The Role of Dopamine in Attentional and Memory Biases for Emotional Information
Abstract
Objective: Cognitive models suggest that biased processing of emotional information may play a role in the genesis and maintenance of psychotic symptoms. The role of dopamine and dopamine antagonists in the processing of such information remains unclear. The authors investigated the effect of a dopamine antagonist on perception of, and memory for, emotional information in healthy volunteers. Method: Thirty-three healthy male volunteers were randomly assigned to a single-blind intervention of either a single dose of the dopamine D 2 /D 3 antagonist amisulpride or placebo. An attentional blink task and an emotional memory task were then administered to assess the affective modulation of attention and memory, respectively. Results: A significant interaction was observed between stimulus valence and drug on recognition memory accuracy; further contrasts revealed enhanced memory for aversive-arousing compared with neutral stimuli in the placebo but not the amisulpride group. No effect of amisulpride was observed on the perception of emotional stimuli. Conclusions: Amisulpride abolished the enhanced memory for emotionally arousing stimuli seen in the placebo group but had no effect on the perception of such stimuli. These results suggests that dopamine plays a significant role in biasing memory toward emotionally salient information and that dopamine antagonists may act by attenuating this bias.
Dopamine antagonists have been used for over half a century to treat the symptoms of psychosis. Yet we have only a limited understanding of how dopamine dysregulation gives rise to symptoms of psychosis or how dopamine antagonist medications exert their antipsychotic therapeutic effects at a cognitive level (1) . Both cognitive and biological approaches have been used to explain the origins of symptoms in psychosis, and these approaches have only recently begun to converge. For example, Kapur (2) proposed a theory of motivational salience to explain how dopamine dysregulation may give rise to cognitive changes that ultimately lead to symptoms such as delusions and hallucinations. This model has not been empirically validated, however. Greater insight into the role of dopamine in human cognitive processes, particularly emotional processing, may facilitate the integration of biological and psychological approaches to understanding psychosis and contribute to novel therapeutic possibilities.
Dopamine is an important modulator of emotional learning and memory in experimental animals, mediated via the amygdala (3 , 4) . However, the role of dopamine in emotional memory in human beings has not yet been adequately explored. Recent evidence highlighting the ability of dopamine to modulate the amygdala response in humans suggests that there may be a similar role for dopamine in human emotional memory (5 , 6) . Additionally, Mehta et al. (7) demonstrated a tendency for the D 2 receptor antagonist sulpiride to impair emotional memory in healthy volunteers, although this result did not reach statistical significance, possibly because of a small sample size.
Dopamine is also thought to play a specific role in drawing attention to emotionally significant or salient events (8) . Such stimuli evoke responses in mesolimbic dopaminergic neurons in experimental animals (9 , 10) , but again, there has been limited investigation in human subjects. Although Franken et al. (11) demonstrated that haloperidol reduced attentional bias to drug-related cues compared with neutral cues in an emotional Stroop task in heroin-dependent patients, these findings cannot necessarily be extrapolated to attentional processing of emotionally salient information in healthy volunteers.
Our study sought to further examine the role of dopamine in the affective modulation of both attention and memory. We investigated healthy male volunteers to avoid confounding by symptoms or gender, and we used a larger sample than did Mehta et al. (7) for increased statistical power. We predicted that dopamine blockade would reduce both attentional and memory biases for emotional stimuli. An antipsychotic with a high specificity for dopamine receptors was used for this purpose to avoid confounding by other neurotransmitter effects.
Method
Participants
Healthy male volunteers (N=33) 18–40 years of age (mean=24.2 years, SD=5.4) were recruited through an advertisement and were paid for their participation. Potential participants were screened for psychiatric and neurological disorders by means of a checklist questionnaire. Exclusion criteria were any current or past history of psychiatric illness, any significant history of substance misuse (including nicotine), and taking regular medication. Participants were randomly assigned to receive either drug (N=17) or placebo (N=16). The study was approved by the Institute of Psychiatry Research Ethics Committee. Participants received a complete description of the study and provided written informed consent.
