Evidence of White Matter Pathology in Bipolar Disorder Adolescents Experiencing Their First Episode of Mania: A Diffusion Tensor Imaging Study
Abstract
OBJECTIVE: Previous diffusion tensor imaging findings have supported suggestions that bipolar disorder is characterized by subtle white matter changes. The chronic nature of the study population, however, has limited interpretation of these findings. In this study the authors utilized diffusion tensor imaging to study white matter tracts of adolescents in their first episode of mania to address whether abnormalities are present in early bipolar disorder. METHOD: Eleven medication-naive adolescents in their first episode of mania and 17 healthy subjects underwent diffusion tensor imaging scans. Fractional anisotropy and trace apparent diffusion coefficients of prefrontal and posterior regions of interest were compared between groups. RESULTS: Bipolar adolescents showed significantly decreased fractional anisotropy only in superior-frontal white matter tracts. Trace apparent diffusion coefficients did not significantly differ in any regions examined. CONCLUSIONS: These findings suggest that prefrontal white matter abnormalities are present early in bipolar disorder and may consist largely of axonal disorganization. The presence of changes in young first-episode patients also suggests that white matter pathology may represent an early marker of bipolar disorder.
Limited evidence, including increased numbers of white matter hyperintensities and decreased whole brain white matter volume and density, has suggested that neurofunctional changes in bipolar patients are related to subtle white matter abnormalities (1, 2). Diffusion tensor imaging, a magnetic resonance technique that measures aspects of water diffusion, may more precisely identify these subtle white matter derangements (3). One diffusion tensor imaging measure, trace apparent diffusion coefficient, represents the distance water molecules may freely diffuse; increases in trace apparent diffusion coefficient have been associated with axonal demyelization and damage, as well as localized edema (3).
Decreased fractional anisotropy, which represents the degree of anisotropic diffusion, may similarly reflect a loss of axonal integrity. A partial loss of structural organization, however, may also decrease gross directional differences in diffusion with a resultant decrease in fractional anisotropy (3, 4). Lower fractional anisotropy values are associated with antidepressant treatment response in elderly, unipolar depressed patients (5). In adult, multiepisode bipolar patients, decreased fractional anisotropy was observed in white matter tracts adjacent to the prefrontal cortex, with only minimal differences in trace apparent diffusion coefficient between bipolar patients and comparison subjects, suggesting that fractional anisotropy changes may represent white matter disorganization rather than a loss of axonal integrity (6).
To address whether white matter changes occur early in the course of bipolar disorder, we compared measures of diffusion tensor imaging in bipolar disorder adolescents experiencing their first episode of mania and a matched group of healthy subjects. Our previous findings suggested that white matter changes represent a loss of bundle coherence and therefore may be developmental in origin. Consequently, we hypothesized that we would observe significant decreases in fractional anisotropy in bipolar adolescents without significant changes in trace apparent diffusion coefficient.
Method
Eleven adolescents (six female and five male) experiencing their first manic or mixed episode and 17 healthy subjects (10 female and seven male) with no psychiatric history in themselves or first-degree relatives were recruited. All patients had a Young Mania Rating Scale score ≥20; mean age at onset was 11 years (SD=3). All subjects (bipolar and healthy) were between 10 and 18 years of age (mean=14 years, SD=2) and were right-handed or ambidextrous with Tanner stage ≥2.5 and IQ ≥70 (7, 8). No subjects had any history of substance dependence or abuse within the preceding 3 months. All subjects were medically and neurologically stable, with no history of head trauma that resulted in loss of consciousness ≥10 minutes. Subjects were mood stabilizer and antipsychotic naive. No subjects were receiving any pharmacotherapy.
Diagnoses, or their absence, were confirmed using the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (9). Demographic information was obtained from adolescents and their legal guardians. Adolescent subjects provided written assent, and legal guardians provided written informed consent after study procedures were fully explained. This study was approved by the institutional review boards of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
Diffusion-weighted and T1-weighted three-dimensional brain scan images were obtained as previously described (6). T1-weighted three-dimensional brain scans provided anatomical localization and were used to derive normalization parameters. Image data were processed as previously described (6). Trace apparent diffusion coefficient and fractional anisotropy were calculated for each data set. Regions of interest included white matter tracts adjacent to portions of the prefrontal and posterior cortex where activation changes have been observed in bipolar patients (10, 11). Regions of interest were 5 voxels in diameter and were placed bilaterally on six adjacent 5-mm slices by a single investigator (J.A.), as previously described (6). Anterior regions of interest extended from 2 mm below the anterior commissure-posterior commissure (AC-PC) to 28 mm above the AC-PC. Posterior regions of interest extended from 2 to 31 mm above the AC-PC. Regions of interest were combined to form inferior, middle, and superior regions as previously described (6).
Mean values for trace apparent diffusion coefficient and fractional anisotropy were determined for each region. A multiple analysis of variance (MANOVA) was performed separately for fractional anisotropy and trace apparent diffusion coefficient for frontal and posterior cortex. Post hoc groupwise regional comparisons were performed separately for fractional anisotropy and trace apparent diffusion coefficient, using Student’s t tests. Laterality was tested only where differences were significant. All statistics were two-tailed.
