Platelet Serotonin Reuptake Inhibition and Response to SSRIs in Depressed Adolescents
Abstract
OBJECTIVE: The authors examined platelet serotonin reuptake inhibition and response to selective serotonin reuptake inhibitor (SSRI) treatment in depressed adolescents. METHOD: Twenty-three depressed adolescents participating in pharmacokinetic studies of SSRIs had platelet serotonin reuptake measured before and after 14–28 days of treatment. The Clinical Global Impression (CGI) improvement rating was determined on the basis of all clinical information and was performed blind to the platelet data. RESULTS: Improvement in depressive symptoms as rated with the CGI improvement subscale was significantly associated with the percentage change in platelet serotonin reuptake inhibition from pre- to posttreatment. Improvement in depression was also associated with absolute decrease in platelet serotonin reuptake when adjusted for the magnitude of baseline reuptake. CONCLUSIONS: Platelet serotonin reuptake inhibition may be an appropriate surrogate biological marker for the pharmacodynamic activity of SSRIs in depressed adolescents.
Selective serotonin reuptake inhibitors (SSRIs) have been shown to be efficacious in children and adolescents for the treatment of depression (1–3). The presumed mechanism of action for the therapeutic effect of SSRIs is blockade of the serotonin reuptake transporter on presynaptic neurons, with the subsequent increase in transmission by serotonergic neurons. Human platelets have a serotonin reuptake transporter that is identical to the one in the brain (4), thus the platelet transporter may be used as a surrogate marker for the effect of SSRIs on serotonergic neurons.
Previous positron emission tomography data have indicated that adults taking 20 mg of paroxetine or 20 mg of citalopram had a 77% mean occupancy of the serotonin transporter in the brain (5). It has also been observed that at the typical minimum therapeutic doses of SSRIs in adults (e.g., 20 mg of fluoxetine or paroxetine, 50 mg of sertraline, 40 mg of citalopram), the mean serotonin reuptake inhibition in patient platelets was 60%–80% (6). However, the extent of platelet serotonin reuptake inhibition has not been quantified during SSRI treatment in adolescents and has not been directly related to clinical response in this population or in adults.
This study determined the change in platelet serotonin reuptake in 23 adolescents with depressive symptoms who participated in a 14–28-day pharmacokinetic study of citalopram (20 mg/day), paroxetine (20 mg/day), or sertraline (50 mg/day). The relation of serotonin reuptake inhibition to clinical response was also examined.
Method
Subjects were participants in pediatric SSRI pharmacokinetic studies that were approved by the University of Pittsburgh Institutional Review Board (7). They were referred to the study by their attending child psychiatrist for initiation of SSRI treatment. The subjects’ parents or guardians as well as those subjects aged 14 years or older provided written informed consent prior to initiation of any study procedures. Verbal assent was obtained from children 13 years of age or younger. Subjects had a pretreatment interview that included the depression section of the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS), present episode version (8). Subjects also completed the Beck Depression Inventory (9) or Children’s Depression Inventory (10) and had a clinical interview with a board-certified child psychiatrist (D.A.A.) to confirm the appropriateness of SSRI treatment. Subjects were included in this analysis if they met DSM-IV criteria for a current depressive diagnosis. Of the 23 subjects who were depressed at intake, 13 had major depressive disorder, two had dysthymic disorder, and eight had depressive disorder not otherwise specified. There were 10 male and 13 female subjects, with ages ranging from 13.1 to 17.7 years (mean=15.1, SD=1.2). Seventeen subjects were white, five were African American, and one was Asian American. All subjects were physically healthy and had normal physical examination results at study entry.
On the morning before starting the first dose of the SSRI, subjects had blood drawn for analysis of pretreatment platelet serotonin reuptake inhibition. They then took a daily morning dose of citalopram, 20 mg (N=13); sertraline, 50 mg (N=8); or paroxetine, 20 mg (N=2). After 14–28 days (mean=16.2) of treatment, subjects returned in the morning to have blood drawn before taking their morning dose to measure posttreatment platelet serotonin reuptake. Repeat assessments with the K-SADS depression interview and either the Beck Depression Inventory or Children’s Depression Inventory were performed at that time. Subjects returned to their referring psychiatrist for follow-up treatment. Subjects did not take other psychotropic medications for at least 2 weeks before and during the study. All had negative urine drug screen results before entering the protocol.
