BDNF Val66Met Polymorphism and GAD67 mRNA Expression in the Prefrontal Cortex of Subjects With Schizophrenia
Abstract
OBJECTIVE: In the prefrontal cortex of subjects with schizophrenia, decreased signaling mediated by brain-derived neurotrophic factor (BDNF) and its receptor tyrosine kinase (TrkB) appears to contribute to the reduced expression of mRNA encoding the 67-kilodalton isoform of glutamate decarboxylase (GAD67), an enzyme for GABA synthesis. The authors examined in subjects with schizophrenia the effect in the human BDNF gene of a single nucleotide polymorphism (Val66Met), which reduces the trafficking and secretion of BDNF protein, on the expression of GAD67 mRNA. METHOD: BDNF Val66Met genotyping was performed in 27 matched pairs of schizophrenia and comparison subjects. The impact of this polymorphism on prefrontal cortex GAD67 mRNA expression in schizophrenia subjects was assessed by comparing within-pair differences in GAD67 mRNA expression between schizophrenia subjects with versus without the Met66 allele after the level of BDNF mRNA expression was controlled. RESULTS: In contrast to expectations, the within-pair reduction in GAD67 mRNA expression was not greater in schizophrenia subjects who were hetero- or homozygous for the Met66 allele. These subjects did tend to exhibit less marked within-pair reductions in both GAD67 and BDNF mRNA expression compared with schizophrenia subjects homozygous for the Val allele. CONCLUSIONS: The presence of the BDNF Met66 allele does not contribute to the decreased level of GAD67 mRNA expression in the prefrontal cortex of subjects with schizophrenia.
In the prefrontal cortex of subjects with schizophrenia, inhibitory neurotransmission appears to be altered as indicated by the decreased expression of mRNA encoding markers of γ-aminobutyric acid (GABA) neurotransmission, such as the 67-kilodalton isoform of glutamate decarboxylase (GAD67), an enzyme for GABA synthesis (1, 2). This mRNA is down-regulated in the subclass of GABA neurons containing the calcium-binding protein, parvalbumin, and the mRNA encoding parvalbumin was also found to be decreased (3). These alterations are likely to contribute to prefrontal cortex dysfunction in schizophrenia, given the evidence that parvalbumin-containing GABA neurons play a critical role in the regulation of prefrontal cortex information processing during working memory tasks (4).
Several lines of evidence suggest that the signaling mediated by brain-derived neurotrophic factor (BDNF) and its receptor tyrosine kinase (TrkB) is involved in these alterations in GABA neurons in schizophrenia. First, BDNF regulates the maturation of GABA neurons in the developing cortex and influences the expression of GABA markers, including GAD67 and parvalbumin (5, 6). Second, the levels of mRNA and protein for BDNF and TrkB are decreased in the prefrontal cortex of subjects with schizophrenia (7, 8). Finally, these decreases in BDNF and TrkB mRNA are significantly correlated with the decreases in GAD67 mRNA levels (8).
A single nucleotide polymorphism (rs6265) in the human BDNF gene (bdnf), which produces a valine to methionine substitution at codon 66 (Val66Met) in the 5′ proregion of BDNF, was reported to reduce intracellular trafficking and activity-dependent secretion of BDNF (9). In addition, subjects with the Met66 allele showed abnormal hippocampal activity and poorer performance during an episodic memory task, consistent with a reduced functional effect of BDNF in hippocampal circuitry (9). Thus, the presence of the Met66 allele in subjects with schizophrenia would be predicted to be associated with a greater decrease of cortical GAD67 mRNA expression relative to subjects with schizophrenia homozygous for the Val66 allele.
Method
The 27 subject pairs examined in this study have been described previously (8) as cohort 1 (subject pairs 1–15) and cohort 2 (subject pairs 16–27). Each pair consisted of a subject with schizophrenia and a comparison subject matched by age, gender, and postmortem interval. In order to determine BDNF Val66Met genotype, genomic DNA was isolated from cerebellar tissue of each subject using DNeasy Tissue Kit (Qiagen, Valencia, Calif.) and analyzed with Taqman 5′-exonuclease assay as described previously (9). Information regarding the PCR conditions and sequences of the primers and Taqman probes is available upon request. The expression levels of GAD67 and BDNF mRNA in the prefrontal cortex of the 27 subject pairs were reported previously (8). Because the strongly negative correlation between age and prefrontal cortex BDNF expression (8, 10) would confound comparisons of mRNA expression, we compared within-pair differences in mRNA expression for two subject pair groups defined by bdnf genotype: pairs in which both subjects carried only the Val66 allele and pairs in which only the schizophrenia subject carried at least one Met66 allele.
