Augmentation of Behavior Therapy With d -Cycloserine for Obsessive-Compulsive Disorder
Abstract
Objective: This study examined whether d -cycloserine, a partial agonist at the N -methyl- d -aspartate (NMDA) glutamatergic receptor, enhances the efficacy of behavior therapy for obsessive-compulsive disorder (OCD). Method: A randomized, double-blind, placebo-controlled trial investigating d -cycloserine versus placebo augmentation of behavior therapy was conducted in 23 OCD patients. Patients first underwent a diagnostic interview and pretreatment evaluation, followed by a psychoeducational/treatment planning session. Then they received 10 behavior therapy sessions. Treatment sessions were conducted twice per week. One hour before each of the behavior therapy sessions, the participants received either d -cycloserine, 100 mg, or a placebo. Results: Relative to the placebo group, the d -cycloserine group’s OCD symptoms were significantly more improved at mid-treatment, and the d -cycloserine group’s depressive symptoms were significantly more improved at posttreatment. Conclusions: These data provide support for the use of d -cycloserine as an augmentation of behavior therapy for OCD and extend findings in animals and other human disorders suggesting that behavior therapy acts by way of long-term potentiation of glutamatergic pathways and that the effects of behavior therapy are potentiated by an NMDA agonist.
Although both exposure-based behavior therapy and medications have proven efficacious for obsessive-compulsive disorder (OCD), the evidence that current pharmacologic strategies enhance the efficacy of behavior therapy has been mixed. Medications for OCD have traditionally targeted serotonergic pathways; generally, such medications have not been shown to substantially improve the efficacy of behavior therapy monotherapy, particularly after treatment is discontinued (1 , 2) . This pattern seems to be fairly consistent across the anxiety disorders (3) .
In recent years, there has been increasing interest in the N -methyl- d -aspartic acid (NMDA) subtype of glutamate receptor and its role in extinction learning such as that purported to take place during exposure-based behavior therapy (4) . Specifically, extinction of fear has been linked to NMDA receptor-dependent neural plasticity within the basolateral amygdala (5 – 11) . The involvement of the NMDA receptor in fear extinction has led to an increasing body of research suggesting that NMDA receptor agonists, such as the partial agonist d -cycloserine, may enhance extinction effects. Animal research indicates that rats receiving d -cycloserine in addition to extinction training show significantly less posttraining conditioned startle than do rats receiving extinction alone. Rats receiving d -cycloserine who did not also receive extinction training did not benefit, suggesting that the findings were due not to any anxiety-attenuating properties of d -cycloserine itself but rather to the facilitative effect of d -cycloserine on the neural mechanisms of extinction (12) .
In the first test of d -cycloserine augmentation of exposure therapy in anxiety-disordered humans, Ressler et al. (13) randomly assigned subjects with a fear of heights to receive placebo; d -cycloserine, 50 mg; or d -cycloserine, 500 mg; 1 hour before two sessions of exposure-based virtual-reality behavior therapy. The patients receiving d -cycloserine appeared to benefit more from exposure therapy than did the patients receiving placebo. At posttreatment, approximately 60% of the participants who received exposure plus d -cycloserine, versus approximately 20% of those receiving exposure plus placebo, rated themselves “much improved” or “very much improved.” These differences were maintained through a 3-month follow-up assessment. More recently, Hofmann and colleagues (14) extended these findings to patients with social anxiety disorder. Participants with significant public speaking anxiety were randomly assigned to receive d -cycloserine, 50 mg, or placebo 1 hour before four public speaking exposure sessions. At posttreatment, the participants receiving exposure plus d -cycloserine reported significantly less social anxiety compared to patients receiving exposure plus placebo. This difference was even greater at the 1-month follow-up, and controlled effect sizes were in the medium to large range.
To date, to our knowledge, two studies have investigated augmentation of behavior therapy with d -cycloserine in individuals with OCD. Storch and colleagues (15) randomly assigned 24 OCD patients to 12 weekly 75–90-minute sessions of behavior therapy plus 250 mg d -cycloserine versus behavior therapy plus placebo. The d -cycloserine or placebo was given 4 hours before the treatment sessions. They did not find any group differences with respect to OCD symptom decrease, as measured by the Yale-Brown Obsessive Compulsive Scale. A recent study by Kushner and colleagues (16) also tested whether d -cycloserine augments the efficacy of behavior therapy in patients with OCD. Twenty-five patients received either d -cycloserine, 125 mg, or placebo 2 hours before each twice-weekly session of exposure-based behavior therapy. The patients received up to 10 sessions of behavior therapy (treatment was terminated earlier if treatment goals were reached sooner). The results revealed no significant difference between the two groups at posttreatment or 3-month follow-up in OCD severity on the Yale-Brown Obsessive Compulsive Scale (17) . However, after four sessions, the patients in the d -cycloserine group reported significantly greater decreases in subjective units of distress associated with certain obsession-related stimuli compared to the placebo group. Unfortunately, this study did not include the Yale-Brown Obsessive Compulsive Scale or other comprehensive assessment midway through treatment.
Thus, so far, the research on d -cycloserine augmentation of behavior therapy in OCD has led to inconsistent results. The aim of the present study was to conduct a double-blind, placebo-controlled trial of d -cycloserine augmentation of 10 hours of twice-weekly behavior therapy in individuals with OCD. Similar to the d -cycloserine-augmented trials for height phobia (13) and social anxiety (14) , we also chose a briefer treatment duration than what would usually be administered for a non- d -cycloserine-augmented OCD treatment trial (1) . It was hypothesized that patients receiving d -cycloserine would show significantly greater improvement in OCD symptoms compared to patients receiving placebo and that the advantage of d -cycloserine would remain after treatment discontinuation. It was further predicted that d -cycloserine would result in a more rapid response to treatment, as evidenced by a mid-treatment assessment.
Method
Subjects
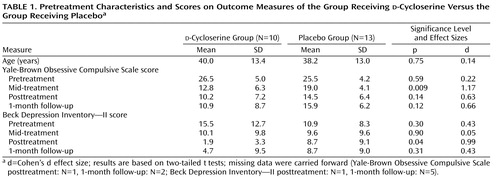
The OCD group was composed of 23 participants with a primary DSM-IV diagnosis of OCD (18) . The following comorbid diagnoses were obtained: major depression (N=3), social phobia (N=3), specific phobia (N=3), dysthymia (N=2), generalized anxiety disorder (N=2), anxiety disorder not otherwise specified (N=1), depressive disorder not otherwise specified (N=1), and panic disorder with agoraphobia (N=1). Sixteen OCD patients (seven patients receiving d -cycloserine, nine patients receiving placebo) were receiving a stable dose of psychotropic medication: paroxetine (N=4), bupropion (N=3), clonazepam (N=3), fluvoxamine (N=2), sertraline (N=2), alprazolam (N=1), buspirone (N=1), duloxetine (N=1), fluoxetine (N=1), risperidone (N=1), and venlafaxine (N=1). Medication doses had to be stable for 2 months before the baseline assessment and were stable over the course of the study. The groups did not differ with respect to the proportion of patients currently receiving psychiatric medications (χ 2 =0.47, df=1, p=0.62). The patients were enrolled at Massachusetts General Hospital, Boston, or the Institute of Living, Hartford, Conn. Therapists followed an abbreviated version of a well-validated behavior therapy treatment protocol by Kozak and Foa (19) that emphasizes exposure and response prevention (formal cognitive restructuring was not part of the protocol). The study therapists were supervised by clinicians specializing in OCD (S.W., D.F.T., and S.A.M.). The participants were recruited through flyers posted in the community and through the OCD clinics at Massachusetts General Hospital and the Institute of Living. Thirty-three OCD patients signed informed consent. Of those 33 patients, three did not meet inclusion criteria, one refused the treatment because he reported that he was “not ready for change,” and six discontinued treatment before the mid-treatment evaluation. Twenty-two patients completed the treatment and were included in the statistical analysis. One patient dropped out after the mid-treatment evaluation; his data were carried forward. Group characteristics are presented in Table 1 .
