Executive Function and Cognitive Subprocesses in First-Episode, Drug-Naive Schizophrenia: An Analysis of N-Back Performance
Abstract
OBJECTIVE: This study investigated changes in the cognitive architecture of N-back performance in schizophrenia. METHOD: N-back performance of 12 patients with first-episode, drug-naive schizophrenia and matched healthy comparison subjects was studied in a reaction-time decomposition paradigm. RESULTS: Imposition of a working memory load led to a significant drop in response accuracy in patients. Reaction-time decomposition suggested slowing of visuomotor and choice reaction processing as well as an inability of parallel processing directed by working memory. DISCUSSION: Although N-back tasks validly access working memory function as a neurocognitive trait in the illness, several additional subprocesses and the ability for cognitive parallel processing are altered and require further study in schizophrenia.
Working memory disturbance represents a core neurocognitive feature of schizophrenia (1). One of the most frequently used methods to assess working memory is the N-back paradigm, in which schizophrenic patients commonly make more errors and need more processing time (1). However, neuroimaging results have been variable, with both prefrontal hypo- and hyperactivity being reported.
The N-back task is cognitively complex and embeds working memory into other subfunctions of information processing. Understanding these subprocesses and their possible differential impairment in schizophrenia might help explain discrepant neuroimaging results and advance our understanding of the cognitive dysfunction. We used a reaction-time decomposition approach comparing two types of N-back tasks widely used in neuroimaging: continuous delayed response (2, 3) and continuous matching (4). We examined drug-naive, first-episode patients to exclude a confounder of neuroleptic medication that can influence working memory performance (5).
Method
Twelve neuroleptic-naive inpatients with a DSM-IV diagnosis of first-onset schizophrenia or schizophreniform disorder were studied after informed written consent was received. Their mean Positive and Negative Syndrome Scale (6) scores were total: 88.6 (SD=19.4), positive: 20.7 (SD=4.8), negative: 22.7 (SD=8.3), and general: 45.2 (SD=10.6).
Healthy comparison subjects were matched pairwise for age (patients: mean=24.6 years, SD=5.8; healthy comparison subjects: mean=25.8 years, SD=6.2) (t=0.48, df=22, p=0.63, independent t test), sex (six men and six women), and years of education. All subjects were right-handed and physically healthy.
To analyze the N-back as cognitive subcomponents, we decomposed it into a hierarchy of five tasks of increasing cognitive complexity (7). If subprocesses are serial and independent, the time for each stage can then be estimated by subtracting the reaction time of the previous stage. Reaction-time decomposition also allows detection of situations where subprocesses are not serial or independent.
The tasks are described elsewhere (8). Thirty squares and triangles were presented at random (equal probability). Response was by moving a cursor as fast as possible from a starting to a target button. Trials were self-paced.
In simple reaction tasks, the subjects had to respond as soon as any stimulus was presented. In stimulus discrimination tasks, the reaction was to triangles only, ignoring squares, and introducing stimulus discrimination and motor no-go components. In choice reaction tasks, two target buttons were displayed that were selected based on the stimulus type, necessitating a response selection stage.
The continuous delayed response task was laid out like the choice reaction task, but the target button was now determined by the stimulus presented in the previous trial, requiring maintenance of stimulus information during the intertrial interval, as well as an inhibitory component to override the tendency to respond to the current stimulus. In the continuous matching task, target buttons were labeled “same” and “different,” additionally requiring the subjects to compare the previous stimulus with the currently presented one.
Dependent variables were the percentage of correct responses and reaction time. Since cognitive processing may continue after the initiation of motor responses (8), reaction time was measured from stimulus onset until reaching the target button. To avoid error recovery confounders, only correct responses were analyzed.
We used two-by-five analysis of variance with Greenhouse-Geisser correction, the independent factor of group (healthy subjects or patients), and the repeated measurement factor of task (simple reaction, stimulus discrimination, choice reaction, continuous delayed response, and continuous matching) followed by post hoc testing (t and Levene’s tests).
Results
Response accuracy showed a significant group-by-task interaction (F=17.24, df=2.1, 45.5, p<0.0001) (Figure 1). Although both groups exhibited equally high accuracy in the simple reaction, stimulus discrimination, and choice reaction tasks, the patients but not the comparison subjects decreased their scores markedly in the continuous delayed response task. This group difference persisted in the continuous matching task, although the comparison subjects also decreased their scores. Thus, the highest group separation occurred during working memory tasks, especially during the continuous delayed response task (85% nonoverlap).
Reaction time showed an effect of group (F=28.21, df=1, 22, p<0.0001), indicating general slowness of patients and a group-by-task interaction (F=11.01, df=2.4, 52.9, p<0.0001). The patients were slower performing the simple reaction task, did not slow further during stimulus discrimination, but showed additional disproportional slowing in the choice reaction task.
Working memory load in the continuous delayed response task led to a marked difference (88% separation), with patients needing 313 msec more for the continuous delayed response task than for the choice reaction task, whereas the healthy comparison subjects required approximately the same time. Differences in continuous delayed response times remained significant when they were corrected for choice reaction time.
The continuous matching task only added about 90 msec to each patient’s reaction time over the continuous delayed response, but processing time increased markedly in healthy subjects (about 267 msec). After correction for choice reaction time, the between-group difference was 110 msec (n.s.).