Drugs
Dopamine blockade was achieved with 400 mg of amisulpride, a substituted benzamide neuroleptic. Amisulpride was selected for this purpose because of its selectivity for D 2 /D 3 receptors and its lack of affinity for nondopaminergic (adrenergic, cholinergic, serotonergic, and histaminergic) receptors. It is also selective for limbic regions (12 , 13) and has low risk of inducing extrapyramidal side effects (14) . A 400 mg dose was chosen because it represents the lower end of the daily dosage range for achieving dopamine blockade and reduction of positive symptoms in schizophrenia (400–1200 mg). It has been suggested that lower doses (50–300 mg) enhance dopaminergic transmission (12 , 15 , 16) . Additionally, a single 400 mg dose of amisulpride has been found to have no effect on general psychomotor or cognitive performance in healthy volunteers (17) .
Eight 50 mg amisulpride tablets (total, 400 mg) were placed in opaque colored gelatin capsules. Placebo capsules contained two 100 mg ascorbic acid tablets (total, 200 mg). Both capsules were prepared by the Maudsley Hospital Pharmacy, London.
Procedure
Participants took part in a between-subjects placebo-controlled study. The study was single-blind; participants were not informed as to whether they were receiving drug or placebo, but for safety reasons the administering physician was aware of the condition to which participants had been assigned. Participants attended two sessions separated by 1 week. They were instructed to abstain from beverages containing alcohol or caffeine for the 24 hours prior to the test sessions. Sessions were conducted between 9 a.m. and 1 p.m.
On arrival at the laboratory for the first session, participants received either amisulpride or placebo. After 1 hour (to allow satisfactory plasma levels to be reached) a peripheral venous blood sample was taken for measurement of amisulpride plasma concentration. To reduce the likelihood of participants anticipating a memory test, they were told that the purpose of the study was to measure their physiological responses to emotional stimuli, and they were connected to a galvanic skin response device for the duration of testing. However, skin conductance measures were not recorded. The National Adult Reading Test was administered to obtain a measure of IQ. The encoding phase of the emotional memory task and the attentional blink task (described below) were then administered. Participants were told that the second session would be exactly the same as the first but without drug administration. Memory tests were not mentioned.
The second session took place 1 week later and consisted of the retrieval phase of the emotional memory task and a debriefing interview to explain the purpose of the study. To assess the effectiveness of blinding, participants were also asked to indicate whether they believed they had received drug or placebo.
Cognitive Tasks
Emotional memory task
This task consisted of an encoding phase in which participants viewed scenes, each with a normative rating for arousal and valence, and a delayed recognition memory test in which they viewed all of the previously seen pictures along with an equal number of new pictures (foils). All pictures were derived from the International Affective Picture System stimulus set (18) . Previous studies with healthy volunteers have demonstrated a recognition memory bias for emotionally arousing compared with neutral pictures (19 , 20) . Our primary hypothesis was that this bias would be reduced or abolished by amisulpride.
During the encoding phase, participants viewed 92 pictures. Half of the scenes were aversive-arousing (mean valence and arousal ratings of 2.6 and 6.1, respectively) and half were neutral (mean valence and arousal ratings of 5.1 and 3.3, respectively). Stimuli were presented on a laptop computer for 3 seconds with a 4-second interstimulus interval during which a fixation cross was present on the screen. The order of presentation was randomized across participants. Participants were asked to judge whether they felt emotionally aroused (i.e., wide awake or jittery) or unaroused (i.e., calm or relaxed) by each scene immediately after its presentation by pressing one of two keys labeled “aroused” and “calm” during the interstimulus interval. This was to ensure that participants attended to the scenes and that our classification of the pictures as arousing and neutral correlated with that of the participants.
One week after the encoding phase, participants returned to the laboratory for an unexpected recognition memory test in which they viewed all of the 92 previously seen pictures and 92 foils. The foils were selected to match the previously presented scenes in their content, valence, and arousal characteristics. During the recognition test, participants were instructed to press marked keys to indicate whether each picture was “old” (previously seen) or “new” (foil). After making the old/new judgment, they were asked to rate the arousal and valence of each picture on a 9-point scale. The hit rate and false alarm rate were calculated for each participant. Signal detection theory was used to calculate measures of recognition memory performance: sensitivity (d′) and response bias (C) (21) . The values for d′ and C were entered as dependent measures in a repeated-measures analysis of variance (ANOVA) with valence (aversive or neutral) as the within-subjects factor and drug (amisulpride or placebo) as the between-subjects factor.