Clinical response of depressive symptoms during the 14–28-day interval between platelet reuptake measurements was rated retrospectively by the lead author (D.A.A.) using the Clinical Global Impression (CGI) improvement subscale. The ratings were based on the K-SADS depression interviews, Beck Depression Inventory/Children’s Depression Inventory self-reports, and clinical progress notes. The CGI was performed blind to the results of the platelet data.
The method of Tuomisto and colleagues (11) was used as the procedure for the platelet serotonin reuptake assay but was modified by reducing the blood volume collected to 20 ml. Platelet concentration was determined by a Coulter count, and samples were diluted to a platelet concentration of 200,000×103/μl. Serotonin uptake was measured at nine standard concentrations of [3H]serotonin within 2.5 hours of obtaining the sample from the subject. The interday coefficient of variation of the assay ranged from 9.1% to 14.6%. For the calculation of maximum velocity (Vmax) of serotonin uptake into the platelet, the raw data (from scintillation counter measurements at each of the nine concentrations) was converted into a V versus serotonin plot that was fit to the Michaelis-Menten equation by nonlinear regression using Enzfitter version 2.0.8 (Biosoft, Ferguson, Mo.). Vmax was measured in picomoles of serotonin/107 platelets/5 minutes. Platelet serotonin reuptake inhibition was determined by the change in Vmax from pre- to posttreatment and was expressed as the percentage change from the baseline Vmax for the primary analysis. SPSS version 11.5 was used for the nonparametric correlation analysis and for an ordinal regression analysis using a logit link function (SPSS, Inc., Chicago).
Results
Two subjects were rated as being very much improved (CGI rating of 1), six were much improved (CGI rating of 2), 10 were minimally improved (CGI rating of 3), and five were rated as not improved (CGI rating of 4); therefore, 35% (N=8) of the 23 subjects were unequivocal responders. The mean platelet reuptake inhibition was 52% (SD=27%, range=0%–87%). There were no associations between demographic variables and either clinical response or platelet serotonin reuptake inhibition. There was a significant correlation between CGI improvement ratings and platelet reuptake inhibition, with greater clinical response associated with higher inhibition (Figure 1). Ordinal regression analysis determined that improvement on the CGI improvement subscale was significantly associated with absolute change in Vmax when baseline Vmax was controlled (beta estimate=0.31; Wald statistic=6.2, df=1, p=0.01).
Conclusions
To our knowledge, this is the first study in adolescents that quantifies the pharmacodynamic effect of SSRIs on a biological marker and shows a relation of the marker to clinical response. This is a preliminary study and should be viewed with the following limitations in mind. The study sample was small and was heterogeneous in the intensity of depressive symptoms at intake. Final clinical response was determined retrospectively, although it was based on data that was obtained prospectively and conducted blind to the results of the platelet assays. The duration of SSRI treatment prior to measuring response was relatively short, so some subjects may not have had time to exhibit a complete response to the medication. Consequently, a substantial proportion of subjects were rated as having minimal improvement, and platelet results were widely distributed in this group. However, the effect size of the difference in platelet serotonin reuptake inhibition between subjects who showed unequivocal clinical improvement (CGI rating of 1 or 2; mean inhibition=66% [SD=17]) and those who had absolutely no response (CGI rating of 4; mean inhibition=33% [SD=33]) was large (d=1.4). Despite the aforementioned limitations, the results demonstrate that platelet serotonin reuptake inhibition may be an appropriate surrogate biological marker of the pharmacodynamic activity of SSRIs.
The development of appropriate surrogate biomarkers of treatment response to SSRIs in youth can have potential use clinically as well as have implications for whether this class of medication has therapeutic effects in this patient population. SSRI blood levels have not been shown to correlate with clinical response in numerous studies of depression in adults (12). As it can take several weeks at the proper dose of an SSRI to show improvement in depressive symptoms, a biological surrogate marker of response that can be measured early in treatment has the potential to substantially reduce titration time to the proper dose.