Study Design/Procedure
Written informed consent was obtained after the procedures had been fully explained. After the psychiatric pretreatment assessment, the patients underwent a medical assessment conducted by a study physician to screen for medical conditions that would contraindicate the use of d -cycloserine. The research pharmacies at Massachusetts General Hospital and the Institute of Living prepared and dispensed the study medication (100 mg d -cycloserine or placebo) and maintained the coded random assignment schedule for the double-blind design. The patients were asked to come to the clinic 1 hour before the treatment sessions to take the study medication in the presence of their therapists. After the pretreatment assessments, the patients underwent a psychoeducational/treatment planning session (90 minutes) during which the treatment was explained and an exposure hierarchy was created based on the patients’ subjective units of distress (see reference 19 ). Thereafter, the patients received 10 individual behavior therapy sessions (60 minutes each) held twice a week. d -Cycloserine (or placebo) was given 1 hour before the 10 exposure-based behavior therapy sessions. Additional assessments were held at mid-treatment (after session five), at posttreatment (after session 10), and at the 1-month follow-up to assess the patients’ improvement over the course of the study.
Outcome Measures
DSM-IV diagnoses were determined by the Structured Clinical Interview for DSM-IV (20) . OCD symptom severity was assessed with the clinician-administered Yale-Brown Obsessive Compulsive Scale (17) . As a secondary outcome measure, depressive symptom severity was examined with the Beck Depression Inventory—II (21) .
Statistical Analysis
Data were entered with SPSS version 10.0 (SPSS, Chicago) and analyzed with two separate two- (condition: d -cycloserine or placebo) by-four (time: pretreatment, mid-treatment, posttreatment, 1-month follow-up) mixed-factor general linear models with time as the repeated measure. The dependent variables were the two outcome measures (the Yale-Brown Obsessive Compulsive Scale and the Beck Depression Inventory—II, respectively). Significant general linear model findings were followed up by post hoc two-tailed t tests. Moreover, effect sizes (Cohen’s d) were computed to examine the magnitude of the effects. Data were analyzed both as intent-to-treat (with last observation carried forward) and completer-only analyses. The pattern of the results did not change, regardless of the kind of analyses. Thus, we only present the intent-to-treat analysis.
Results
Adverse Effects
None of the participants reported any adverse side effects during the course of the treatment.
OCD and Depressive Symptoms
The study outcome measures and raw scores are shown in Table 1 . The groups did not differ with respect to their pretreatment Yale-Brown Obsessive Compulsive Scale (T=0.54, df=21, p=0.59) and Beck Depression Inventory—II total scores (T=1.06, df=21, p=0.30). For the OCD symptoms (as measured with the Yale-Brown Obsessive Compulsive Scale), general linear models indicated a main effect of time (F=53.17, df=3, 63, p<0.001) and a time-by-condition interaction (F=3.53, df=3, 63, p=0.02). See Figure 1 . However, the main effect for condition did not reach significance (F=3.03, df=1, 21, p=0.10). Follow-up t tests indicated that the d -cycloserine group had significantly lower Yale-Brown Obsessive Compulsive Scale scores at mid-treatment than the placebo group (T=2.87, df=21, p=0.009). This was a large effect (Cohen’s d effect size=1.17). No significant group differences with respect to posttreatment (T=1.52, df=21, p=0.04) and 1-month follow-up were obtained (T=1.61, df=21, p=0.12); however, this may have been caused by the limited power (Cohen’s d effect sizes were d=0.63 and d=0.66, respectively).

a p=0.009.
With respect to depressive symptoms (as measured with the Beck Depression Inventory—II), general linear models indicated a main effect of time (F=9.53, df=3, 63, p<0.001) and a significant time-by-condition interaction (F=4.91, df=3, 63, p=0.004). See Figure 2 . However, no significant main effect for condition was obtained (F=0.18, df=1, 21, p=0.68). Follow-up t tests indicated that the d -cycloserine group had significantly fewer depressive symptoms at posttreatment (T=2.23, df=21, p=0.04); this was a large effect (Cohen’s d effect size=0.99). However, no significant group differences with respect to mid-treatment (T=0.13, df=21, p=0.90) and 1-month follow-up were obtained (T=1.03, df=21, p=0.31).

a p=0.04.
Discussion
The results of this double-blind, randomized, controlled trial indicate that patients in the d -cycloserine group had significantly lower OCD symptom severity scores than did patients in the placebo group at mid-treatment and that the effect size was large. At posttreatment and 1 month after the end of treatment, group differences in OCD severity no longer reached statistical significance, but effect sizes were still in the moderate range. d -Cycloserine-augmented behavior therapy was superior to placebo-augmented behavior therapy for depressive symptoms at posttreatment. Thus, our study showed that d -cycloserine augmentation may accelerate and potentiate behavior therapy for OCD.