Attentional blink task
This task was based on dual-target rapid serial visual presentation methodology. Identification of a first target (T1) in a rapid stream of stimuli leads to transient impairment in identification of a second target (T2)—an effect known as the attentional blink(22) . Studies with healthy volunteers indicate that there is a bias toward detection of emotionally salient T2 targets compared with neutral targets (23 – 25) . Our primary hypothesis was that this bias would be reduced or abolished by amisulpride.
The task comprised 168 trials, each consisting of 13 white distractor words and two green target words (T1 and T2) presented sequentially in the center of a laptop computer screen (see Figure 1 ). T1 stimuli were 56 neutral words used by Anderson (24) averaging 4.8 letters in length. T2 stimuli were 56 words derived from the Affective Norms for English Words (26) . Half of the T2 words were aversive-arousing words (mean valence and arousal ratings of 2.5 and 7.0, respectively) and half were neutral (mean valence and arousal ratings of 5.1 and 3.5, respectively). Aversive-arousing and neutral T2 words did not differ significantly in letter length (mean=5.1 versus 4.8, respectively, p=0.21) or written word frequency (mean=67.1 versus 87.9, respectively, p=0.50) (27) (word frequency was accessed from the MRC Psycholinguistic Database, http://www.psy.uwa.edu.au/MRCDataBase/uwa_mrc.htm). Distractor items were 92 words of longer length (mean number of letters=12.5) to facilitate masking of the targets. Each item was presented for 100 msec and was immediately followed by the subsequent item. The lag between the T1 and T2 targets was varied to contain one, three, or five intervening distractors (lag 2, lag 4, or lag 6) with corresponding stimulus onset asynchronies of 200 msec, 400 msec, or 600 msec. Participants were instructed to ignore the words in white (distractors) and identify the two green words (T1 and T2). They responded by writing down the two target words in any order immediately after each trial. The critical outcome measure was the percentage accuracy of T2 report, contingent on the correct identification of T1. This was to ensure that proper attention had been devoted to T1. An independent-samples t test was used to compare the percentage accuracy of T1 report between the groups. T2 accuracy was entered as the dependent measure in a repeated-measures ANOVA with valence (aversive or neutral) and lag (2, 4, and 6) as within-subjects factors and drug (amisulpride or placebo) as the between-subjects factor.
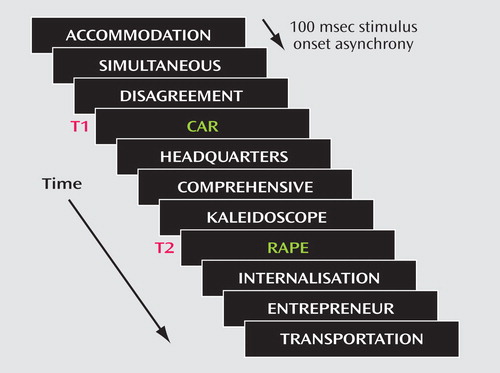
a Adapted with permission from Macmillan Publishers Ltd, Nature Neuroscience (reference 23), copyright 2001.
Results
IQ, Blinding, and Amisulpride Levels
Participants in the amisulpride and placebo groups did not differ in IQ (mean=116.5, SD=6.3). There was no association between what participants believed they had received (drug or placebo) and what they had actually received, which suggests that blinding was effective. The plasma amisulpride concentrations in the drug group ranged from 30 mg/liter to 1,030 mg/liter (mean=354 mg/liter, SD=261).
Cognitive Tasks
Emotional memory task
Data for one participant had to be excluded from the analysis because a significant delay in the participant’s response time on a trial during the recognition phase created an error in the stimulus presentation program. Participants’ emotional classification of the pictures at encoding was highly correlated with our categorization (χ 2 =1,137.5, df=2, p<0.001), and the two groups did not differ in their classification of the pictures. The valence and arousal ratings participants made during the recognition memory phase were highly correlated with the standardized ratings (r=0.70, p<0.001 and r=0.62, p<0.001, respectively), and there was no significant difference between the groups.
Repeated-measures ANOVA revealed a significant valence-by-drug interaction on d′ (F=6.76, df=1, 30, p=0.014). Contrasts revealed that d′ was significantly higher for aversive pictures than for neutral pictures in the placebo group (see Figure 2 ) but not the amisulpride group.

a Significant difference between test conditions, t=3.54, df=15, p=0.003.
