Sleep-Related Functioning in Euthymic Patients With Bipolar Disorder, Patients With Insomnia, and Subjects Without Sleep Problems
Abstract
OBJECTIVE: The authors investigated sleep-related functioning in euthymic patients with bipolar disorder. METHOD: Euthymic patients with bipolar disorder (N=20), patients with insomnia (N=20), and subjects with good sleep (N=20) were compared on data from interviews and questionnaires and on findings from eight consecutive days and nights of sleep diary keeping (subjective sleep estimate) and actigraphy (objective sleep estimate). RESULTS: Seventy percent of the euthymic patients with bipolar disorder exhibited a clinically significant sleep disturbance. Compared with the other groups, the bipolar disorder group exhibited impaired sleep efficiency, higher levels of anxiety and fear about poor sleep, lower daytime activity levels, and a tendency to misperceive sleep. The bipolar disorder group held a level of dysfunctional beliefs about sleep that was comparable to that in the insomnia group and significantly higher than that in the good sleeper group. CONCLUSIONS: Insomnia is a significant problem among euthymic patients with bipolar disorder. Components of cognitive behavior therapy for insomnia, especially stimulus control and cognitive therapy, may be a helpful adjunct to treatment for patients with bipolar disorder.
Bipolar disorder is a common, severe, chronic, and often life-threatening condition. The lifetime prevalences of bipolar I disorder and bipolar II disorder are 0.8% and 0.5%, respectively (DSM-IV), although more liberal definitions of hypomania identify many more patients with bipolar spectrum disorders (1). Treatment of bipolar I disorder typically involves pharmacotherapy. However, even in patients with continued adherence to medication regimens, the risk of relapse over a 5-year period has been estimated to be as high as 73% (2). The risk of relapse is similar in both bipolar I disorder and bipolar II disorder, is higher in bipolar disorder than in unipolar depression, and persists across the lifespan in patients with bipolar disorder (1).
Three lines of evidence point to the importance of sleep in bipolar disorder. First, experimentally induced sleep deprivation is associated with the onset of hypomania or mania in a considerable proportion of patients (e.g., reference 3). Second, in a systematic review of 11 studies involving 631 patients with bipolar disorder, sleep disturbance was the most common prodrome of mania (reported by 77% of patients) and the sixth most common prodrome of bipolar depression (reported by 24% of patients) (4). Third, the sleep/wake cycle has been a core component of theoretical conceptualizations of bipolar disorder. It has been hypothesized that bipolar disorder patients have a genetic diathesis that may take the form of circadian rhythm instability. Psychosocial stresses are proposed to cause disrupted routine and sleep, which, in turn, disrupts the vulnerable circadian rhythm and triggers an episode (5, 6).
As a result of these findings, psychosocial treatments for bipolar disorder, such as cognitive behavior therapy and interpersonal and social rhythm therapy, include education and monitoring of the sleep/wake cycle and include the aim of promoting regularity in daily activities. Although these interventions have been shown to be efficacious adjuncts to pharmacotherapy (7, 8), they focus on a wide variety of behaviors, making them potentially time-consuming, and have not yet drawn on the large and impressive literature documenting the effectiveness of cognitive behavior therapy in the treatment of insomnia (9).
In the present study, we sought to establish whether core components of cognitive behavior therapy for insomnia have the potential to enhance interventions for bipolar disorder patients by providing a specific emphasis on sleep. Three components were investigated: 1) stimulus control to reverse maladaptive conditioning between the bed/bedroom and not sleeping by limiting sleep-incompatible behaviors within the bedroom environment, 2) sleep hygiene to provide education about behaviors that interfere with sleep, and 3) cognitive therapy to alter unhelpful beliefs about sleep. An additional aim of this study was to observe the sleep-related functioning of bipolar disorder patients in the nonacute, so-called euthymic or interepisode phase of the disorder. Although it is recognized that patients with bipolar disorder often continue to be symptomatic between episodes (10), to our knowledge the research to date on sleep disturbance has often focused exclusively on sleep just prior to and during episodes of mania or depression.
