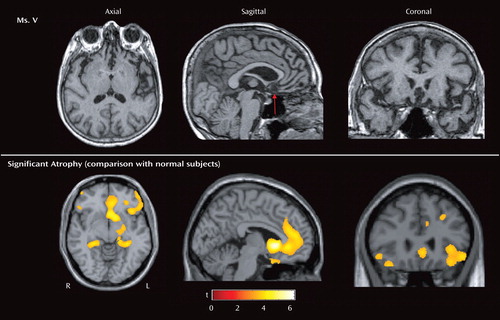
a The red arrow indicates the area of most severe atrophy. Voxel-based morphometry on high-resolution T 1 -weighted MRI images was used to identify brain regions that showed significantly greater voxelwise gray matter volume loss in Ms. V in relation to 94 normal comparison subjects (1, 2). The technique comprises an image-preprocessing step (spatial normalization, segmentation, modulation, and smoothing) followed by statistical analysis. Both stages were implemented in the SPM2 software package (www.fil.ion.ucl.ac.uk/spm) with standard procedures (2). Areas of significant atrophy are displayed on axial, sagittal, and coronal sections of a standard brain (bottom) and thresholded at p<0.001, uncorrected for multiple comparisons (coordinates: x=–4.1, y=31.4, z=–6.2). The most significantly atrophied area is the subgenual cingulate gyrus in the ventromedial orbitofrontal cortex (p<0.05, corrected for multiple comparisons). Other areas of less severe atrophy include the lateral orbitofrontal cortex, anterior cingulate, and hippocampi bilaterally.
Discussion
Although medical causes must always be ruled out in the case of new-onset mania, this is particularly important in the elderly. Older patients with new-onset mania are more than twice as likely to suffer from a comorbid neurological disorder (3) , including silent cerebral infarcts (65% in new-onset versus 25% in chronic illness) (4) . In addition to a standard history and a physical, these patients require a thorough neurologic examination, neuroimaging, and other selected tests (5) . Reported neurological causes of late-onset mania include stroke, tumors, epilepsy, Huntington’s disease and other movement disorders, multiple sclerosis and other white matter diseases, head trauma, infections such as neurosyphilis, Creutzfeldt-Jakob disease, and frontotemporal dementia (for a review, see references 6–8).
Ms. V’s symptoms of irritability, pressured speech, expansiveness, decreased need for sleep, impulsivity, and psychomotor agitation all suggested mania. Clues that she had a neurodegenerative disease included her age as well as the chronic and progressive nature of her symptoms. The profound loss of sympathy and empathy for others, along with severe disinhibition and the repetitive motor behaviors (e.g., checking locks), suggested frontotemporal dementia. Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices, has several clinical subtypes (for a comprehensive review, see references 9 and 10 ), and is a common cause of dementia in patients younger than 65 (11) . The frontal lobe variant of frontotemporal dementia (also known as behavioral variant or social/executive disorder variant) is characterized by insidious behavioral and personality changes, and often the initial presentation lacks any clear neurological signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy for others, repetitive motor behaviors, overeating, and frontal/executive deficits on neuropsychological testing. Because of these features, in one series (12) , patients with frontotemporal dementia were more likely to initially present to a psychiatrist than to a neurologist and often received erroneous psychiatric diagnoses before frontotemporal dementia was correctly diagnosed.