Attentional blink task
The amisulpride group had impaired detection of T1 stimuli compared with the placebo group (86.2% [SD=16.7] versus 94.1% [SD=5.3], respectively), but the difference did not reach statistical significance (t=1.187, df=31, p=0.077). Repeated-measures ANOVA of T2 accuracy revealed significant main effects of valence (F=6.39, df=1, 31, p=0.017) and lag (F=50.78, df=1.1, 35.0, p<0.001). Mauchly’s test indicated that the assumption of sphericity had been violated for the main effect of lag (χ 2 =44.4, df=2, p<0.001); therefore, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (28) . There was a significant lag-by-valence interaction (F=11.31, df=2, 62, p<0.001). There was no significant main effect of drug or any interactions. Contrasts revealed that T2 detection was significantly higher for aversive words than neutral words at lag 4 (t=2.81, df=16, p=0.013) and lag 6 (t=2.23, df=16, p=0.04) in the amisulpride group and at lag 4 (t=2.77, df=15, p=0.014) in the placebo group ( Figure 3 ). There was no difference in detection of aversive versus neutral words at lag 2 in either group.

a Significant difference between test conditions (p<0.05).
Discussion
Several studies with human subjects have demonstrated attentional (23 – 25 , 29) and memory (20 , 30 – 32) biases for emotionally arousing information. We used a randomized, placebo-controlled experimental design to investigate the role of dopamine and dopamine antagonism in such biases. We found a significant interaction between stimulus valence and drug on recognition memory accuracy, with further contrasts revealing enhanced memory for aversive-arousing compared with neutral pictures in the placebo but not the amisulpride group. This result indicates that amisulpride was effective in abolishing the memory bias for emotionally arousing material. We did not find any effect of amisulpride on the perception of emotional stimuli. These results provide preliminary evidence for the hypothesis that dopamine is involved in modulating human memory for emotional stimuli, but not the perception of such stimuli.
Amisulpride Modulation of Emotional Memory
Amisulpride may contribute to the attenuation of the memory bias for emotional stimuli in at least two ways: via encoding (attention and perception) or postencoding (consolidation) effects. Given that our results do not support a role for amisulpride in the affective modulation of attentional processes, it seems unlikely that its effects on emotional memory derive from impairment of the selective encoding of emotional material. The trend toward impaired T1 detection suggests that amisulpride may cause subtle disruption of general selective attentional processes, which could contribute to the observed impairment in emotional memory. However, processing of the arousing properties of stimuli and the associated enhancement of memory are preserved even when attentional resources are restricted (33 , 34) . It therefore seems more likely that amisulpride’s effects on emotional memory occur via disruption of consolidation processes. This is in keeping with the evidence derived from both animal and human studies indicating that emotional enhancement of memory arises from amygdala-mediated postencoding consolidation effects (35 – 39) . The effect of amisulpride cannot be localized to the amygdala in this study. However, recent evidence of the attenuation of the limbic response to emotional stimuli after administration of the D 2 antagonist sultopride lends support to the notion that dopamine blockade may attenuate the emotional memory bias by disrupting amygdala-dependent consolidation processes (40) .
Amisulpride Modulation of Attention to Emotional Stimuli
In the first study to investigate the role of dopamine in the affective modulation of attention in human subjects, Franken et al. (11) reported that an acute dose of the dopamine antagonist haloperidol produced a reduction in attentional bias to drug-related cues in an emotional Stroop task in a group of heroin-dependent patients. Their finding suggests that dopamine may be involved in directing attention toward emotionally salient information. The absence of a drug effect in our study does not support a role for dopamine in the affective modulation of attention.
There is substantial evidence that both attentional and memory biases for emotional information are mediated by the arousal characteristics of stimuli as opposed to valence per se (20 , 24 , 25) . There is also evidence to suggest that aversive stimuli are associated with greater arousal properties than their positive counterparts (41) . We therefore chose to use aversive stimuli in order to maximize these biases and increase the likelihood of detecting drug effects. However, while there is clear evidence for a dopaminergic response to emotional stimuli associated with reward, the response to nonrewarding stimuli remains controversial (42) . The rewarding nature of the drug-related cues in the Franken et al. study (11) , as opposed to the aversive stimuli used in our study, could partly explain the different findings. Greater clarity would be achieved by examining the effect of dopamine antagonism on attention to both rewarding and aversive stimuli.
Clinical and Research Implications
A number of studies suggest that psychotic patients show exaggerated attentional and memory biases for emotionally salient stimuli compared with healthy volunteers (43 , 44) . Such biases are thought to play a role in the emergence and maintenance of symptoms such as delusions (45) . Our findings suggest that amisulpride attenuates the memory bias for emotional stimuli in healthy volunteers. The therapeutic effect of dopamine antagonists may therefore derive in part from their ability to “dampen down” emotional memory. This potential mechanism of action in turn implies that other neurotransmitter systems known to modulate emotional memory, such as the noradrenergic system (46) , may present additional therapeutic targets.
The inability of amisulpride to modulate the attentional bias toward emotionally salient information in our study suggests that this aspect of emotional processing may not be relevant to the mechanism of action of antipsychotic medication. However, our study was carried out in healthy volunteers. It may be the case that while dopamine antagonists have no effect on the ordinary attentional bias toward emotional stimuli, they can reduce the exaggerated responses to such stimuli that may occur in pathological hyperdopaminergic states such as psychosis or drug addiction. Such a view would be in keeping with the findings of Franken et al. (11) but requires further investigation.
Our findings may also be useful in interpreting studies of emotional processing in patients receiving antipsychotic medication. Several studies have demonstrated impaired emotional processing, along with abnormal neural responses, in schizophrenia. However, the majority of these studies have investigated medicated patients (47) . Our study suggests that the potential confounding effect of antipsychotic medication in studies of emotional processing should be taken into account. An understanding of the potential effects of psychotropic medications on the cognitive functions to be investigated in clinical groups would be of significant value in the design and execution of such studies.
Methodological Considerations
There are a number of limitations to our study. First, while the impairment of selective attention in the amisulpride group is in keeping with central dopaminergic antagonism, we did not directly assess dopamine receptor occupancy in this study. To the best of our knowledge, there are no published studies of dopamine receptor occupancy after a single 400 mg dose of amisulpride in healthy volunteers. However, a number of studies with schizophrenia patients treated with amisulpride suggest that plasma concentrations are highly positively correlated with D 2 /D 3 receptor occupancy in both striatal and extrastriatal regions (48 , 49) . Xiberas et al. (50) found that relatively low plasma concentrations (28–92 mg/liter) induced marked extrastriatal occupancy, and Bressan et al. (13) found that a mean plasma concentration of 278 mg/liter was associated with 82% receptor occupancy in the temporal cortex. These findings were based on steady-state plasma concentrations after repeated dosing and therefore cannot be directly extrapolated to our sample. However, they lend support to the likelihood of effective dopamine blockade being achieved with the range of plasma concentrations produced in our sample.
A second limitation is that we manipulated the dopaminergic system in only one direction. More conclusive evidence of dopaminergic involvement in emotional memory would be obtained by demonstrating its enhancement with a dopaminergic agonist.
Finally, given the increasing evidence of gender differences in emotional processing (51 , 52) , we chose to use an all-male sample to avoid confounding by gender or loss of power associated with a mixed-gender group of the same size. This limits our appreciation of the extent to which our results are applicable to women. Larger studies using mixed-gender groups would clarify this issue.
Conclusions
Our findings in this study provide the first demonstration of impairment of human emotional enhancement of memory by an antipsychotic. They suggest that dopamine very likely plays a significant role in biasing memory toward emotionally salient information and that dopamine antagonists may act by attenuating this bias. This study thus contributes to our understanding of the neurobiology of human emotional processing and may contribute to our understanding of the mechanism of action of antipsychotic medication. Pharmacological manipulation of cognitive processes represents a powerful yet underutilized tool for understanding the neurochemical basis of normal and abnormal cognition as well as drug mechanisms of action.