The efficacy of SSRIs for depression in the pediatric population has recently been called into question. A recently issued FDA advisory stated that despite submissions of randomized controlled trials of sertraline, paroxetine, citalopram, venlafaxine (a serotonin-norepinephrine reuptake inhibitor), and fluoxetine for pediatric depression, only fluoxetine had sufficient evidence to establish effectiveness for the treatment of pediatric depression (13). Therefore, data that clinical improvement of depressive symptoms in adolescents is correlated with changes in serotonin reuptake add indirect evidence that SSRIs may indeed be helpful for this patient population.
Presented in part at the 49th annual meeting of the American Academy of Child and Adolescent Psychiatry, San Francisco, Oct. 22–27, 2002. Received July 8, 2003; revision received April 21, 2004; accepted May 26, 2004. From the Department of Psychiatry, University of Pittsburgh Medical Center-Western Psychiatric Institute and Clinic. Address correspondence and reprint requests to Dr. Axelson, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213. Supported in part by NIMH grants MH-01878, MH-70008, and MH-55123.
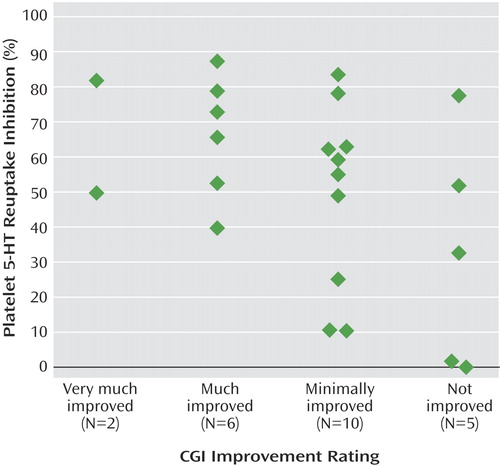
Figure 1. Platelet Serotonin Reuptake Inhibition in 23 Depressed Adolescents Treated With SSRIs, by Posttreatment CGI Improvement Ratinga
aSignificant correlation between platelet serotonin reuptake inhibition and CGI improvement rating (rs=–0.44, p<0.04).
1. Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 1997; 54:1031–1037Crossref, Medline, Google Scholar
2. Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown EB, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 2002; 41:1205–1215Crossref, Medline, Google Scholar
3. Keller MB, Ryan ND, Strober M, Klein RG, Kutcher SP, Birmaher B, Hagino OR, Koplewicz H, Carlson GA, Clarke GN, Emslie GJ, Feinberg D, Geller B, Kusumakar V, Papatheodorou G, Sack WH, Sweeney M, Wagner KD, Weller EB, Winters NC, Oakes R, McCafferty JP: Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2001; 40:762–772Crossref, Medline, Google Scholar
4. Lesch KP, Wolozin BL, Murphy DL, Reiderer P: Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem 1993; 60:2319–2322Crossref, Medline, Google Scholar
5. Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S: Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [11C]DASB PET imaging study. Am J Psychiatry 2001; 158:1843–1849Link, Google Scholar
6. Preskorn SH: Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet 1997; 32(suppl 1):1–21Google Scholar
7. Axelson DA, Perel JM, Birmaher B, Rudolph GR, Nuss S, Bridge J, Brent DA: Sertraline pharmacokinetics and dynamics in adolescents. J Am Acad Child Adolesc Psychiatry 2002; 41:1037–1044Crossref, Medline, Google Scholar
8. Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrazi MA, Davies M: The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Arch Gen Psychiatry 1985; 42:696–702Crossref, Medline, Google Scholar
9. Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
10. Kovacs M: Children’s Depression Inventory: Manual. Tonawanda, NY, Multi-Health Systems, 1992Google Scholar
11. Tuomisto J, Tukianen E, Ahlfors UG: Decreased uptake of 5-hydroxytryptamine in blood platelets from patients with endogenous depression. Psychopharmacology (Berl) 1979; 65:141–147Crossref, Medline, Google Scholar
12. Rasmussen BB, Brosen K: Is therapeutic drug monitoring a case for optimizing clinical outcome and avoiding interaction of the selective serotonin reuptake inhibitors? Ther Drug Monit 2000; 22:143–154Crossref, Medline, Google Scholar
13. US Food and Drug Administration: FDA Issues Public Health Advisory: Reports of Suicidality in Pediatric Patients Being Treated With Antidepressant Medications for Major Depressive Disorder (MDD). FDA Talk Paper TO3–70. Washington, DC, FDA, Oct 27, 2003Google Scholar