The frequency of the Met66 allele and the proportion of subjects with this allele were compared between schizophrenia and comparison subjects using a chi-square test. To assess the impact of the Met66 allele on the change in GAD67 mRNA expression, within-pair differences in BDNF and GAD67 mRNA expression were subjected to a mixed-model two-way analysis of variance (ANOVA) with bdnf genotype of the subject pair (i.e., pairs in which both subjects were homozygous for the Val66 allele versus those in which only the schizophrenia subject had one or more Met66 alleles) as a between-pair factor and mRNA type (BDNF versus GAD67) as a within-pair factor. Because there was a significant correlation between within-pair differences in GAD67 and BDNF mRNA levels across subject pairs (8), the net effect of the Met66 allele on within-pair differences in GAD67 mRNA levels, after within-pair differences in BDNF mRNA levels were controlled, was assessed by the interaction between the two factors.
Results
The proportion of subjects with the Met66 allele was significantly greater (χ2=5.3, df=1, p<0.03) among subjects with schizophrenia (six heterozygous and one homozygous subject) than among comparison subjects (one heterozygous subject) (Figure 1). The frequency of the Met66 allele in the schizophrenia group (15%) and the comparison group (2%) also significantly differed (χ2=5.9, df=1, p<0.02).
Within-pair percentage changes in BDNF and GAD67 mRNA expression levels for each pair are shown in Figure 1. In pair 20, the schizophrenia subject showed a 310% increase in BDNF mRNA expression level. As reported previously, this abnormally high expression of BDNF mRNA (more than seven standard deviations above the comparison subject mean) appeared to be due to a tricyclic antidepressant overdose (8). We excluded pair 20 from the analysis. Pair 15 was also excluded because the schizophrenia and comparison subjects both had one Met66 allele and one Val66 allele.
The mean within-pair percentage changes in BDNF and GAD67 mRNA expression levels in the schizophrenia subjects were –15.0% and –9.9%, respectively, for the five subject pairs with a Met66 allele in the schizophrenia subject, and –38.5% and –31.2%, respectively, for the 20 pairs homozygous for the Val66 allele (Figure 2). Two-way ANOVA failed to reveal a significant interaction between pair genotype and mRNA type (F=0.22, df=1, 23, p=0.88), indicating that the presence of a Met66 allele in schizophrenia subjects did not contribute to the decreased level of GAD67 mRNA expression. The ANOVA did reveal a tendency for pair genotype to affect within-pair differences in mRNA expression (F=3.76, df=1, 23, p<0.07), suggesting that BDNF and GAD67 mRNA expression changes might be smaller in the subject pairs with the Met allele relative to those without a Met allele.
Discussion
Although the primary focus of this study was to assess the effect of bdnf genotype on GAD67 mRNA expression, we unexpectedly encountered a significantly lower frequency of the bdnf Met66 allele in the comparison group (2%) than in the schizophrenia group (15%). However, previous case/control studies using larger populations reported much higher frequencies (19%–22%) of the Met66 allele in comparison subjects that were similar to (9) or even higher than (11) the frequency found in schizophrenia subjects. Considering the limited size of our cohort, it seems likely that the low frequency of the Met66 allele in the comparison group represents a chance finding.
Because BDNF has been shown to induce GAD67 expression in cortical neurons in vitro and in vivo (5, 6), the decreased expression of GAD67 mRNA in the prefrontal cortex of subjects with schizophrenia might reflect a reduced availability of BDNF. BDNF signaling in the cortex is determined not only by its synthesis in neurons but also by its release from them. Thus, GAD67 expression levels might be modified by the BDNF Val66Met genotype, given that the Val to Met substitution in the 5′ prodomain of pro-BDNF peptide reportedly causes abnormal intracellular trafficking and a subsequent reduction of activity-dependent secretion of BDNF (9). Thus, we expected that the magnitude of decrease in GAD67 mRNA would be greater in schizophrenia subjects with the Met allele after the level of BDNF mRNA expression was controlled. However, no evidence for such an effect was found, suggesting that the possible deficit in activity-dependent release of BDNF caused by the presence of the Met allele does not contribute to the decrease in GAD67 mRNA expression in subjects with schizophrenia. It is interesting that the decreases in both GAD67 and BDNF mRNA expression in the prefrontal cortex appear to be less severe in schizophrenia subjects with the Met66 allele than in those without the Met66 allele. Further studies are needed to both confirm this observation and to illuminate potential mechanisms underlying the association between the presence of the Met allele and a tempering of the deficit in GAD67 and BDNF mRNA expression in subjects with schizophrenia. These findings, together with our previous observation of no change in cortical GAD67 mRNA expression in mice with a neuron-specific inducible knockout of bdnf(8), suggest that the reduced availability of BDNF may not be a primary cause of decreased GAD67 expression in the prefrontal cortex of subjects with schizophrenia. In contrast, other factors such as the availability of TrkB (8) or allelic variants in the GAD67 gene (12) may be more important.
Received May 18, 2005; revision received Aug. 17, 2005; accepted Sept. 19, 2005. From the Department of Psychiatry and the Department of Neuroscience, University of Pittsburgh. Address correspondence and reprint requests to Dr. Lewis, Department of Psychiatry, University of Pittsburgh, W1650 Biomedical Science Tower, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail).Supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression’s Essel Foundation (Dr. Hashimoto) and by NIMH grants MH-43784 and MH-45156 (Dr. Lewis).The authors thank Dr. Daniel Weinberger (NIMH) for providing technical information regarding human BDNF Val66Met genotyping.
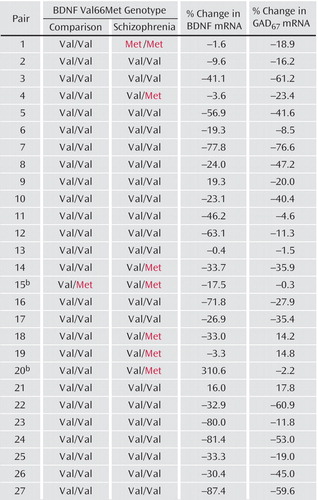
Figure 1. Effect of BDNF Val66Met Genotype on Changes in GAD67 mRNA Expression in Schizophrenia Subjects Relative to Their Matched Comparison Subjects a
aBDNF Val66Met genotype of the comparison and schizophrenia subjects in each pair is shown along with the percentage changes in BDNF and GAD67 mRNA expression in the schizophrenia subjects relative to their matched comparison subjects.
bExcluded from the analyses.
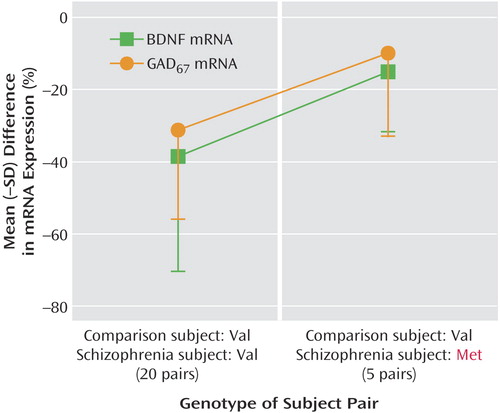
Figure 2. Changes in BDNF and GAD67 mRNA Expression in Subject Pairs Without the Met66 Allele and Pairs With the Met66 Allele in the Schizophrenia Subject Onlya
aNote that the presence of nonintersecting lines connecting the mean percentage changes in the BDNF and GAD67 mRNA indicates the absence of an effect of bdnf genotype on GAD67 mRNA expression after controlling the level of BDNF mRNA expression (F=0.22, df=1, 23, p=0.88).
1. Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr, Jones EG: Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 1995; 52:258–266Crossref, Medline, Google Scholar
2. Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA: Decreased GAD67 mRNA expression in a subset of prefrontal cortical GABA neurons in subjects with schizophrenia. Arch Gen Psychiatry 2000; 57:237–245Crossref, Medline, Google Scholar
3. Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA: Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23:6315–6326Crossref, Medline, Google Scholar
4. Lewis DA, Hashimoto T, Volk DW: Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312–324Crossref, Medline, Google Scholar
5. Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S: BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 1999; 98:739–755Crossref, Medline, Google Scholar
6. Cotrufo T, Viegi A, Berardi N, Bozzi Y, Mascia L, Maffei L: Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. J Neurosci 2003; 23:3566–3571Crossref, Medline, Google Scholar
7. Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE: Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2003; 8:592–610Crossref, Medline, Google Scholar
8. Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA: Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci 2005; 25:372–383Crossref, Medline, Google Scholar
9. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR: The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112:257–269Crossref, Medline, Google Scholar
10. Hayashi M, Yamashita A, Shimizu K: Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. Brain Res 1997; 749:283–289Crossref, Medline, Google Scholar
11. Neves-Pereira M, Cheung JK, Pasdar A, Zhang F, Breen G, Yates P, Sinclair M, Crombie C, Walker N, St Clair DM: BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol Psychiatry 2005; 10:208–212Crossref, Medline, Google Scholar
12. Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, Greenstein D, Lenane M, Gochman P, Baker N, Balkissoon R, Vakkalanka RK, Weinberger DR, Rapoport JL, Straub RE: GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry 2005; 10:581–588Crossref, Medline, Google Scholar