Method
Participants
Twenty individuals who met the DSM-IV criteria for bipolar I disorder, as assessed by the Structured Clinical Interview for DSM-IV (SCID) (11), and 20 individuals who met the DSM-IV criteria for primary insomnia, as assessed by the Insomnia Diagnostic Interview (unpublished 2004 manuscript of A.G. Harvey et al.), were recruited for this study, along with 20 volunteers without sleep problems. The insomnia and good sleeper groups provided a baseline index of poor sleep and good sleep, respectively. The bipolar disorder patients were recruited from a group of outpatients who met the DSM-IV diagnostic criteria for bipolar I disorder but were currently euthymic. The 20 patients with a diagnosis of primary insomnia were recruited from a group of outpatients seeking treatment for insomnia. The 20 volunteers in the good sleeper group were recruited from the subject pool in the Department of Experimental Psychology, University of Oxford. Inclusion criteria for the good sleeper group were an indication of sleeping “very well” on the Insomnia Diagnostic Interview, no difficulties with sleep in the past month, and no current use of medication for sleep.
For the bipolar disorder group, the average age at onset of the disorder was 22.3 years (SD=10.1), the average number of manic episodes was 4.5 (SD=2.2), and the average number of depressive episodes was 4.7 (SD=1.6). At the time of the study, all participants were being treated with two to three pharmacological agents, including lithium (700–1500 mg/day), carbamazepine (400–800 mg/day), sodium valproate (500–800 mg/day), venlafaxine (225–400 mg/day), fluoxetine (20–70 mg/day), and lamotrigine (50–100 mg/day). In addition, ten patients were taking medication to help them sleep (eight were taking benzodiazepine receptor agonists, and two were taking over-the-counter medications). It is noteworthy that a drug-free group of bipolar disorder patients would likely be unfeasible or unrepresentative. The bipolar disorder patients were confirmed to be euthymic according to their average scores on the Hamilton Depression Rating Scale (mean=1.4, SD=1.9) (12) and the Young Mania Rating Scale (mean=2.3, SD=2.8) (13). For the insomnia group, the average length of sleep disturbance was 10.55 years (SD=7.13). Six patients with insomnia were taking medication to help them sleep (two were taking sedative antidepressants, and four were taking over-the-counter medications).
As for most psychiatric disorders (14), both bipolar disorder and insomnia are known to commonly co-occur with one or more other disorders (15, 16). As such, we did not select “pure” cases, because this selection criterion would reduce the representativeness of the study groups (17, 18). However, we ensured that bipolar disorder was the primary diagnosis of the patients in the bipolar disorder group and that insomnia was the primary diagnosis for the insomnia group. The primary diagnosis was defined as the disorder that was reported to be currently most distressing and disabling (19). Current comorbid axis I disorders, assessed with the SCID, included problem alcohol/drug/medication dependence (N=4), specific phobia (N=1), generalized anxiety disorder (N=2), and posttraumatic stress disorder (N=2) for the bipolar disorder group; social phobia (N=1), specific phobia (N=3), and generalized anxiety disorder (N=1) for the insomnia group; and specific phobia (N=3) for the good sleeper group.
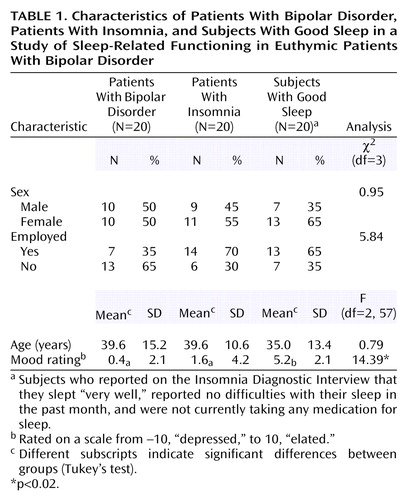
The three groups did not differ in sex, age, or employment status, although the difference between groups for the latter variable approached significance (p=0.06). It is unsurprising that bipolar disorder subjects may be less likely to be in employment appropriate to their education. At the time of the interview, the good sleeper group rated their mood as more positive, relative to the bipolar disorder and insomnia groups, who did not differ from each other in their mood ratings (Table 1).
Procedure
After written informed consent was obtained from the participants, as required by the ethics committee that approved this study, the SCID and Insomnia Diagnostic Interview were administered, demographic data were recorded, and a current mood rating was made (on a scale from –10, “depressed,” to 10, “elated”).
Measures of the sleep/wake cycle
In view of the complex issues involved in accurately assessing sleep (20), the sleep/wake cycle was indexed by using three different methods.
Retrospective self-report
Three measures were taken. First, the Insomnia Diagnostic Interview was administered, as described earlier. Second, the Pittsburgh Sleep Quality Index (21) was administered. This 19-item questionnaire comprises seven component scores, including 1) subjective sleep quality, 2) sleep onset latency, 3) sleep duration, 4) habitual sleep efficiency, 5) sleep disturbance, 6) use of sleep medications, and 7) daytime functioning. The Pittsburgh Sleep Quality Index refers to the majority of days and nights over the previous month. The seven component scores are summed to provide a global score that ranges from 0 to 21, with higher scores indicating worse sleep. The Pittsburgh Sleep Quality Index has good internal consistency (α=0.83) and test-retest reliability (r=0.85) (22). A total score >5 identifies a clinically significant sleep disturbance with 89.6% sensitivity and 86.5% specificity (21). Third, the participants were asked to report the number of nights in the past week that they had trouble falling asleep and staying asleep and were asked to provide a rating of their sleep quality on a scale from 1, “very restless,” to 10, “very sound.”
Retrospective self-report measures have the advantages of being quick and easy to complete and of capturing the individual’s subjective global impression of his/her sleep. The disadvantages of these measures include 1) that subjects must understand the concept of an average, as they are asked to report their “typical sleep;” 2) that their answers rely on long-term memory, which can be subject to inaccuracy (23, 24); and 3) that the answers are open to reasoning biases, such as answering on the basis of saliency (the worst night) or recency (the last night) (20). To compensate for the disadvantages of the self-report measures, we also included two prospective measures.
Prospective self-report
The participants were asked to keep a sleep diary (subjective estimate of the sleep/wake cycle) over 8 consecutive days. They were asked to record, in the morning immediately on waking, how long it took them to get to sleep (sleep onset latency), the number of times they woke during the night and the duration of these awakenings (wake after sleep onset), and the length of time they slept in total (total sleep time).
One advantage of this measure is that it constitutes an index of the considerable night-to-night variability in sleep (25). In addition, it retains the advantages associated with retrospective self-report measures while overcoming several of the disadvantages. However, a disadvantage of prospective self-reports of sleep is that sleep is very difficult to estimate accurately; it is defined by the absence of memories (26, 27). Accordingly, an objective estimate of sleep that did not require memory, perception, or judgment was also included.
Prospective actigraphy estimate
While keeping the sleep diary, the participants were asked to wear an Actigraph for 8 consecutive days and nights. The Actigraph (Ambulatory Monitoring Inc., Ardsley, N.Y.) is a small wristwatch-like device worn continuously on the nondominant wrist. Embedded within the device is a miniaturized piezoelectric acceleration sensor that detects and stores information about physical motion. This information is downloaded into compatible software to generate an estimation of the sleep/wake cycle. For adults without sleep complaints, there is strong agreement between sleep estimates generated by actigraphy and those generated by polysomnography, which is the “gold standard” sleep assessment technique. The epoch-by-epoch agreement rate for sleep and wakefulness detection ranges from 74% to 98%) (28–30). However, the rate of agreement is lower in individuals who lie immobile for long periods (e.g., clinically depressed patients and insomnia patients), as actigraphy is less accurate in differentiating quiet presleep wakefulness from sleep. Data were collected in the Actigraph’s Zero-Crossing Mode, which allowed for the recording of a daytime activity count for each 60-second epoch. This count reflects the number of times movement was recorded above the preset threshold within each epoch and has previously been found to provide an effective measure of daytime activity level (31). It is noteworthy that six individuals in the bipolar disorder group did not complete the 8-day prospective monitoring task. Thus, the analysis was based on data for 14 patients with bipolar disorder. There were no significant differences in the Pittsburgh Sleep Quality Index global score between the 14 completers and the six noncompleters (t=–1.42, df=18, n.s.).
Sleep-related cognitive and behavioral processes
A semistructured interview (32) was administered to assess the constructs that underpin the stimulus control and sleep hygiene intervention. The topics covered were those listed in previous research as indicators of poor sleep hygiene and poor stimulus control (e.g., references 33–36). As an index of the need for a cognitive intervention, the Sleep Disturbance Questionnaire (37, 38) and the Dysfunctional Attitudes and Beliefs About Sleep Questionnaire (36) were administered. The Sleep Disturbance Questionnaire requires participants to rate the extent to which 12 attributions for inability to sleep are true for them on a scale from “never true” to “very often true.” The items assess several potential causes of sleep disturbance, including physical tension (average of three items), sleep pattern problem (average of three items), cognitive arousal (average of three items), and sleep effort (average of three items) (37). Because the Sleep Disturbance Questionnaire is used to inquire about sleep disturbance, it was not considered meaningful to administer it to the good sleeper group. The Dysfunctional Attitudes and Beliefs About Sleep Questionnaire is a 30-item questionnaire designed to assess beliefs and attitudes relating to sleep. For each item the participant is asked to mark his/her extent of agreement on a Likert-type scale from 0, “strongly disagree,” to 10, “strongly agree.” The Dysfunctional Attitudes and Beliefs About Sleep Questionnaire generates a total score and scores on five subscales (39): 1) unhelpful beliefs about the consequences of insomnia (average of six items), 2) unhelpful beliefs about the control and predictability of sleep (average of nine items), 3) unhelpful beliefs about sleep requirements (average of three items), 4) unhelpful causal attributions for insomnia (average of two items), and 5) unhelpful sleep promoting practices (average of eight items).
Immediately after this session, the Hamilton Rating Scale for Depression and the Young Mania Rating Scale were completed for the participants in the bipolar disorder group. The participants returned for a second session to hand in the sleep diary and Actigraph and to receive a debriefing about the aims of the study.
Data Analytic Plan
For continuous variables, data analysis comparing all three groups was based on one-way analysis of variance with post hoc Tukey’s tests to explore significant effects. Data analysis comparing two groups was based on t tests for independent samples. For categorical variables, chi-square analyses were conducted. For analyses involving multiple comparisons, a Bonferroni adjustment was conducted to control for the possibility of inflated error (0.05/number of comparisons).
Both the bipolar disorder group and the insomnia group reported experiencing more nights of difficulty falling asleep and staying asleep over the past week, relative to the good sleepers, but the two patient groups did not differ on these variables. The insomnia patients rated their sleep quality as poorer, compared to the bipolar disorder patients, whose ratings of sleep quality were poorer than those of the good sleeper subjects (Table 2).
Results
Measures of Sleep/Wake Cycle
Retrospective self-report
Based on the Insomnia Diagnostic Interview, the percentage of participants who met the full diagnostic criteria for primary insomnia was 55% in the bipolar disorder group, 100% in the insomnia group, and 0% in the good sleeper group. Criterion D for primary insomnia, which specifies that insomnia must not occur exclusively because of another psychiatric disorder, was not used in making the diagnosis of primary insomnia in the bipolar disorder group.
The percentage of participants scoring >5 on the Pittsburgh Sleep Quality Index, a level indicative of a clinically significant sleep disturbance (21), was 70% for the bipolar disorder group, 100% for the insomnia group, and 0% for the good sleeper group. The mean values for the Pittsburgh Sleep Quality Index variables are summarized in Table 2. The mean global score on the Pittsburgh Sleep Quality Index was higher for the insomnia group than for the bipolar disorder group, which scored higher than the good sleeper group. On the sleep onset latency and daytime dysfunction subscales, the insomnia group and the bipolar disorder group did not differ from each other, but both scored higher than the good sleeper group. For the subjective sleep quality, sleep duration, and habitual sleep efficiency subscales, the insomnia group scored higher than the bipolar disorder group, which scored higher than the good sleeper group.
Prospective self-report
Over 8 consecutive days and nights, the bipolar disorder group reported longer sleep onset latency, relative to the good sleeper group. The insomnia group’s subjective rating of time awake after sleep onset was longer and of total sleep time was shorter, relative to the good sleeper group.
Prospective actigraphy estimate
For the bipolar disorder group, the objective estimate of total sleep time was longer and the daytime activity mean lower, relative to both the insomnia and good sleeper groups (Table 2). Sleep onset latency and wake after sleep onset did not differ significantly across the groups.
Sleep-Related Cognitive and Behavioral Processes
The bipolar disorder and insomnia groups had lower sleep efficiency scores, relative to the good sleeper group; otherwise, the three groups scored similarly on the sleep hygiene and stimulus control variables (Table 3).
As for the Sleep Disturbance Questionnaire subscales (Table 3), the highest scores for both patient groups were obtained on the cognitive arousal subscale. Comparisons of the bipolar disorder group and the insomnia group on each of the subscales indicated that the insomnia group scored higher on both the physical tension and cognitive arousal subscales, relative to the bipolar disorder group. The three Sleep Disturbance Questionnaire items endorsed most frequently by the bipolar disorder group were “I can’t get into a proper routine” (40% endorsed either “often true” or “always true”), “My mind keeps turning things over” (45% endorsed either “often true” or “always true”), and “I am unable to empty my mind” (30% endorsed either “often true” or “always true”). The three Sleep Disturbance Questionnaire items endorsed most frequently by the insomnia group were “My mind keeps turning things over” (75% endorsed either “often true” or “always true”), “My thinking takes a long time to unwind” (75% endorsed either “often true” or “always true”), and “I am unable to empty my mind” (75% endorsed either “often true” or “always true”).
For the total score on the Dysfunctional Attitudes and Beliefs About Sleep Questionnaire, the insomnia and bipolar disorder groups did not differ from each other but both scored higher than the good sleeper group. The differences for only one of the five subscales of the Dysfunctional Attitudes and Beliefs About Sleep Questionnaire reached significance (Table 3). For the subscale measuring control and predictability of sleep, the insomnia and bipolar disorder groups did not differ from each other, but both scored significantly higher than the good sleeper group. The correlation between the total score on the Dysfunctional Attitudes and Beliefs About Sleep Questionnaire and the Pittsburgh Sleep Quality Index global score was significant for the bipolar disorder group (r=0.76, N=20, p<0.001) but not for the insomnia group (r=0.35, N=20, n.s.) or the good sleeper group (r=0.004, N=20, n.s.).
Discussion
One aim of the present study was to investigate the sleep-related functioning of euthymic bipolar disorder patients. The rationale was that while patients with bipolar disorder are known to be symptomatic even when euthymic (10), to our knowledge there are no published data documenting their sleep/wake cycle during nonacute periods, despite the importance of sleep in theories about bipolar disorder (e.g., references 5, 6). Taken together, the sleep estimates from the retrospective, prospective, subjective, and objective sources in the current study indicate that sleep disturbance was, indeed, a significant problem in the bipolar disorder group, even when the patients were euthymic. Seventy percent of the bipolar disorder group had a clinically significant sleep problem, and 55% met the strict diagnostic criteria for insomnia (excluding criterion D). These findings add to previous evidence indicating that bipolar disorder patients may experience considerable symptoms during periods when their illness is nonacute (10). Furthermore, the sleep of the bipolar disorder patients, as a group, was more similar to that of the insomnia patients than to that of the good sleeper group on most measures. This pattern was even more evident in analyses comparing the 11 patients with bipolar disorder plus insomnia with the patients who had insomnia only. However, we make these observations cautiously, as the number of subjects was small; hence the study may have lacked sufficient statistical power to detect minor but important differences.
The bipolar disorder and insomnia groups were heterogeneous in 1) their sleep characteristics (see standard deviations in Table 2), 2) the medications they were taking, and 3) their patterns of comorbidity. However, correlations between the Pittsburgh Sleep Quality Index global score and demographic variables were not significant, suggesting that baseline differences in demographic characteristics were unrelated to sleep problems.
