Evidence for Sensory Prediction Deficits in Schizophrenia
Abstract
OBJECTIVE: Patients with schizophrenia experiencing delusions and hallucinations can misattribute their own actions to an external source. The authors test the hypothesis that patients with schizophrenia have defects in their ability to predict the sensory consequences of their actions. METHOD: The authors measured sensory attenuation of self-produced stimuli by patients with schizophrenia and by healthy subjects. RESULTS: Patients with schizophrenia demonstrated significantly less sensory attenuation than healthy subjects. CONCLUSIONS: Patients with a diagnosis of schizophrenia have a dysfunction in their predictive mechanisms.
Self-monitoring is fundamental to normal cognitive functions in planning, controlling, and anticipating the consequences of motor acts (1). Prediction plays a key role in such monitoring, allowing a comparison between expected and actual outcomes of an action to be computed (2, 3). An efference copy of the outgoing motor command in conjunction with a predictive model can be used to generate such a prediction (4). One role of this predictive process is to permit the sensory consequences of a movement to be anticipated and used to attenuate the perceptions related to these sensations, thereby enhancing the salience of sensations that have an external cause (5–7). An additional role may be to label movements as generated by oneself or by an external source. If predicted sensory consequences match the actual sensory consequences, the movement is labeled as one’s own. However, if the predicted and actual sensory consequences are discordant, as when one’s arm is passively moved by someone else, the movement is labeled as externally generated.
A dysfunctional predictive mechanism would lead to incorrect predictions, causing the misattribution of self-generated actions as externally generated. Patients with schizophrenia can demonstrate just such difficulties, when self-generated actions are experienced as being of alien origin—delusions of control or the misperception of self-generated speech as an auditory hallucination (8). Both psychophysical and neuroimaging studies have suggested that self-monitoring may be dysfunctional in patients with schizophrenia (9, 10). Here we directly test the hypothesis that patients with schizophrenia have a defect in their ability to predict the sensory consequences of their actions.
We have previously shown (11) that although healthy subjects are accurate at reproducing a target force when using a joystick, they significantly overestimate the force required when it is directly self-generated. In other words, they exert a greater force than the target when they are using their index finger to make the match. This occurs because self-generated forces are perceived as weaker than externally generated forces of the same magnitude, which arises from a predictive process in which the sensory consequences of the movement are anticipated and partially removed from the perception. Our hypothesis in the current study was that patients with a diagnosis of schizophrenia would be more accurate than healthy subjects in reproducing the external force. This increased accuracy would reflect the failure of the normal sensory attenuation mechanism due to a dysfunctional sensory predictive process.
During the joystick condition, subjects reproduce the external force by using their right index finger to move a joystick that controls the force output of a torque motor. In this situation the active hand is not generating the force directly, but the movement of the hand is translated into a force through the torque motor. It has been shown that in this unusual situation, predictive mechanisms are not employed (12); we anticipated that both patients and healthy subjects should be accurate at this task.
Method
We used a recently developed force-matching task that allows us to quantify the sensory attenuation of self-produced stimuli (11) (Figure 1). A target force is applied to the subject’s left index finger by a torque motor. Subjects are then required to reproduce the force they just experienced, either directly by pressing with the index finger of their right hand or indirectly by using a joystick controlling the torque motor. Forty right-handed subjects—20 with a DSM-IV diagnosis of schizophrenia and 20 healthy volunteers—participated in the study after providing informed consent and after all experimental protocols were approved by a local ethics board.
Results
The 20 patients and 20 healthy volunteer subjects were well matched for age (patients’ mean age=36.4 years, SD=13.4; volunteers’ mean age=35.9, SD=14), gender (12 in each group were men), handedness, and premorbid IQ (13) (patients’ mean=110, SD=8; volunteers’ mean=114, SD=6). All patients except one were treated with antipsychotic medication, the majority with atypical antipsychotic medication, but all were still moderately symptomatic; their mean score on the Brief Psychiatric Rating Scale (14) was 36. Most patients had prominent positive symptoms: 16 subjects had a score of 4 or more on items related to suspiciousness or hallucinatory behavior. Two participants (one patient and one healthy volunteer) were found to have produced matching forces that did not significantly correlate with the corresponding target forces in either task (r2<0.04, p>0.10). We concluded that they had not properly understood the instructions and removed their data from further analysis.
All participants consistently applied a greater force when using their right index finger to directly match the externally applied target force; they consistently underestimated the force they were applying because their perception of the force was likely to be attenuated. To quantify attenuation in the patients and healthy subjects, we calculated the percentage of the matched force by which the matched force exceeded the target force. The patients were more accurate at the task, showing 27.5% attenuation (Figure 1, red circles), compared with 43.5% in the comparison group (Figure 1, blue circles). When subjects matched the target force using the joystick, both patients and healthy volunteers reproduced the original force much more accurately (Figure 1, diamonds). A repeated-measures two-way analysis of variance (patients versus healthy subjects and direct versus joystick) of the mean matching force normalized by the mean target force showed a significant interaction (F=4.88, df=1, 36, p<0.04). This interaction arose from the patients having significantly less attenuation in the direct force-generation task (simple main effect F=4.71, df=1, 36, p<0.04) but no significant difference from the healthy subjects in the joystick task (simple main effect F=0.16, df=1, 36, p=0.69).