Results
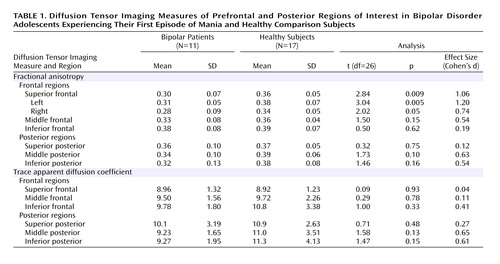
A MANOVA comparing regional fractional anisotropy group differences in frontal brain regions was not significant (F=2.70, df=3, 24, p=0.07). Fractional anisotropy of inferior and middle frontal regions did not significantly differ by group. However, fractional anisotropy of the superior frontal region was significantly lower in bipolar adolescents, particularly on the left (Table 1). There were no significant overall group effects (F=1.47, df=3, 24, p=0.25) for fractional anisotropy in the posterior cortex. While the middle posterior region of bipolar patients showed a somewhat decreased fractional anisotropy relative to healthy subjects, no posterior regions showed a significant difference (Table 1).
There were no significant overall group effects for trace apparent diffusion coefficient (frontal: F=0.56, df= 3, 24, p=0.65, posterior: F=0.95, df=3, 24, p=0.43), nor were there any significant differences between bipolar and healthy subjects in trace apparent diffusion coefficient for any frontal or posterior regions (Table 1).
Discussion
Our observation of decreased prefrontal fractional anisotropy in bipolar adolescents is consistent with suggestions that white matter neuropathology may play a role in previously observed prefrontal neurofunctional changes (12). Differences in fractional anisotropy were not significant in any posterior regions of interest, although the medium to strong effect size in the middle region suggests possible white matter pathology in this area. The lack of differences in trace apparent diffusion coefficient, even in regions with decreased fractional anisotropy, supports our hypothesis that this loss of anisotropic diffusion in prefrontal white matter is more related to axonal disorganization than axonal demyelization or apoptosis.
No subject was receiving psychotropic medications at the time of the scan, all subjects were mood stabilizer and antipsychotic naive, and no subject had previously required psychiatric hospitalization. Some limitations, however, arise from the relatively small study group size. The lack of differences in trace apparent diffusion coefficient may reflect inadequate power to detect subtle losses of axonal integrity, although the low effect sizes observed suggest otherwise. Although gender ratios were not precisely matched, previous studies have failed to correlate gender and measures of diffusion tensor imaging (13). In addition, no studies have examined potential effects of mood state on diffusion tensor imaging.
This study extends previous diffusion tensor imaging findings to a population of young, mood stabilizer- and antipsychotic-naive first-episode patients. Our findings provide support for hypotheses suggesting that a loss of network connectivity represents an element of the neuropathology of bipolar disorder. Decreased fractional anisotropy in bipolar adolescents, early in their illness course, suggests that white matter neuropathology constitutes an intrinsic aspect of bipolar disorder that may constitute an early illness marker that precedes the relatively late appearance of functional deficits (14).
 |
Presented in part at the 157th annual meeting of the American Psychiatric Association, New York, May 1–6, 2004. Received March 15, 2005; revision received June 20, 2005; accepted July 15, 2005. From the Center for Bipolar Disorders Research and the Center for Imaging Research, University of Cincinnati College of Medicine; and the Imaging Research Center, Children’s Hospital Medical Center, University of Cincinnati. Address correspondence and reprint requests to Dr. Adler, Department of Psychiatry, University of Cincinnati College of Medicine, 231 Albert Sabin Way, Cincinnati, OH 45267-0559; [email protected] (e-mail).Support was provided by the Stanley Medical Research Institute (C.M.A., S.M.S), the Theodore and Vada Stanley Foundation (A.L.), the National Alliance for Research on Schizophrenia and Depression (C.M.A.), the Heinz C. Prechter Bipolar Research Fund (M.P.D.), and NIH grants MH-58170 (S.M.S.), MH-63373 (M.P.D.), and MH-64086 (C.M.A.).
1. Figiel GS, Krishnan KR, Rao VP, Doraiswamy M, Ellinwood EH Jr, Nemeroff CB, Evans D, Boyko O: Subcortical hyperintensities on brain magnetic resonance imaging: a comparison of normal and bipolar subjects. J Neuropsychiatry Clin Neurosci 1991; 3:18–22Crossref, Medline, Google Scholar
2. Strakowski SM, Wilson DR, Tohen M, Woods BT, Douglass AW, Stoll AL: Structural brain abnormalities in first-episode mania. Biol Psychiatry 1993; 33:602–609Crossref, Medline, Google Scholar
3. Beaulieu C: The basis of anisotropic diffusion in the nervous system—a technical review. NMR Biomed 2002; 15:435–455Crossref, Medline, Google Scholar
4. Foong J, Symms MR, Barker GJ, Maier M, Miller DH, Ron MA: Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport 2002; 13:333–336Crossref, Medline, Google Scholar
5. Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO: Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry 2002; 159:1929–1932Link, Google Scholar
6. Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, Strakowski SM: Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord 2004; 6:197–203Crossref, Medline, Google Scholar
7. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
8. Crovitz HF, Zener K: A group test for assessing hand- and eye-dominance. Am J Psychol 1962; 75:271–276Crossref, Medline, Google Scholar
9. Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C: Reliability of the Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS). J Am Acad Child Adolesc Psychiatry 2001; 40:450–455Crossref, Medline, Google Scholar
10. Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP: A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology 2004; 29:1734–1740Crossref, Medline, Google Scholar
11. Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM: Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord 2004; 6:540–549Crossref, Medline, Google Scholar
12. Strakowski SM, DelBello MP, Adler CM: The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry 2005; 10:105–116Crossref, Medline, Google Scholar
13. Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A: Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport 2001; 12:99–104Crossref, Medline, Google Scholar
14. Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS: Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann NY Acad Sci 2004; 1021:376–383Crossref, Medline, Google Scholar