Previous research has established a discrepancy between subjective and objective sleep estimates in patients with insomnia, who tend to overestimate the time it takes them to get to sleep (sleep onset latency) and underestimate how much sleep they obtain overall (total sleep time) (e.g., references 40, 41). In the present study, the bipolar disorder group showed a similar pattern of results: they overestimated how long it took them to fall asleep (by an average of 40.6 minutes [Table 2]) and they underestimated how much sleep they obtained overall (by an average of 1.3 hours [Table 2]). Although these findings should be verified by using polysomnography as the objective sleep measure, we offer several tentative interpretations. First, research on insomnia has highlighted that cognitive arousal is a mechanism that underpins the tendency to misperceive sleep in insomnia (42). Given the results for the Sleep Disturbance Questionnaire and Dysfunctional Attitudes and Beliefs About Sleep Questionnaire in the present study, it is possible that this mechanism also operates to distort the perception of sleep in patients with bipolar disorder. Second, misperception of sleep may contribute to anxiety because it leads the person to believe that the sleep he/she is getting is inadequate. Because anxiety is antithetical to sleep, this difficulty may then serve to maintain the insomnia (43). A behavioral intervention can reduce the tendency to underestimate the amount of sleep obtained and reduce sleep-related anxiety in insomnia patients (44); similar results from use of such interventions would be predicted in euthymic bipolar disorder patients.
Finally, the bipolar disorder group had lower daytime activity levels, relative to both the insomnia group and the good sleeper group. The lower activity level in the bipolar disorder group may be related to their use of medication and may not be important for sleep. On the other hand, it could also be relevant to their insomnia. For example, lower activity levels might serve to maintain nighttime sleep problems, as the patients may not be sufficiently tired to get to sleep. The latter finding raises the possibility that components from the behavioral activation intervention (45) may be worth considering as an adjunct to psychosocial treatment for bipolar disorder.
An additional aim of this study was to assess the constructs that underpin three components of cognitive behavior therapy for insomnia. The finding that the insomnia group had poorer sleep efficiency is consistent with previous findings (see reference 25). Sleep efficiency was the only stimulus control variable to reach significance in the comparison of bipolar disorder patients and good sleepers. This finding raises the possibility that improved sleep efficiency, by means of a stimulus control intervention, may be a useful objective for patients with bipolar disorder (46, 47). By contrast, the bipolar disorder group did not have poor sleep hygiene. This finding brings into question the value of sleep hygiene training in the treatment of insomnia in bipolar disorder patients. By analogy with behavioral approaches to primary insomnia (32), our analysis represents a first step in the process of empirically deriving appropriate interventions for the treatment of sleep disturbance in patients with bipolar disorder.
The results from the Sleep Disturbance Questionnaire and Dysfunctional Attitudes and Beliefs About Sleep Questionnaire were striking. The most commonly endorsed item on the Sleep Disturbance Questionnaire—“I can’t get in a proper routine”—confirms the importance of regularizing the sleep/wake cycle, a treatment component that is already included in cognitive behavior therapy and interpersonal and social rhythm therapy for bipolar disorder (7, 8). The other most frequently endorsed items (“My mind keeps turning things over” and “I am unable to empty my mind”), along with the relatively high score on the cognitive arousal subscale of the Sleep Disturbance Questionnaire, parallel results found in the insomnia groups in this study and in previous research (32, 37). These findings raise the possibility that excessive cognitive arousal while trying to get to sleep may be one process that maintains the sleep disturbances experienced by euthymic bipolar patients.