Discussion
The results show that self-generated forces were attenuated less in the patient group, suggesting a dysfunction in their ability to predict the sensory consequences of their actions. This would be in accord with previous imaging data in the verbal domain (10). Although most of the patients were treated with antipsychotic medication, the absence of any difference between patients and healthy volunteers in the joystick matching task suggests that there was no systematic effect of medication on motor performance; indeed, there was no significant correlation between the chlorpromazine equivalents of medication and the degree of attenuation demonstrated by patients. The present study provides support for the presence of a dysfunctional sensory predictive mechanism in schizophrenia. Future work planned to follow up this interesting finding will concentrate on clarifying the state versus trait nature of this deficit by examining patients longitudinally over time, with changes in symptom profile, and the specificity of this deficit for schizophrenia by examining patients with a diagnosis of bipolar disorder also treated with antipsychotic medication.
Received Sept. 22, 2004; revision received Dec. 17, 2004; accepted Jan. 10, 2005. From the Institute of Neurology, University College London; and the Institute of Psychiatry Kings College London. Address correspondence and reprint requests to Dr. Shergill, Institute of Psychiatry Kings College London, De Crespigny Park, London, SE5 8AF; [email protected] (e-mail). Supported in part by the Wellcome Trust, the McDonnell Foundation, and the Human Frontiers Science Programme.
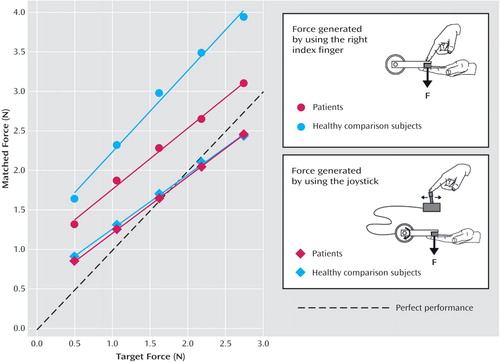
Figure 1. Matching Force Generated by 19 Patients With Schizophrenia and 19 Healthy Volunteers Using the Right Index Finger or Joystick as a Function of the Externally Generated Target Forcea
aDotted line represents perfect performance. On each trial the torque motor generated a force between 0.5 and 2.75 Newtons on the left index finger for 3 seconds (80 trials in a pseudo-randomized order). Subjects were then required to reproduce the force either by pushing with their right index finger or by using a joystick that controlled the torque motor. Each subject participated in both conditions in a counterbalanced order. The applied forces were measured by using a force transducer mounted in the lever of the torque motor.
1. Jeannerod M: The Cognitive Neuroscience of Action. Oxford, UK, Blackwell, 1997Google Scholar
2. Sperry RW: Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 1950; 43:482–489Crossref, Medline, Google Scholar
3. von Holst E: Relations between the central nervous system and the peripheral organs. Br J Animal Behaviour 1954; 2:89–94Crossref, Google Scholar
4. Wolpert DM, Ghahramani Z, Jordan MI: An internal model for sensorimotor integration. Science 1995; 269:1880–1882Crossref, Medline, Google Scholar
5. Claxton G: Why can’t we tickle ourselves? Percept Mot Skills 1975; 41:335–338Crossref, Medline, Google Scholar
6. Blakemore SJ, Wolpert DM, Frith CD: Central cancellation of self-produced tickle sensation. Nat Neurosci 1998; 1:635–640Crossref, Medline, Google Scholar
7. Curio G, Neuloh G, Numminen J, Jousmaki V, Hari R: Speaking modifies voice-evoked activity in the human auditory cortex. Hum Brain Mapp 2000; 9:183–191Crossref, Medline, Google Scholar
8. Shergill SS, Brammer MJ, Williams S, Murray RM, McGuire PK: Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000; 57:1033–1038Crossref, Medline, Google Scholar
9. Frith CD, Blakemore S, Wolpert DM: Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Rev 2000; 31:357–363Crossref, Medline, Google Scholar
10. Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT: Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry 2001; 158:2069–2071Link, Google Scholar
11. Shergill SS, Bays PM, Frith CD, Wolpert DM: Two eyes for an eye: the neuroscience of force escalation. Science 2003; 301:187Crossref, Medline, Google Scholar
12. Blakemore SJ, Goodbody SJ, Wolpert DM: Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci 1998; 18:7511–7518Crossref, Medline, Google Scholar
13. Nelson HE, Willison J: National Adult Reading Test (NART). Windsor, UK, National Foundation for Educational Research-Nelson, 1991Google Scholar
14. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar



