Pupillary Reactivity to Emotional Information in Child and Adolescent Depression: Links to Clinical and Ecological Measures
Abstract
Objective: Pupil dilation provides a quantitative index of the temporal pattern of brain reactivity to emotional stimuli. Previous reports indicate that depressed adults show sustained pupil dilation to emotional words, but this phenomenon has not been investigated in children. This study investigated pupil dilation in children with depression and examined how differences in pupillary responses to emotional stimuli correlate with self-rated emotional experiences in participants’ natural environments in everyday life. Method: Participants were 20 children with major depressive disorder and 22 comparison children ages 8–17. Pupil dilation was measured during a valence identification task. Participants also rated positive and negative affect in their natural environments as part of an ecological momentary assessment protocol. Results: Children showed greater pupil dilation to negative words than to neutral or positive words. Children with major depression had diminished late pupil dilation relative to comparison children 9–12 sec after a negative word was presented. Diminished late pupil dilation to negative words was associated with greater severity of depression and with higher levels of negative affect and lower levels of positive affect in the natural environment. Conclusions: Depressed children exhibit a dynamic change in cognitive-affective resources devoted to processing negative emotional words, with more dramatic decreases than in comparison children after a negative word is initially processed, a pattern that differs markedly from that observed in depressed adults. Diminished late pupil dilation in children with major depression could be a marker for problems in emotional reactivity and/or regulation associated with pediatric depression.
Behavioral studies suggest that child and adolescent depression involves problems in emotional reactivity and regulation (1) . However, little research has examined underlying mechanisms of alterations in emotion processing in depressed children and adolescents. In this study, we examined the temporal pattern of emotional information processing in depressed and healthy youths.
Depressed adults exhibit altered neural and physiological responses to emotional material, particularly greater activation in limbic regions, such as the amygdala, and dysfunction in prefrontal cortical systems that modulate limbic activity (2 , 3) . However, other studies have reported reduced neural responses to emotional stimuli in adults with major depression, possibly reflecting “emotional blunting” (4 , 5) . These central disruptions are often reflected in peripheral physiological indicators of emotional information processing, such as event-related potentials or eye blinks (e.g., references 6 , 7) .
Few studies have examined physiological reactivity to emotional stimuli in depressed children or adolescents, and the extent to which models derived from adult studies can be applied to children is unclear. Initial data suggest developmental differences. For example, Thomas et al. (8) found that depressed children had a blunted amygdala response to fearful faces relative to healthy comparison children, and others (9) have reported a blunted sensitivity to affective feedback in depressed adolescents. Thus, it is possible that whereas adults are characterized by hyperreactivity to emotional stimuli, depressed children may have decreased reactions.
Pupillometry is one promising method for investigating the temporal pattern of emotional reactions. The pupil becomes more dilated in response to stimuli requiring greater cognitive load or emotional intensity (10 , 11) . The pupil is innervated by brain structures involved in both cognitive and emotional processing. Inhibition of the pupillary constrictor muscle occurs through parasympathetic innervation of the Edinger-Westphal nucleus, which receives extensive inputs from cortical and limbic regions. Stimulation of the pupillary dilator muscle occurs through a hypothalamic pathway that also receives corticolimbic inputs. Thus, stimulation of limbic regions, such as the amygdala, increases pupil dilation (12) , as does stimulation of the midbrain reticular formation (13) , which receives afferent projections from the frontal cortex and sends efferent projections to the ocular motor nuclei, particularly structures such as the anterior cingulate cortex, which are implicated in emotion regulation (14) . Concurrent pupillary and functional magnetic resonance imaging (fMRI) studies suggest that pupil dilation provides a summative index of task-related cognitive-affective brain activity (15) .
Pupil dilation also provides information about the time course of brain activation in response to a stimulus. The pupil remains dilated as long as the processing demand persists, and because pupil dilation can be sampled every 16 msec, it provides a dynamic measure of changes in cognitive-affective load following exposure to the stimulus. We have shown (11 , 16) that in adults with depressive disorders, pupil dilation in tasks requiring identification of the valence of emotional words is increased and sustained for up to 30 sec after behavioral responses.
In this study, we investigated pupil dilation to emotional stimuli in depressed children and adolescents during a valence identification task. Our first goal was to replicate findings of altered pupil dilation in response to emotional words in a child and adolescent sample with depression. Our second goal was to address whether this physiological response in the laboratory directly applies to real-life affective functioning. We examined associations between pupil dilation and children’s affect ratings during social interactions collected via ecological momentary assessment, an ecologically valid method of gathering representative data on affect and behavior in natural environments.
Method
Participants
Participants were 42 children (28 females, ages 8–17 years [mean=13.1 years, SD=2.57]) from a longitudinal study of neurobehavioral factors in pediatric affective disorder (see reference 17 for details). Twenty participants had a current primary diagnosis of major depressive disorder based on DSM-IV criteria, and 22 participants were low-risk comparison children. Eleven (55%) participants in the major depression group also had a comorbid anxiety disorder (generalized anxiety disorder, social phobia, and/or separation anxiety disorder). None of the participants had ever taken psychotropic medications. Comparison children had no lifetime psychopathology, no first-degree relatives with a lifetime episode of mood or psychotic disorder, and no second-degree relatives with a lifetime history of childhood-onset, recurrent, psychotic, or bipolar depression or schizoaffective or schizophrenic disorder, and no more than 20% of their second-degree relatives had a lifetime single episode of major depression.
Recruitment and Diagnostic Interviews
Children with major depression were recruited from inpatient and outpatient clinics and community advertisements. Comparison children were recruited from community advertisements. Lifetime and present DSM-IV diagnoses were assessed using the Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime Version (18) . Parents were interviewed with the Structured Clinical Interview for DSM-IV (19) as well as a family history interview (20) . For most participants the pupil dilation assessment was completed during the initial visit to the laboratory within the longitudinal study, but for some it was completed during a follow-up visit. For this report, we drew on data from 35 initial assessments and seven 1-year follow-ups. At each follow-up, the interview covered the period from the previous assessment to the time of the interview.
Pupil Dilation Assessment
Participants completed a two-night assessment battery (see methods described in reference 17 ). The pupil dilation assessment was conducted in the morning in a moderately lit room (1.54 foot-candles illuminance). The stimuli were in lowercase letters approximately 1.59 cm high subtending 0.76° of visual angle, displayed in dark gray on a light gray computer screen. Reaction times were recorded via a modified game pad containing three buttons arranged in a triangle so that respondents’ fingers were nearly equidistant from each possible response. The mapping of game pad buttons to responses was counterbalanced across participants. Pupil size was recorded with an ISCAN head-mounted RK-726 or a table-mounted RK-464 eye-tracker at 60 Hz (every 16.7 msec). The resolution for a typical participant was better than 0.05 mm pupil diameter.
Word Valence Identification Task
Participants were instructed to identify the emotional valence of 22 positive, 22 negative, and 22 neutral words, chosen from a corpus of emotional words normed for use with children (21) , by pressing a corresponding button for each valence. Words from each category were balanced for length and frequency. Each trial included a 1-sec fixation mask, followed by the word for 5 sec, followed by a mask for 6 sec ( Figure 1 ).

Data Selection, Cleaning, and Reduction
Data were cleaned by our lab’s standard methods, based on those described by Granholm et al. (e.g., reference 22 ). Trials with reaction times below 100 msec and above 4.9 sec were discarded as outliers, and trials comprising more than 50% blinks were removed. Linear interpolations replaced blinks throughout the data set. Data were smoothed using a 10-point weighted average filter. Linear trends in pupil dilation calculated over blocks of trials were removed to eliminate effects of slow drift in pupil diameter not related to trial characteristics. The average pupil diameter over the 167 msec (10 samples) preceding the onset of the stimulus was subtracted from pupil diameter after stimulus onset to produce stimulus-related pupil dilation waveforms.
Self-Reports
Children provided self-report data on depressive symptoms on the day of the assessment via the Mood and Feelings Questionnaire (23) . Socioeconomic status was measured via parent report using the Hollingshead Four-Factor Index of Social Status (24) .
Ecological Momentary Assessment
A subset of 35 participants (19 in the major depression group, 16 in the comparison group) completed an ecological momentary assessment protocol designed to obtain information on children’s affective behavior in the naturalistic environment. Participants were given answer-only cell phones on which they received calls from an interviewer 12 times between 4 p.m. Friday and 10 p.m. Monday on the weekend preceding the laboratory visit. At each call, participants were asked to rate their current affect on a subset of 5-point scales from the Positive and Negative Affect Schedule for Children (25) . Global negative affect and positive affect scores were created by averaging across each of the negative and positive subscales, and a ratio of global positive to negative affect was computed (26) .
Results
Demographic Characteristics and Reaction Times
There were no group differences in age or gender. The families of the children in the major depression group were lower in socioeconomic status and more likely to have minority status than the families of children in the comparison group. There were no differences in harmonic mean reaction times by valence and no diagnostic group-by-valence interactions, although children in the major depression group responded more slowly than those in the comparison group to all words (t =2.29, df=40, p<0.05; Cohen’s d=0.64).
Pupil Dilation
Hypotheses were tested using mixed-effects models with an autoregressive covariance structure (AR1) using pupil dilation waveforms as the dependent variable. Models included mean pupil dilation at each second for each valence per subject, with subject treated as a random effect and time and valence as repeated measures. Fixed effects included diagnostic group, valence, segment, and all interactions. Segment 1, the “early” segment, was defined as the latter 3 sec of the period during which the word was on-screen (3 sec to 6 sec), and segment 2, the “late” segment, was defined as the latter 3 sec of the period during which the word was off-screen (9 sec to 12 sec). Straightforward methods for calculating statistical power for repeated-measures mixed-effects models are not yet available. Using standard general linear model methods, our sample of 42 provides power ≥0.80 to detect only large effect sizes; however, as mixed-effects models take advantage of all degrees of freedom for repeated measurements, this is likely a conservative estimate.
Mixed-effects analyses indicated main effects for valence (F=7.25, df=2, 674, p<0.01) and segment (F=26.74, df=1, 312, p<0.001), which were qualified by a group-by-valence interaction (F=8.66, df=2, 674, p<0.001) and a group-by-valence-by-segment interaction (F=7.73, df=2, 681, p<0.001). Because the two groups differed in mean socioeconomic status and proportion who had minority status, we ran the mixed-effects model covarying socioeconomic status and minority status. We also included a covariate for age to account for the wide age range of participants. In the model including these three covariates, effects remained significant for valence (F=16.37, df=2, 605, p<0.001), segment (F=25.43, df=1, 273, p<0.001), and the group-by-valence-by-segment interaction (F=6.71, df=2, 612, p<0.01), and the diagnostic group-by-valence interaction was no longer significant (F=1.74, df=2, 605, p=0.18). Pairwise comparisons of estimated marginal means revealed that pupil dilation was greater during segment 1 (estimated marginal mean=0.07 mm) than segment 2 (estimated marginal mean=0.01 mm; Cohen’s d=0.59) and was greater to negative words (estimated marginal mean=0.05 mm) than to positive (estimated marginal mean=0.03 mm) or neutral words (estimated marginal mean=0.03 mm; Cohen’s d=0.21; see Figure 2 ).
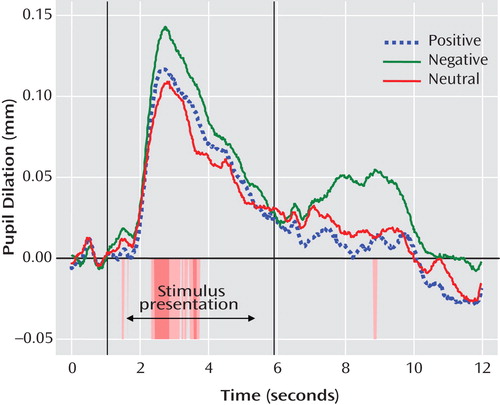
a Statistically significant t tests are indicated by color bands below the x-axis. Areas shaded darker are significant at p<0.05; areas shaded lighter are significant at p<0.1.
The diagnostic group-by-valence-by-segment interaction was probed by examining group effects at each time point along the waveform for each valence. To control type I error, we used Guthrie and Buchwald’s technique (27) to identify regions of the waveform over which an entire series of contiguous point-by-point tests, each significant at p<0.1, could be considered significant at p<0.05, given the temporal autocorrelation of the waveform. This analysis revealed that diagnostic groups differed primarily in their pupil dilation following negative words, after the word was no longer on-screen. As shown in Figure 3 , children with major depression showed less late pupil dilation relative to comparison children from approximately 9 sec to 12 sec after the beginning of the trial (8.93–9.78 sec: t=2.02, df=40, p<0.05; Cohen’s d=0.62; 9.80–11.90 sec: t=2.08, df=40, p<0.05, Cohen’s d=0.64).

a Regions of statistically significant differences are indicated by color bands below the x-axis. Areas shaded darker (red) are significant at p<0.05; areas shaded lighter (yellow) are significant at p<0.1. Underlined regions (on the right) have ≥1.17 sec of consecutive tests statistically significant at p<0.1 (39).
Because children in the major depression group had slower reaction times than children in the comparison group, reaction-time-aligned waveforms were examined ( Figure 4 ). Participants in the major depression group had less pupil dilation to negative words relative to those in the comparison group for the majority of the period 5–10 sec after their response (t=1.83–2.46, df=40; p=0.02–0.07; Cohen’s d=0.57–0.76).

a Regions of statistically significant differences are indicated by color bands below the x-axis. Areas shaded darker (red) are significant at p<0.05; areas shaded lighter (yellow) are significant at p<0.1. Underlined regions (on the right) have ≥1.17 sec of consecutive tests statistically significant at p<0.1 (39).
The two groups did not differ on pupil dilation to neutral or positive words at any point along the waveform, with the exception of a lower degree of dilation to positive words in comparison children relative to depressed children during the last second of the trial (t=–2.53, df=40, p<0.05, Cohen’s d=–0.78).
Late Pupil Dilation and Depressive Symptoms With Comorbid Anxiety
As expected, children in the major depression group had more depressive symptoms than comparison children ( Table 1 ). Depressive severity was inversely related to late pupil dilation to negative words, such that children with greater depressive severity had less late pupil dilation to negative words (r= –0.35, p<0.05). Depressed children with and without comorbid anxiety disorders both displayed less late pupil dilation to negative words (t=0.76, df=18, p=0.46; Cohen’s d=0.34).
Correlation of Late Pupil Dilation With Self-Reported Emotions in the Natural Environment in Everyday Life
In the ecological momentary assessment, children in the major depression group reported higher levels of negative affect (t=3.64, df=33, p<0.01; Cohen’s d=1.27) and had a lower ratio of positive to negative affect (t=–2.62, df=33, p<0.05; Cohen’s d=0.90) in the natural environment during the weekend preceding the laboratory visit relative to comparison children. Negative affect (r=0.83, p<0.001) and the ratio of positive to negative affect (r=–0.57, p<0.001) were highly related to severity of depression, although positive affect was less strongly related to severity of depression (r=–0.32, p=0.07).
Late pupil dilation after negative words was strongly associated with positive and negative affect in the natural environment reported via ecological momentary assessment ( Figure 5 ). Participants with less late pupil dilation to negative words reported higher levels of global negative affect (r=–0.49, p<0.01) and lower levels of global positive affect (r=0.41, p<0.05) and had a lower ratio of positive to negative affect (r=0.53, p<0.01). Hierarchical linear regressions indicated that there were no interactions between pupil dilation and diagnostic group in positive affect ratings or ratio of positive to negative affect, but there was a pupil dilation-by-group interaction in predicting negative affect (ΔR 2 =0.11, F=7.16, p<0.05, Cohen’s f 2 =0.23). As shown in Figure 5 , post hoc tests revealed that late pupil dilation to negative words was associated with negative affect in children with major depression (β=–0.59, p<0.01) but not comparison children (β=–0.25, p=0.36). This difference is likely driven by low levels of variability in negative affect in ecological momentary assessments in comparison children (see Figure 5 ).

a A lower degree of pupil dilation to negative words was strongly associated with depressed children’s reports of negative affect, positive affect, and the ratio of positive to negative affect as described to researchers in cell phone interviews over a 4-day period outside the lab.
b Linear R 2 =0.35, p<0.01.
c Linear R 2 =0.06, p=0.36.
d Linear R 2 =0.17, p=0.12.
e Linear R 2 =0.14, p=0.11.
f Linear R 2 =0.28, p<0.05.
g Linear R 2 =0.20, p=0.08.
Discussion
To our knowledge, this is the first study to assess pupil dilation in response to emotional stimuli in children and adolescents with major depression. Pupil dilation in children was greater after negative words than neutral or positive words, which suggests that pupil dilation in response to negative words during a valence identification task can index emotional information processing in pediatric populations. Group differences in pupil dilation to negative words occurred long after the stimulus onset, 5 to 10 sec after the participant’s response. Participants with major depression showed the expected initial reaction to negative words but, contrary to our expectations, showed diminished pupil dilation to negative words compared with children in the comparison group after the word was no longer on-screen. This effect was not observed with neutral or positive words. Moreover, the diminishment in late pupil dilation to negative words was more marked in children with greater severity of depressive symptoms.
This normal initial pupillary response paired with a later blunted or decreased pupillary response could indicate a more dramatic decrease in cognitive-affective resources devoted to processing negative emotional words after a negative word is initially processed in depressed children than in nondepressed children. Multiple alterations in emotional reactivity or regulation could be responsible for such decreased resource recruitment and should be investigated in future research. For example, decreased cognitive-affective load could reflect avoidance of emotional information or affective blunting or could be the consequence of earlier overcompensatory regulation. Since the pupil is innervated by both sympathetic and parasympathetic fibers, it is not possible to draw conclusions about neural mechanisms underlying this finding. Potential brain mechanisms could include blunted or diminished reactivity of emotional circuitry (e.g., the amygdala). Alternatively, prefrontal or anterior cingulate regulatory structures may fail to engage or may, after initial engagement, yield decreased sustained limbic activity. This lack of neural specificity is a limitation to the pupillometry approach that could be addressed in future research by use of concurrent collection of pupillometric and neuroimaging data, which is now available in many fMRI laboratories and can be used to better delineate the time course of activation in specific brain regions (see reference 15 ).
Although these interpretations remain speculative until the underlying neural circuitry can be better delineated, they are consistent with clinical and research reports. For example, devotion of less resources to processing negative emotional information after the initial response in depressed children is consistent with clinical reports of patients who are easily upset but “shut down” emotionally when facing too much arousal. The possibility that this pattern of findings reflects avoidance processes is consistent with evidence that adolescents with more severe depressive symptoms use more avoidance and disengagement strategies to regulate negative emotions (28 , 29) . The hypothesis that limbic reactivity may be blunted in depressed children is consistent with the finding by Thomas et al. (8) of blunted amygdala reactivity to fearful faces in depressed children and the finding by Jazbec et al. (9) of blunted affective sensitivity in adolescents with major depression during an antisaccade task. Our potential explanations are also consistent with reports of reduced neural and physiological responses to emotional stimuli in adults with major depression (e.g., references 4 , 5 , 30) .
The ecological momentary assessment data also suggest that the lower pupil dilation response is related to problems in emotional reactivity and/or regulation in everyday life. We found that children with lower levels of late pupil dilation in response to negative words in the laboratory reported higher levels of negative emotion and lower levels of positive emotion and had a lower ratio of positive to negative emotion in their everyday lives. The relationship between late pupil dilation to negative words and daily negative emotion was particularly pronounced in depressed children, suggesting that this tool may have particular utility in understanding emotional functioning in this population. These ecological momentary assessment findings show that our measure of pupil dilation to negative words has ecological validity and has implications for children’s everyday emotion regulation and social functioning.
Our finding that group differences in pupil dilation to negative words appear primarily in the late processing period is consistent with findings from studies of adults with major depression (11 , 16) . However, the direction of this effect differed from that of the adult studies, in which major depression is associated with increased late pupil dilation to emotional words (11 , 16) . One possible interpretation of this finding is that children and adolescents with affective disorders may have an ability that is lost later in development to “shut off” or avoid the processing of negative information. By blunting, avoiding, or overregulating negative emotions, the depressed child would lose the opportunity to develop and practice more adaptive skills for tolerating and regulating negative emotions, potentially leading to greater difficulty managing emotion as an adult. Another possibility is that the increased late pupil dilation observed in adults is indicative of ruminative processes (11) that may be less developed in children and young adolescents.
This potential developmental difference is consistent with evidence that patterns of emotional processing change across development, including several instances in which these patterns have been reported to reverse direction. For example, amygdala reactivity to affective stimuli has been shown to be increased in depressed adults but decreased in depressed children (8) , and activity to fearful versus neutral faces has been shown to be increased in adults but decreased in children (31) . There is also evidence that brain regions involved in emotion regulation undergo functional and anatomical reorganization during puberty and continue to mature into early adulthood (see reference 32 ) and that adolescents and adults differ in the way they allocate neural resources to emotional information (33) . Cross-sectional research with larger numbers of children and adults, as well as longitudinal research, is needed to better delineate the trajectory of pupillary responses across development.
Another possibility is that the difference between our findings and findings with adults is a function of stimulus timing rather than development. Because children take longer to read words than adults, we increased the word presentation period from 150 msec (11 , 16) to 5,000 msec. Emerging evidence suggests that patterns of emotional processing may depend on whether stimuli are presented for long enough to engage effortful processing (approximately >500 msec; see reference 34 ). For example, anxious children and adults have been shown to display vigilant reactions to quickly presented or subliminal threat stimuli but avoidant reactions to longer presentations (34 – 36) . Similarly, children with recurrent abdominal pain have shown attentional biases toward pain-related words presented subliminally (20 msec) and attentional biases away from pain-related words presented for 1,250 msec (37) . The relatively long word presentation in our study may have allowed children sufficient time to engage top-down regulatory resources. Research is needed to compare children’s pupillary response to words presented for both brief and long durations.
Several limitations of this study should be noted. First, the study was powered sufficiently to detect only large effect sizes, and it was underpowered to detect group-by-development interactions. Second, as described above, pupillometry does not provide direct information about specific brain regions involved in the observed response. Yet, significant conclusions may be drawn from this study given its unique strengths, which include a rigorously diagnosed clinical sample of depressed children and adolescents, strong temporal precision regarding the pattern of early and late emotional processing afforded by pupil dilation (not available with self-report), and sampling of ecologically valid information about children’s affective functioning in their daily life. If replicated, these findings could have clinical and methodological implications, as pupillometry is a practical and inexpensive method for collecting information about emotional processing that could be administered in clinical settings and is feasible with pediatric populations. Research is needed to evaluate whether this pupillary response could be a marker of risk for depression in vulnerable children or whether it is a state marker associated with the experience of depression.
1. Sheeber L, Allen N, Davis B, Sorensen E: Regulation of negative affect during mother-child problem-solving interactions: adolescent depressive status and family processes. J Abnorm Child Psychol 2000; 28:467–479Google Scholar
2. Mayberg HS: Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003; 65:193–207Google Scholar
3. Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS: Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 2002; 51:693–707Google Scholar
4. Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML: Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Google Scholar
5. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ: The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 2002; 59:597–604Google Scholar
6. Deldin P, Deveney C, Kim A, Casas BR: A slow wave investigation of working memory biases in mood disorders. J Abnorm Psychol 2001; 110:267–281Google Scholar
7. Ohira H: Analysis of eyeblink activity during self-referent information processing in mild depression. Percept Mot Skills 1995; 81:1219–1229Google Scholar
8. Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ: Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 2001; 58:1057–1063Google Scholar
9. Jazbec S, McClure E, Hardin M, Pine DS, Ernst M: Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry 2005; 58:632–639Google Scholar
10. Beatty J: Task-evoked pupillary responses processing load and the structure of processing resources. Psychol Bull 1982; 91:276–292Google Scholar
11. Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME: Do the seconds turn into hours? relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognit Ther Res 2003; 27:365–382Google Scholar
12. Koikegami H, Yoshida K: Pupillary dilation induced by stimulation of amygdaloid nuclei. Folia Psychiatr Neurol Jpn 1953; 7:109–125Google Scholar
13. Beatty J: The pupil system, in Psychophysiology: Systems, Processes, and Application. Edited by Coles MGH, Donchin E, Porges SW. New York, Guilford, 1986, pp 43–50Google Scholar
14. Szabadi E, Bradshaw CM: Autonomic pharmacology of 2-adrenoceptors. J Psychopharmacol 1996; 10(suppl 3):6–18Google Scholar
15. Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS: Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage 2003; 20:114–124Google Scholar
16. Siegle GJ, Granholm E, Ingram RE, Matt GE: Pupillary and reaction time measures of sustained processing of negative information in depression. Biol Psychiatry 2001; 49:624–636Google Scholar
17. Birmaher B, Dahl RE, Williamson DE, Perel JM, Brent DA, Axelson DA, Kaufman J, Dorn LD, Stull S, Rao U, Ryan ND: Growth hormone secretion in children and adolescents at high risk for major depressive disorder. Arch Gen Psychiatry 2000; 57:867–872Google Scholar
18. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Google Scholar
19. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
20. Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M: Offspring of depressed parents: 10 years later. Arch Gen Psychiatry 1997; 54:932–940Google Scholar
21. Neshat-Doost HT, Moradi AR, Taghavi MR, Yule W, Dalgleish T: The development of a corpus of emotional words produced by children and adolescents. Pers Individ Dif 1999; 27:433–451Google Scholar
22. Granholm E, Asarnow RF, Sarkin AJ, Dykes KL: Pupillary responses index cognitive resource limitations. Psychophysiology 1996; 33:457–461Google Scholar
23. Angold A, Costello EJ, Messer SC, Pickles A, Winder F, Silver D: Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res 1995; 5:237–249Google Scholar
24. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
25. Laurent J, Catanzaro SJ, Joiner TE Jr, Rudolph KD, Potter KI, Lambert S, Osborne L, Gathright T: A measure of positive and negative affect for children: scale development and preliminary validation. Psychol Assess 1999; 11:326–338Google Scholar
26. Fredrickson BL, Losada MF: Positive affect and the complex dynamics of human flourishing. Am Psychologist 2005; 60:678–686Google Scholar
27. Guthrie D, Buchwald JS: Significance testing of difference potentials. Psychophysiology 1991; 28:240–244Google Scholar
28. Compas BE, Connor Smith JK, Saltzman H, Thomsen AH, Wadsworth ME: Coping with stress during childhood and adolescence: problems, progress, and potential in theory and research. Psychol Bull 2001;127:87–127Google Scholar
29. Silk JS, Steinberg L, Morris AS: Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev 2003; 74:1869–1880Google Scholar
30. Rottenberg J, Gross JJ, Wilhelm FH, Najmi S, Gotlib IH: Crying threshold and intensity in major depressive disorder. J Abnorm Psychol 2002; 111:302–312Google Scholar
31. Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey B: Amygdala response to facial expressions in children and adults. Biol Psychiatry 2001; 49:309–316Google Scholar
32. Nelson EE, Leibenluft E, McClure E, Pine DS: The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med 2005; 35:163–174Google Scholar
33. Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS: Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage 2003; 20:420–428Google Scholar
34. Mogg K, Bradley BP: Time course of attentional bias for fear-relevant pictures in spider-fearful individuals. Behav Res Ther 2006; 44:1241–1250Google Scholar
35. Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E, Guyer AE, Ernst M, Charney DS, Kaufman J: Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry 2005; 162:291–296Google Scholar
36. Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJR, Chen G, Charney DS, Ernst M, Pine DS: Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry 2006; 163:1091–1097Google Scholar
37. Boyer MC, Compas BE, Stanger C, Colletti RB, Konik BS, Morrow SB, Thomsen AH: Attentional biases to pain and social threat in children with recurrent abdominal pain. J Pediatr Psychol 2006; 31:209–220Google Scholar