1. Kapur S, Mamo D: Half a century of antipsychotics and still a central role for dopamine D 2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27:1081–1090 Google Scholar
2. Kapur S: Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003; 160:13–23Google Scholar
3. Greba Q, Gifkins A, Kokkinidis L: Inhibition of amygdaloid dopamine D 2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res 2001; 899:218–226 Google Scholar
4. Guarraci FA, Frohardt RJ, Young SL, Kapp BS: A functional role for dopamine transmission in the amygdala during conditioned fear. Ann N Y Acad Sci 1999; 877:732–736Google Scholar
5. Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR: Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology 2002; 27:1036–1040Google Scholar
6. Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, Hyde TM, Weinberger DR, Mattay VS: Dopamine modulates the response of the human amygdala: a study in Parkinson’s disease. J Neurosci 2002; 20:9099–9103Google Scholar
7. Mehta MA, Hinton EC, Montgomery AJ, Bantick RA, Grasby PM: Sulpiride and mnemonic function: effects of a dopamine D 2 receptor antagonist on working memory, emotional memory, and long-term memory in healthy volunteers. J Psychopharmacol 2005; 19:29–38 Google Scholar
8. Wickelgren I: Getting the brain’s attention. Science 1997; 278:35–37Google Scholar
9. Horvitz JC: Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 2000; 96:651–656Google Scholar
10. Horvitz JC, Stewart T, Jacobs BL: Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res 1997; 759:251–258Google Scholar
11. Franken IHA, Hendriks VM, Stam C, van den Brink W: A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol 2004; 14:503–508Google Scholar
12. Schoemaker H, Claustre Y, Fage D, Rouquier L, Chergui K, Curet O, Oblin A, Gonon F, Carter C, Benavides J, Scatton B: Neurochemical characteristics of amisulpride, an atypical dopamine D 2 /D 3 receptor antagonist with both presynaptic and limbic selectivity. J Pharmacol Exp Ther 1997; 280:83–97 Google Scholar
13. Bressan RA, Erlandsson K, Jones HM, Mulligan R, Flanagan RJ, Ell PJ, Pilowsky LS: Is regionally selective D 2 /D 3 dopamine occupancy sufficient for atypical antipsychotic effect? an in vivo quantitative [ 123 I]epidepride SPET study of amisulpride-treated patients. Am J Psychiatry 2003; 160:1413–1420 Google Scholar
14. Leucht S, Pitschel-Walz G, Engel RR, Kissling W: Amisulpride, an unusual “atypical” antipsychotic: a meta-analysis of randomized controlled trials. Am J Psychiatry 2002; 159:180–190Google Scholar
15. Boyer P, Lecrubier Y, Puech AJ, Dewailly J, Aubin F: Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 1995; 166:68–72Google Scholar
16. Paillère-Martinot M-L, Lecrubier Y, Martinot J-L, Aubin F: Improvement of some schizophrenic deficit symptoms with low doses of amisulpride. Am J Psychiatry 1995; 152:130–133Google Scholar
17. Ramaekers JG, Louwerens JW, Muntjewerff ND, Milius H, de Bie A, Rosenzweig P, Patat A, O’Hanlon JF: Psychomotor, cognitive, extrapyramidal, and affective functions of healthy volunteers during treatment with an atypical (amisulpride) and a classic (haloperidol) antipsychotic. J Clin Psychopharmacology 1999; 19:209–221Google Scholar
18. Lang PJ, Bradley MM, Cuthbert BN: International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, University of Florida, Center for Research in Psychophysiology, 1998Google Scholar
19. Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L: Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neuroscience 2000; 20:1–5Google Scholar
20. Bradley MM, Greenwald MK, Petry MC, Lang PJ: Remembering pictures: pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn 1992; 18:379–390Google Scholar
21. Macmillan NA, Creelman DC: Detection Theory: A User’s Guide. Cambridge, UK, Cambridge University Press, 1991Google Scholar
22. Raymond JE, Shapiro KL, Arnell KM: Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform 1992; 18:849–860Google Scholar
23. Anderson AK, Phelps EA: Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 2001; 411:305–309Google Scholar
24. Anderson AK: Affective influences on the attentional dynamics supporting awareness. J Exp Psychol Gen 2005; 134:258–281Google Scholar
25. Keil A, Ihssen N: Identification facilitation for emotionally arousing verbs during the attentional blink. Emotion 2004; 4:23–35Google Scholar
26. Bradley M, Lang P: Affective Norms for English Words. Gainsville, Fla, NIMH Centre for the Study of Emotion and Attention, University of Florida, 1999Google Scholar
27. Kucera H, Francis W: Computational Analysis of Present-Day American English. Providence, RI, Brown University Press, 1967Google Scholar
28. Greenhouse S, Geisser S: On methods in the analysis of profile data. Psychometrika 1959; 24:95–112Google Scholar
29. Richards A, Blanchette I: Independent manipulation of emotion in an emotional Stroop task using classical conditioning. Emotion 2004; 4:275–281Google Scholar
30. LaBar KS, Phelps EA: Arousal-mediated memory consolidation: role of the medial temporal lobe. Psychol Sci 1998; 9:490–493Google Scholar
31. Heuer F, Riesberg D: Vivid memories of emotional events: the accuracy of remembered minutiae. Mem Cognit 1990; 18:496–506Google Scholar
32. Williams JM, Mathews A, MacLeod C: The emotional Stroop task and psychopathology. Psychol Bull 1996; 120:3–24Google Scholar
33. Vuilleumier P, Armony JL, Driver J, Dolan RJ: Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 2001; 30:829–841Google Scholar
34. Sharot T, Phelps EA: How arousal modulates memory: disentangling the effects of attention and retention. Cogn Affect Behav Neurosci 2004; 4:294–306Google Scholar
35. Tabert MH, Borod JC, Tang CY, Lange G, Wei TC, Johnson R, Nusbaum AO, Buchsbaum MS: Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia 2001; 39:556–573Google Scholar
36. Kleinsmith LJ, Kaplan S: Paired-associate learning as a function of arousal and interpolated interval. J Exp Psychol Gen 1963; 65:190–193Google Scholar
37. LaLumiere RT, Buen T, McGaugh JL: Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci 2003; 23:6754–6758Google Scholar
38. LaLumiere RT, Nguyen LT, McGaugh JL: Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: involvement of noradrenergic and cholinergic systems. Eur J Neurosci 2004; 20:2804–2810Google Scholar
39. Hamann SB, Ely TD, Grafton ST, Kilts CD: Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci 1999; 2:289–293Google Scholar
40. Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T, Okubo Y: Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological study. Neuroimage 2005; 27:991–1001Google Scholar
41. Bradley MM, Codispoti M, Cuthbert BN, Lang PJ: Emotion and motivation, I: defensive and appetitive reactions in picture processing. Emotion 2001; 1:276–298Google Scholar
42. Ungless MA: Dopamine: the salient issues. Trends Neurosci 2004; 27:702–706Google Scholar
43. Bentall R, Kaney S: Content specific information processing and persecutory delusions: an investigation using the emotional Stroop test. Br J Med Psychol 1989; 62:355–364Google Scholar
44. Bentall R, Kaney S, Bowen-Jones K: Persecutory delusions and recall of threat-related, depression-related, and neutral words. Cogn Ther Res 1995; 19:331–343Google Scholar
45. Bentall R: Cognitive biases and abnormal beliefs: towards a model of persecutory delusions, in The Neuropsychology of Schizophrenia. Edited by David A, Cutting J. London, Lawrence Erlbaum Associates, 1994, pp 337–360Google Scholar
46. Cahill L, Prins B, Weber M, McGaugh JL: b-adrenergic activation and memory for emotional events. Nature 1994; 371:702–704Google Scholar
47. Aleman A, Kahn RS: Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol 2005; 77:283–298Google Scholar
48. la Fougère C, Meisenzahl E, Schmitt G, Stauss J, Frodl T, Tatsch K, Hahn K, Möller HJ, Dresel S: D 2 receptor occupancy during high- and low-dose therapy with the atypical antipsychotic amisulpride: a 123I-iodobenzamide SPECT study. J Nucl Med 2005; 46:1028–1032 Google Scholar
49. Martinot JL, Paillere-Martinot ML, Poirier M, Dao-Castellana M, Loc’h C, Maziere B: In vivo characteristics of dopamine D 2 receptor occupancy by amisulpride in schizophrenia. Psychopharmacology (Berl) 1996; 124:154–158 Google Scholar
50. Xiberas X, Martinot JL, Mallet L, Artiges E, Canal M, Loc’h C, Maziere B, Paillere-Martinot ML: In vivo extrastriatal and striatal D 2 receptor blockade by amisulpride in schizophrenia. J Clin Psychopharmacol 2001; 21:207–214 Google Scholar
51. Bradley MM, Codispoti M, Sabatinelli D, Lang PJ: Emotion and motivation, II: sex differences in picture processing. Emotion 2001; 1:300–319Google Scholar
52. Cahill L: Why sex matters for neuroscience. Nat Rev Neurosci 2006; 7:477–484Google Scholar