Discussion
We found deficits in N-back performance at the onset of schizophrenia before neuroleptic treatment, strengthening the case for the use of N-back tasks to access a valid cognitive trait of schizophrenia. That this trait is related to working memory proper was indicated by response accuracy: the patients’ performance only deteriorated when a working memory load was imposed, not at any previous step. However, our fine-grained analyses of cognitive architecture with a reaction-time decomposition uncovered additional disturbances in earlier stages that, while not impairing performance by themselves, may contribute to decompensation under working memory demands.
The slowing of visuomotor performance in simple reaction was expected from the literature (8). We observed additional significant differential slowing with normal accuracy in choice reaction tasks, indicating a subtle abnormality in first-episode schizophrenia, replicating previous results (4, 8, 9). Since stimulus discrimination was not differentially slowed, this favors disturbances in choice reaction over problems with response selection.
In the continuous delayed response task, a significant reaction-time increase and loss of accuracy were seen in patients but not comparison subjects. We propose that comparison subjects might deviate from simple serial processing at this stage by selecting and preparing the upcoming motor response in the intertrial interval based on the single stimulus held in working memory. In marked contrast, no indication for this strategy was found in patients, possibly indicating an inability to use working memory to flexibly prepare motor responses in parallel.
In the continuous matching task, parallel processing during the intertrial interval is impossible because comparison with the current stimulus determines motor response. Consequently, this led to a large increase in reaction time in comparison subjects (who then had to revert to serial processing) but not patients. With correction for choice reaction, processing times for this stage were not significantly different, indicating that it was of limited accuracy and that the absence of parallel processing capability differentiates working memory processing in schizophrenia but not time requirement as such. Of interest, in schizophrenia patients, reaction time in choice reaction tasks predicted error rates in the continuous matching task (r=0.730, p<0.007) but not the continuous delayed response task, which argues that problems with choice reaction directed by working memory may contribute to impaired performance.
Neuroimaging data show that parallel, or dual task, requirements differentially affect the prefrontal cortex. Therefore, conflicting N-back findings in schizophrenia may be partially due to the degree in which various paradigms allow parallel processing. Further studies are necessary to decide this issue.
Reaction-time decomposition may be too simplistic to capture complex cognitive aspects involved, e.g., when order effects are present or processes are not independent. Also, some studies have not shown increased reaction time during working memory in schizophrenia (10). However, the approach showed heuristic value by indicating that cognitive deficits in schizophrenia can be parsed into differentially impaired subfunctions, which contribute to our understanding of the illness.
Received May 19, 2003; revision received May 28 and June 9, 2004; accepted Aug. 11, 2004. From the Department of Psychiatry, Justus-Liebig-University Medical School, Giessen, Germany; the Neuroimaging Core Facility and Unit on Systems Neuroscience in Psychiatry, Genes, Cognition, and Psychosis Program, NIMH. Address correspondence and reprint requests to Dr. Meyer-Lindenberg, 10-4C101, NIMH, 9000 Rockville Pike, Bethesda, MD 20892-1365; [email protected] (e-mail).
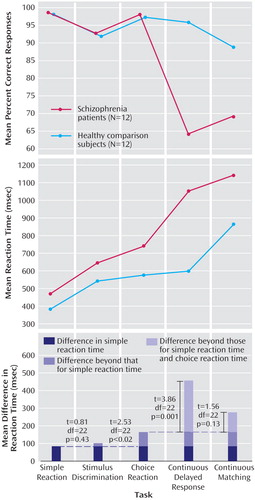
Figure 1. Correct Responses and Reaction Times Measured During Working Memory Tasks for Inpatients With First-Episode Schizophrenia or Schizophreniform Disorder and Healthy Comparison Subjects
1. Keefe RSE: Working memory dysfunction and its relevance in schizophrenia, in Cognitive Functioning in Schizophrenia. Edited by Sharma T, Harvey PD. Oxford, Oxford University Press, 2000, pp 16–49Google Scholar
2. Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger D: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
3. Meyer-Lindenberg A, Poline JP, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158:1809–1817Link, Google Scholar
4. Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD: Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry 1998; 155:1285–1287Link, Google Scholar
5. Kravariti E, Morris RG, Rabe-Hesketh S, Murray RM, Frangou S: The Maudsley Early-Onset Schizophrenia Study: cognitive function in adolescent-onset schizophrenia. Schizophr Res 2003; 65:95–103Crossref, Medline, Google Scholar
6. Kay SR, Fizbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
7. Donders FC: Die Schnelligkeit psychischer Prozesse. Reicherts’s & Dubois-Reymond’s Archiv für Anatomie, Physiologie und Wissenschaftliche Medizin 1868; 657–681Google Scholar
8. Krieger S, Lis S, Gallhofer B: Cognitive subprocesses and schizophrenia: a reaction-time decomposition. Acta Psychiatr Scand Suppl 2001; 104:18–27Crossref, Google Scholar
9. Badcock JC, Michie PT, Johnson L, Combrinck J: Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med 2002; 32:287–297Crossref, Medline, Google Scholar
10. Barch DM, Csernansky JG, Conturo T, Snyder AZ: Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? J Abnorm Psychol 2002; 111:478–494Crossref, Medline, Google Scholar