Our positive findings differ somewhat from those of Storch et al. (15) , who found no significant benefit of d -cycloserine augmentation of behavior therapy for OCD at any point over the course of the treatment. It is likely that ceiling effects may have contributed to the results obtained in the study by Storch et al., which yielded an usually large reduction in score on the Yale-Brown Obsessive Compulsive Scale of over 70% in the behavior therapy plus placebo group (versus a 44% reduction in the present study). It is also possible that patient or therapist differences between the two studies contributed to a greater overall treatment effect in the study by Storch et al., thus obscuring any effect of d -cycloserine.
However, several methodological differences might also account for this discrepancy. For example, it is possible that the high doses resulted in diminished learning effects (22) . The present study used a dose of 100 mg, whereas Storch et al. (15) used a dose of 250 mg to augment behavior therapy for OCD. Previous studies investigating d -cycloserine for behavior therapy augmentation of anxiety disorders in humans have used a dose of 50 mg (13 , 14) , and Kushner and colleagues (16) used a dose of 125 mg. In the earliest d -cycloserine-augmented behavior therapy trial in humans, Ressler and colleagues (13) did not find evidence of a difference between a dose of 50 mg and a dose of 500 mg; however, other research suggests that there may be a narrow therapeutic range of d -cycloserine when used to enhance learning. For example, animal models suggest that the NMDA receptor can become desensitized after prolonged exposure to d -cycloserine (22) . Thus, it is possible that a high dose of d -cycloserine can have an attenuated effect on fear extinction compared to a lower dose, such as that used in the present study.
Another key variable appears to be the timing of d -cycloserine administration. Comparable to the promising study by Hofmann et al. on social anxiety (14) , in our OCD study, the medication was given 1 hour before each session. The OCD patients of Kushner et al. (16) took the medication 2 hours before each session, and in their study, the significant group differences occurred at session four. In contrast, Storch et al. (15) administered d -cycloserine 4 hours before each session of behavior therapy for OCD, and the groups did not differ significantly at any point during or after treatment. Thus, d -cycloserine might lose efficacy if given too early before extinction training; indeed, animal research suggests that d -cycloserine is most effective when administered immediately before or even immediately after extinction training (23) .
In addition to dosing and timing of medication administration, another important factor might be the number of behavior therapy sessions and the related frequency of d -cycloserine administration. Although the number of behavior therapy sessions was comparable across the two studies (10 sessions in the present study, 12 sessions in the study by Storch et al. [15] ), the present study used twice-weekly sessions, whereas Storch et al. provided weekly sessions. Therefore, the total duration of behavior therapy with concurrent d -cycloserine administration was 5 weeks in the present study versus 12 weeks in the study by Storch et al. The longer duration of the study by Storch et al. translates to a greater amount of between-session homework assignments that were not augmented with d -cycloserine; and this greater frequency of non- d -cycloserine-paired homework exposures may have obscured the effects of d -cycloserine.
It is also possible that the extinction-augmenting effects of d -cycloserine are time limited. Indeed, close examination of the results of Storch et al (15) suggests that there was a (nonsignificant) tendency favoring d -cycloserine in that study at week 6. Kushner et al. (16) found group differences at session 4, and in the current study, we found a large effect after five behavior therapy sessions. At posttreatment, none of the studies showed significant d -cycloserine versus placebo group differences. Thus, an inspection of all studies on the effects of d -cycloserine augmentation of behavior therapy for OCD indicates that effects of d -cycloserine for OCD are most powerful early in treatment, and over time, differences between d -cycloserine and placebo augmentation seem to decrease. Longer-term use of d -cycloserine might parallel the strategy used for treating schizophrenia and Alzheimer’s disease, which has yielded generally disappointing results (24 – 28) . Studies of isolated versus long-term dosing of d -cycloserine in animals revealed positive effects on spatial learning only for isolated dosing (29) , and preexposure to d -cycloserine eliminates the enhancing effects of d -cycloserine on the extinction of conditioned freezing (30) . Thus, d -cycloserine administration over a shorter time period may be critical for augmenting exposure-based treatment.
The present study offers stronger evidence for the treatment-enhancing effects of d -cycloserine than does the study by Kushner et al. (16) . Indeed, in our d -cycloserine group, the patients’ OCD symptom severity scores were in the severe range before treatment, and after only five sessions, the scores had dropped to the mild range. Kushner et al. included only subjective fear ratings at mid-treatment, whereas the present study employed a comprehensive mid-treatment assessment using the gold standard rating scale in OCD (the Yale-Brown Obsessive Compulsive Scale). The rapid improvement within only five sessions, assessed with a reliable, valid, clinician-administered OCD symptom severity measure, is an important difference from the study by Kushner et al. Our research suggests that the acceleration of treatment response is a major advantage of d -cycloserine augmentation of behavior therapy. Future research should include more assessment points and process-focused measures (e.g., on OCD-related dysfunctional beliefs) to precisely determine when group differences occur and how treatment augmentation might have accelerated treatment response.
It is also informative to examine how our findings compare to the findings of previous studies of non- d -cycloserine-augmented behavior therapy for OCD. In one of the largest and best trials of behavior therapy to date (1) , patients received an intensive behavior therapy treatment consisting of two information sessions, 30 hours of behavior therapy (15 2-hour sessions), and two relapse-prevention sessions. Patients in that study showed a 55% decrease in OCD symptom severity on the Yale-Brown Obsessive Compulsive Scale. In the present study, using 1-hour behavior therapy sessions held twice a week, the patients receiving d -cycloserine plus behavior therapy showed a 52% Yale-Brown Obsessive Compulsive Scale score decrease at mid-treatment (5 hours of exposure) and a 62% decrease at posttreatment (10 hours of exposure), underscoring d -cycloserine’s significant potential as an augmentation strategy for behavior therapy.
The present study has important clinical implications for patients who often experience significant anxiety over the course of prolonged exposure treatments. Currently, the refusal and dropout rates for behavioral treatments in OCD tend to be high (e.g., reference 1 ). However, patients might be more likely to initiate, and less likely to discontinue, a treatment that reduces anxiety faster and is less time intensive. Thus, future large-scale studies will need to investigate whether d -cycloserine-augmented behavior therapy will be more acceptable to patients than conventional behavior therapy. Future studies will also need to investigate whether more powerful and efficient d -cycloserine-augmented treatments have lower dropout rates. d -cycloserine is an inexpensive augmentation strategy, and faster behavioral treatments reduce the cost of care, which is an important consideration in managed health care environments.
Of interest, in the current study, we found that d -cycloserine augmentation of behavior therapy for OCD also had positive effects on depression. This is of note given the evidence suggesting that acute high doses of d -cycloserine might have an antidepressant effect in their own right (31) . However, our findings suggest that improvements in depression might have been secondary to the initial improvement in OCD symptoms, as has been found previously (32) . It would be useful for future research to investigate whether d -cycloserine accelerates or increases the modification of dysfunctional beliefs, which might point to a mechanism of change of relevance to both OCD and depression.
In conclusion, although treatment strategies for OCD have improved, overall, the success rate of treatment is still rather low. Previous research has simply combined behavior therapy with medications that are designed to reduce obsessions, compulsions, and avoidance, and this research has yielded limited results (1) .
The current study yielded promising results when behavior therapy for OCD was combined with d -cycloserine. d -cycloserine has been used and evaluated frequently. It was originally approved by the Food and Drug Administration for the treatment of tuberculosis. More recently, d -cycloserine has been used to augment exposure-based behavior therapy for several anxiety disorders. The present study, as well as others, has shown that short-term dosing seems to be associated with minimal adverse effects, and the low dose we used was well tolerated. Thus, clinicians might be eager to use d -cycloserine for behavior therapy augmentation in OCD. However, the opinion of the present authors is that it is premature to recommend this unapproved drug for routine clinical use at this early stage of testing. Although several small studies have now indicated that d -cycloserine might show promise as an augmentor of exposure-based psychotherapy, as with all pilot studies, these findings first need to be confirmed in larger studies.

1. Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, Huppert JD, Kjernisted K, Rowan V, Schmidt AB, Simpson HB, Tu X: Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry 2005; 162:151–161Google Scholar
2. Simpson HB, Liebowitz MR, Foa EB, Kozak MJ, Schmidt AB, Rowan V, Petkova E, Kjernisted K, Huppert JD, Franklin ME, Davies SO, Campeas R: Post-treatment effects of exposure therapy and clomipramine in obsessive-compulsive disorder. Depress Anxiety 2004; 19:225–233Google Scholar
3. Foa EB, Franklin ME, Moser J: Context in the clinic: how well do cognitive-behavioral therapies and medications work in combination? Biol Psychiatry 2002; 52:987–997Google Scholar
4. Davis M, Myers KM: The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry 2002; 52:998–1007Google Scholar
5. Baker JD, Azorlosa JL: The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci 1996; 110:618–620Google Scholar
6. Royer S, Pare D: Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 2002; 115:455–462Google Scholar
7. Cox J, Westbrook RF: The NMDA receptor antagonist MK-801 blocks acquisition and extinction of conditioned hypoalgesic responses in the rat. Q J Exp Psychol B 1994; 47:187–210Google Scholar
8. Fanselow MS, LeDoux JE: Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 1999; 23:229–232Google Scholar
9. Goosens KA, Maren S: Long-term potentiation as a substrate for memory: evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus 2002; 12:592–599Google Scholar
10. Rogan MT, Staubli UV, LeDoux JE: Fear conditioning induces associative long-term potentiation in the amygdala. Nature 1997; 390:604–607Google Scholar
11. Davis M: Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. Eur J Neurosci 2002; 16:395–398Google Scholar
12. Walker DL, Ressler KJ, Lu KT, Davis M: Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci 2002; 22:2343–2351Google Scholar
13. Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M: Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004; 61:1136–1144Google Scholar
14. Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW: Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 2006; 63:298–304Google Scholar
15. Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, Jacob ML, Larson M, Hirsh A, Fernandez M, Geffken GR, Goodman WK: D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol 2007; 22:230–237; correction, 22:312Google Scholar
16. Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB: D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry 2007; 62:835–838Google Scholar
17. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Google Scholar
18. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC, APA, 2000Google Scholar
19. Kozak MJ, Foa EB: Mastery of Obsessive-Compulsive Disorder: A Cognitive-Behavioral Approach. San Antonio, Tex, Psychological Corporation, 1997Google Scholar
20. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P, version 2.0). New York, New York State Psychiatric Institute Biometrics Research Department, 1995Google Scholar
21. Beck AT, Steer RA, Brown GK: Beck Depression Inventory, 2nd ed, Manual. San Antonio, Tex, Psychological Corporation, 1996Google Scholar
22. Boje KM, Wong G, Skolnick P: Desensitization of the NMDA receptor complex by glycinergic ligands in cerebellar granule cell cultures. Brain Res 1993; 603:207–214Google Scholar
23. Ledgerwood L, Richardson R, Cranney J: Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci 2003; 117:341–349Google Scholar
24. Goff DC, Herz L, Posever T, Shih V, Tsai G, Henderson DC, Freudenreich O, Evins AE, Yovel I, Zhang H, Schoenfeld D: A six-month, placebo-controlled trial of D-cycloserine co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacology (Berl) 2005; 179:144–150Google Scholar
25. Duncan EJ, Szilagyi S, Schwartz MP, Bugarski-Kirola D, Kunzova A, Negi S, Stephanides M, Efferen TR, Angrist B, Peselow E, Corwin J, Gonzenbach S, Rotrosen JP: Effects of D-cycloserine on negative symptoms in schizophrenia. Schizophr Res 2004; 71:239–248Google Scholar
26. Fakouhi TD, Jhee SS, Sramek JJ, Benes C, Schwartz P, Hantsburger G, Herting R, Swabb EA, Cutler NR: Evaluation of cycloserine in the treatment of Alzheimer’s disease. J Geriatr Psychiatry Neurol 1995; 8:226–230Google Scholar
27. Tuominen HJ, Tiihonen J, Wahlbeck K: Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res 2005; 72:225–234Google Scholar
28. Laake K, Oeksengaard AR: D-cycloserine for Alzheimer’s disease. Cochrane Database Syst Rev 2002; CD003153Google Scholar
29. Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH: Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol 1994; 257:7–12Google Scholar
30. Parnas AS, Weber M, Richardson R: Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem 2005; 83:224–231Google Scholar
31. Britton JC, Gold AL, Feczko EJ, Rauch SL, Williams D, Wright CI: D-cycloserine inhibits amygdala responses during repeated presentations of faces. CNS Spectr 2007; 12:600–605Google Scholar
32. Whittal ML, Thordarson DS, McLean PD: Treatment of obsessive-compulsive disorder: cognitive behavior therapy vs exposure and response prevention. Behav Res Ther 2005; 43:1559–1576Google Scholar