Affective blunting, loss of insight and social awareness, lack of concern about the disease process (so-called la belle indifference ), coldness, and lack of empathy for family and friends were prominent symptoms in Ms. V and are common symptoms in frontal lobe variant frontotemporal dementia (9 , 13 , 14) . Furthermore, damage to the ventromedial portion of the frontal cortex, as seen in Ms. V, leads to disinhibition, poor impulse control, and antisocial behavior in patients with discrete lesions (15) and frontotemporal dementia (16 , 17) . Similarly, atrophy to a frontal-temporal circuit, including the ventromedial frontal cortex in frontotemporal dementia, has been linked to loss of empathy (18) . Patients with frontotemporal dementia with asymmetric atrophy of the nondominant frontal lobe have also been shown to exhibit dramatic alterations in their self as defined by changes in political, social, or religious values (19) . Stereotyped behaviors, such as compulsive cleaning, pacing, and collecting are also common in frontotemporal dementia; in addition to often presenting as acute mania (e.g., reference 20 ), patients with frontotemporal dementia frequently present solely with compulsive symptoms (21) . In dementia, these repetitive motor behaviors are associated with atrophy in the right supplementary motor region of the frontal lobes (17) . Ms. V was also apathetic (e.g., not seeking a new job), which is a common symptom in frontotemporal dementia associated with atrophy of the anterior cingulate and medial frontal lobes (22) . This can be confused with depression, which is not common in frontotemporal dementia; patients with frontotemporal dementia are apathetic and emotionally withdrawn but not sad or suicidal. Often (up to one-third) of patients with frontotemporal dementia exhibit euphoria, which can take the form of elevated mood, inappropriate jocularity, and exaggerated self-esteem that can be indistinguishable from hypomania or mania (for a review, see reference 23 ). Gluttonous overeating is also common in frontotemporal dementia (24 – 26) and appears to be due to disruption of a right-side orbitofrontal-insular-striatal circuit (27 , 28) . Similarly, an exaggerated craving for carbohydrates has also been demonstrated in frontotemporal dementia by questionnaire (24 , 25 , 29) and laboratory (27) studies. The absence of these symptoms in Ms. V may be due to relative sparing of her right ventral insula and striatum.
Therapeutically, serotonin reuptake inhibitors are common first-line agents given the well-described serotonergic deficits in frontotemporal dementia (29 , 30) . A recent meta-analysis demonstrated these agents are effective in the treatment of frontotemporal dementia (31) (but see reference 32 ). Atypical antipsychotics and mood stabilizers can also be used to control the behavioral symptoms of frontotemporal dementia (33) , although data on their efficacy is limited. Furthermore, patients with frontotemporal dementia are known to have dopaminergic deficits (31) and are particularly prone to developing extrapyramidal side effects (34) . Although acetylcholine deficits are well described in Alzheimer’s disease (35) and have led to successful therapeutic use of acetylcholinesterase inhibitors in this disease (36 , 37) , the acetylcholine system in frontotemporal dementia appears to be relatively intact (30 , 31) . In our and others’ clinical experience, acetylcholinesterase inhibitors are ineffective in frontotemporal dementia and contraindicated since they can worsen patients’ agitation, anxiety, behavioral alteration, and restlessness (34 , 38 , but see reference 39 ). The N -methyl- d -aspartic acid (NMDA) antagonist memantine has also been tried, and one small, open-label, uncontrolled study has shown it may be helpful for behavioral disturbances in frontotemporal dementia (40) .
Historically, the clinical features of what would first be termed Pick’s disease and later called frontotemporal dementia were first delineated by the German psychiatrist Arnold Pick in 1892 (41) . He described a 71-year-old gentleman with progressive aphasia, apraxia, and behavior change in association with frontotemporal atrophy. In 1926, pathologists found that round intraneuronal inclusions, when, stained with silver, were characteristic of this illness (42) . In honor of Arnold Pick, these inclusions were later called Pick bodies (43) . More recently, these inclusions were found to contain aggregations of insoluble hyperphosphorylated tau protein. Thus, frontotemporal dementia came to be grouped in a class of neurodegenerative diseases called the tauopathies (others include semantic dementia, primary progressive aphasia, progressive supranuclear palsy, and corticobasal degeneration).
The understanding of frontotemporal dementia has improved over the past 15 years. During this time, large autopsy studies have found that the “classic” tau pathology of frontotemporal dementia is actually absent in 60% of patients with clinically diagnosed frontotemporal dementia. Instead, many of these patients’ brains display ubiquitin-immunoreactive neuronal cytoplasmic inclusions and neuritic changes in the cerebral cortex and the hippocampus (44 , 45) . This neuropathologic pattern was labeled frontotemporal lobar degeneration with ubiquitin inclusions (46) . Until very recently, both the identity of the ubiquinated protein that comprises the inclusions and the underlying pathophysiology of frontotemporal lobar degeneration with ubiquitin inclusions have remained a mystery.
A clue to better understanding frontotemporal lobar degeneration with ubiquitin inclusions lay in the fact that overall, frontotemporal dementia has a strong genetic component with up to 20% of cases showing a highly penetrant, autosomal dominant pattern of disease transmission (47 – 49) . In 1998, in a subset of patients with frontotemporal dementia demonstrating classic tau pathology, mutations in the microtubule-associated protein tau gene on chromosome 17q21 were identified (50) . These mutations remain relatively uncommon, even in familial forms of frontotemporal dementia (51) .
In 2006, two back-to-back articles in Nature reported that mutations in the gene for a widely expressed growth factor called progranulin caused multiple cases of familial and even some cases of sporadic frontotemporal lobar degeneration with ubiquitin inclusions (52 , 53) . The investigators described a variety of frameshift mutations, initiation codon, and nonsense mutations, all leading to a null mutation in one of the two progranulin alleles, resulting in haploinsufficiency (i.e., loss of functional progranulin). In an apparent coincidence, the progranulin gene is also found in chromosome region 17q21, only 1.7 Mb upstream of microtubule-associated protein tau gene, explaining why a number of frontotemporal dementia families who had been shown to have mutations linked to the chromosome 17q21 region lacked any abnormalities in their microtubule-associated protein tau gene (54) . Overall, progranulin mutations account for approximately 10% of frontotemporal dementia cases and approximately 20% of cases with a family history (53 , 55 , 56) .
Progranulin is known to play systemic roles in development, wound repair, and inflammation through its effects on cell cycle progression and motility (57 – 59) . In the CNS, it is most highly expressed in pyramidal neurons and activated microglia, but its specific role in the brain remains unknown (60) . Of interest, when progranulin is overexpressed, it has been linked to tumor genesis, possibly through induction of vascular endothelial growth factor (58 , 59 , 61) , whereas in circumstances of haploinsufficiency, it leads to neurodegeneration.
Frontotemporal dementia patients are approximately evenly divided between those with tau or frontotemporal lobar degeneration with ubiquitin inclusions. In those with the frontotemporal lobar degeneration with ubiquitin inclusions histopathologic pattern, one subset has lentiform intranuclear neuronal inclusions. It is this subset of frontotemporal lobar degeneration with ubiquitin inclusions patients with both neuronal cytoplasmic inclusions and lentiform intranuclear neuronal inclusions that have been found to have mutations in the progranulin gene. Of importance, neuronal cytoplasmic inclusions and lentiform intranuclear neuronal inclusions do not stain for progranulin and instead were recently found to stain for another protein, TAR DNA binding protein-43 (62) . This is consistent with the finding that the progranulin mutations that cause frontotemporal dementia are null alleles leading to a dearth of wild-type progranulin and little to no production of mutant progranulin protein or sometimes even its mRNA. In addition, the discovery that the nuclear protein TAR DNA binding protein-43 is found in the inclusions of all frontotemporal lobar degeneration with ubiquitin inclusions subtypes (i.e., in both neuronal cytoplasmic inclusions and lentiform intranuclear neuronal inclusions) suggests that a common pathogenic pathway unites frontotemporal dementia’s disparate histopathologic forms.
There is great clinical variability, even within families sharing the same progranulin mutation (63 , 64) . Also, a recent study comparing patients with frontotemporal dementia with progranulin mutations to those with microtubule-associated protein tau gene mutations found few consistent phenotypic differences between the two groups (65) . Previous work has shown that 15% of patients with frontotemporal dementia have clinical and electromyograph findings consistent with amyotrophic lateral sclerosis (66) . However, in a large genetic survey, no patients with frontotemporal dementia-amyotrophic lateral sclerosis were found to have progranulin mutations, despite the fact that frontotemporal dementia-amytrophic lateral sclerosis patients also develop a frontotemporal lobar degeneration with ubiquitin inclusions histopathology (67) . Rather, the motor difficulties experienced by patients with frontotemporal dementia with progranulin mutations more frequently take the form of mild parkinsonism (65) .
Can this clinical variability even among patients with frontotemporal dementia with progranulin mutations be explained by the many different progranulin mutations already identified? This explanation appears unlikely in light of the considerable intrafamily phenotypic variability as well as the fact that although many progranulin mutations have been described, they all seem to result in the same endpoint, which is decreased levels of wild-type progranulin. Rather, the phenotypic variability most likely arises out of the variable anatomic distribution of the frontotemporal dementia pathology, which likely results from unknown genetic or environmental modifiers.
The recent advances in our understanding of the genetics and pathophysiology of frontotemporal dementia stimulate great hope for future research and therapies. In particular, the finding that insufficient levels of progranulin can result in frontotemporal dementia suggests a potential simple replacement treatment modality that awaits further investigation.
1. Ashburner J, Friston KJ: Voxel-based morphometry—the methods. Neuroimage 2000; 11(part 1):805–821Google Scholar
2. Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN, Frackowiak RS: Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage 2002; 17:29–46Google Scholar
3. Tohen M, Shulman KI, Satlin A: First-episode mania in late life. Am J Psychiatry 1994; 151:130–132Google Scholar
4. Fujikawa T, Yamawaki S, Touhouda Y: Silent cerebral infarctions in patients with late-onset mania. Stroke 1995; 26:946–949Google Scholar
5. Arciniegas DB: New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006; 163:198–203Google Scholar
6. Mendez MF: Mania in neurologic disorders. Curr Psychiatry Rep 2000; 2:440–445Google Scholar
7. Brooks JO III, Hoblyn JC: Secondary mania in older adults. Am J Psychiatry 2005; 162:2033–2038Google Scholar
8. Almeida OP: [Bipolar disorder with late onset: an organic variety of mood disorder?] Rev Bras Psiquiatr 2004; 26(suppl 3):27–30Google Scholar
9. Boxer AL, Miller BL: Clinical features of frontotemporal dementia. Alzheimer Dis Assoc Disord 2005; 19(suppl 1):S3–S6Google Scholar
10. McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ: Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on frontotemporal dementia and Pick’s disease. Arch Neurol 2001; 58:1803–1839Google Scholar
11. Ratnavalli E, Brayne C, Dawson K, Hodges JR: The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621Google Scholar
12. Gregory CA, Hodges JR: Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl 1996; 47:103–123Google Scholar
13. Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL: Self awareness and personality change in dementia. J Neurol Neurosurg Psychiatry 2005; 76:632–639Google Scholar
14. Rankin KP, Kramer JH, Mychack P, Miller BL: Double dissociation of social functioning in frontotemporal dementia. Neurology 2003; 60:266–271Google Scholar
15. Saver JL, Damasio AR: Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia 1991; 29:1241–1249Google Scholar
16. Rosen HJ, Lengenfelder J, Miller B: Frontotemporal dementia. Neurol Clin 2000; 18:979–992Google Scholar
17. Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL: Neuroanatomical correlates of behavioural disorders in dementia. Brain 2005; 128(part 11):2612–2625Google Scholar
18. Rankin KP, Kramer JH, Miller BL: Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol 2005; 18:28–36Google Scholar
19. Miller BL, Seeley WW, Mychack P, Rosen HJ, Mena I, Boone K: Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology 2001; 57:817–821Google Scholar
20. Gafoor R, O’Keane V: Three case reports of secondary mania: evidence supporting a right frontotemporal locus. Eur Psychiatry 2003; 18:32–33Google Scholar
21. Ames D, Cummings JL, Wirshing WC, Quinn B, Mahler M: Repetitive and compulsive behavior in frontal lobe degenerations. J Neuropsychiatry Clin Neurosci 1994; 6:100–113Google Scholar
22. Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, Phengrasamy L, Weiner M, Rosen HJ: Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 2004; 62:742–748Google Scholar
23. Perry RJ, Miller BL: Behavior and treatment in frontotemporal dementia. Neurology 2001; 56(suppl 4):S46–S51Google Scholar
24. Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D: Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 2001; 70:323–332Google Scholar
25. Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D: Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand 2001; 103:367–378Google Scholar
26. Miller BL, Darby AL, Swartz JR, Yener GG, Mena I: Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia 1995; 6:195–199Google Scholar
27. Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller BL: Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology (in press)Google Scholar
28. Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, Warren JD: VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage 2007; 35:207–213Google Scholar
29. Procter AW, Qurne M, Francis PT: Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord 1999; 10(suppl 1):80–84Google Scholar
30. Sparks DL, Markesbery WR: Altered serotonergic and cholinergic synaptic markers in Pick’s disease. Arch Neurol 1991; 48:796–799Google Scholar
31. Huey ED, Putnam KT, Grafman J: A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 2006; 66:17–22Google Scholar
32. Deakin JB, Rahman S, Nestor PJ, Hodges JR, Sahakian BJ: Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl) 2004; 172:400–408Google Scholar
33. Curtis RC, Resch DS: Case of Pick’s central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J Clin Psychopharmacol 2000; 20:384–385Google Scholar
34. Pijnenburg YA, Sampson EL, Harvey RJ, Fox NC, Rossor MN: Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. Int J Geriatr Psychiatry 2003; 18:67–72Google Scholar
35. Talesa VN: Acetylcholinesterase in Alzheimer’s disease. Mech Ageing Dev 2001; 122:1961–1969Google Scholar
36. Weiner MF, Martin-Cook K, Foster BM, Saine K, Fontaine CS, Svetlik DA: Effects of donepezil on emotional/behavioral symptoms in Alzheimer’s disease patients. J Clin Psychiatry 2000; 61:487–492Google Scholar
37. Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E: A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology 2001; 57:613–620Google Scholar
38. Merrilees JJ, Miller BL: Long-term care of patients with frontotemporal dementia. J Am Med Dir Assoc 2003; (suppl):S162–S164Google Scholar
39. Lampl Y, Sadeh M, Lorberboym M: Efficacy of acetylcholinesterase inhibitors in frontotemporal dementia. Ann Pharmacother 2004; 38:1967–1968Google Scholar
40. Swanberg MM: Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord 2007; 21:164–166Google Scholar
41. Pick A: Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prague, Prager Medicinische Wochenschrift, 1892, pp 165–167Google Scholar
42. Onari K, Spatz H: Anatomische beitrage zur lehre von der pickschen umschriebenen grosshirnrinden-atrophie (“Picksche krankheit”). Z Gesamte Neurol Psych 1926; 101:470–511Google Scholar
43. Brun A: Frontal lobe degeneration of non-Alzheimer type: I, neuropathology. Arch Gerontol Geriatr 1987; 6:193–208Google Scholar
44. Shi J, Shaw CL, Du Plessis D, Richardson AM, Bailey KL, Julien C, Stopford C, Thompson J, Varma A, Craufurd D, Tian J, Pickering-Brown S, Neary D, Snowden JS, Mann DM: Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol (Berl) 2005; 110:501–512Google Scholar
45. Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, Khan N, Al-Sarraj S, Revesz T: Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 2004; 30:369–373Google Scholar
46. The Lund and Manchester Groups: Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994; 57:416–418Google Scholar
47. Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, Lomen-Hoerth C, Wilhelmsen KC, Lee VM, Grossman M, Miller BL: Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 2005; 65:1817–1819Google Scholar
48. Rosso SM, Donker Kaat L, Baks T, Joosse M, de Koning I, Pijnenburg Y, de Jong D, Dooijes D, Kamphorst W, Ravid R, Niermeijer MF, Verheij F, Kremer HP, Scheltens P, van Duijn CM, Heutink P, van Swieten JC: Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain 2003; 126 (part 9):2016–2022Google Scholar
49. Chow TW, Miller BL, Hayashi VN, Geschwind DH: Inheritance of frontotemporal dementia. Arch Neurol 1999; 56:817–822Google Scholar
50. Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P: Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393:702–705Google Scholar
51. Rademakers R, Cruts M, van Broeckhoven C: The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 2004; 24:277–295Google Scholar
52. Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M: Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442:916–919Google Scholar
53. Cruts M, Kumar-Singh S, Van Broeckhoven C: Progranulin mutations in ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Curr Alzheimer Res 2006; 3:485–491Google Scholar
54. Pickering-Brown SM, Baker M, Gass J, Boeve BF, Loy CT, Brooks WS, Mackenzie IR, Martins RN, Kwok JB, Halliday GM, Kril J, Schofield PR, Mann DM, Hutton M: Mutations in progranulin explain atypical phenotypes with variants in MAPT. Brain 2006; 129 (part 11):3124–3126Google Scholar
55. Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Pickering-Brown SM, Graff-Radford N, Uitti R, Dickson D, Wszolek Z, Gonzalez J, Beach TG, Bigio E, Johnson N, Weintraub S, Mesulam M, White CL III, Woodruff B, Caselli R, Hsiung GY, Feldman H, Knopman D, Hutton M, Rademakers R: Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 2006; 15:2988–3001Google Scholar
56. van der Zee J, Le Ber I, Maurer-Stroh S, Engelborghs S, Gijselinck I, Camuzat A, Brouwers N, Vandenberghe R, Sleegers K, Hannequin D, Dermaut B, Schymkowitz J, Campion D, Santens P, Martin JJ, Lacomblez L, De Pooter T, Peeters K, Mattheijssens M, Vercelletto M, Van den Broeck M, Cruts M, De Deyn PP, Rousseau F, Brice A, Van Broeckhoven C: Mutations other than null mutations producing a pathogenic loss of progranulin in frontotemporal dementia. Hum Mutat 2007; 28:416Google Scholar
57. He Z, Ong CH, Halper J, Bateman A: Progranulin is a mediator of the wound response. Nat Med 2003; 9:225–229Google Scholar
58. He Z, Bateman A: Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res 1999; 59:3222–3229Google Scholar
59. He Z, Bateman A: Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 2003; 81:600–612Google Scholar
60. Mackenzie IR: The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol (Berl) 2007; 114:49–54Google Scholar
61. Tangkeangsirisin W, Serrero G: PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis 2004; 25:1587–1592Google Scholar
62. Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM: Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314:130–133Google Scholar
63. Spina S, Murrell JR, Huey ED, Wassermann EM, Pietrini P, Baraibar MA, Barbeito AG, Troncoso JC, Vidal R, Ghetti B, Grafman J: Clinicopathologic features of frontotemporal dementia with progranulin sequence variation. Neurology 2007; 68:820–827Google Scholar
64. Huey ED, Grafman J, Wassermann EM, Pietrini P, Tierney MC, Ghetti B, Spina S, Baker M, Hutton M, Elder JW, Berger SL, Heflin KA, Hardy J, Momeni P: Characteristics of frontotemporal dementia patients with a progranulin mutation. Ann Neurol 2006; 60:374–380Google Scholar
65. Snowden JS, Pickering-Brown SM, Mackenzie IR, Richardson AM, Varma A, Neary D, Mann DM: Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain 2006; 129(part 11):3091–3102Google Scholar
66. Lomen-Hoerth C, Anderson T, Miller B: The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 2002; 59:1077–1079Google Scholar
67. Le Ber I, van der Zee J, Hannequin D, Gijselinck I, Campion D, Puel M, Laquerriere A, De Pooter T, Camuzat A, Van den Broeck M, Dubois B, Sellal F, Lacomblez L, Vercelletto M, Thomas-Antérion C, Michel BF, Golfier V, Didic M, Salachas F, Duyckaerts C, Cruts M, Verpillat P, Van Broeckhoven C, Brice A, French Research Network on FTD/FTD-MND: Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 2007; 28:846–855Google Scholar