The bipolar disorder group held a level of dysfunctional beliefs about sleep that was comparable to that of the insomnia group and significantly higher than that of the good sleepers. Although these findings need to be confirmed with a larger group of subjects, we found that holding more dysfunctional beliefs about sleep was associated with more severe sleep disturbance (r=0.76). This association is consistent with previous research findings (48) and suggests that dysfunctional beliefs about sleep may be important in the maintenance of insomnia in patients with bipolar disorder. In addition, relative to the good sleeper group, both the bipolar disorder group and the insomnia group scored higher on the control and predictability of sleep subscale of the Dysfunctional Attitudes and Beliefs About Sleep Questionnaire. The highly scored items of this subscale for the bipolar disorder group were “I am worried that I may lose control over my abilities to sleep,” “I can’t ever predict whether I’ll have a good or poor night’s sleep,” and “I get overwhelmed by my thoughts at night and often feel I have no control over this racing mind.” In addition to confirming the Sleep Disturbance Questionnaire findings just discussed, these items suggest that the bipolar disorder subjects are anxious and fearful about their sleep. Informally, most of the patients in the bipolar disorder group reported that the cause of their anxiety about sleep was the awareness that sleep loss can herald an episode of mania or depression.
The clinical implications of these findings are threefold. First, they raise the possibility that interventions designed to reduce cognitive arousal while trying to get to sleep may be warranted (49). Second, as anxiety and fear are antithetical to sleep onset, they are worthy targets for intervention. Future studies should explore the utility of education (25, 50) and behavioral experiments (51) designed to alter dysfunctional beliefs. Finally, when working with patients to enhance their ability to recognize prodromes and develop effective coping strategies for prodromes (8), care should be taken not to increase anxiety about sleep.
 |
 |
 |
Received Oct. 20, 2003; revision received March 1, 2004; accepted March 22, 2004. From the Departments of Experimental Psychology and Psychiatry, University of Oxford, Oxford, U.K. Address correspondence and reprint requests to Dr. Harvey, Department of Psychology, University of California, Berkeley, 3210 Tolman Hall, No. 1650, Berkeley, CA 94720-1650; [email protected] (e-mail). Supported by a grant from the Jules Thorn Charitable Trust (project number O1SC/02). The authors thank Alison Bugg for assistance with data entry.
1. Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rossler W: Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord 2003; 73:133–146Crossref, Medline, Google Scholar
2. Gitlin MJ, Swendsen J, Heller TL, Hammen C: Relapse and impairment in bipolar disorder. Am J Psychiatry 1995; 152:1635–1640Link, Google Scholar
3. Wu JC, Bunney WE: The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry 1990; 147:14–21Link, Google Scholar
4. Jackson A, Cavanagh J, Scott J: A systematic review of manic and depressive prodromes. J Affect Disord 2003; 74:209–217Crossref, Medline, Google Scholar
5. Goodwin F, Jamison K: Manic-Depressive Illness. New York, Oxford University Press, 1990Google Scholar
6. Wehr TA, Sack DA, Rosenthal NE: Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry 1987; 144:201–204; correction, 144:542Link, Google Scholar
7. Frank E, Swartz HA, Kupfer DJ: Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry 2000; 48:593–604Crossref, Medline, Google Scholar
8. Lam DH, Watkins ER, Hayward P, Bright J, Wright K, Kerr N, Parr-Davis G, Sham P: A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder. Arch Gen Psychiatry 2003; 60:145–152Crossref, Medline, Google Scholar
9. Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR: Nonpharmacologic treatment of chronic insomnia. Sleep 1999; 22:1134–1156Crossref, Medline, Google Scholar
10. Hlastala SA, Frank E, Mallinger AG, Thase ME, Ritenour AM, Kupfer DJ: Bipolar depression: an underestimated treatment challenge. Depress Anxiety 1997; 5:73–83Crossref, Medline, Google Scholar
11. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC, American Psychiatric Press, 1996Google Scholar
12. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
13. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
14. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS: Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19Crossref, Medline, Google Scholar
15. Harvey AG: Insomnia: symptom or diagnosis? Clin Psychol Rev 2001; 21:1037–1059Crossref, Medline, Google Scholar
16. Kessler RC, Rubinow DR, Holmes JM, Abelson JM, Zhao S: The epidemiology of DSM-III-R bipolar disorder in a general population survey. Psychol Med 1997; 27:1079–1089Crossref, Medline, Google Scholar
17. McCrae CS, Lichstein KL: Secondary insomnia: a heuristic model and behavioral approaches to assessment, treatment, and prevention. Appl Prev Psychol 2001; 10:107–123Google Scholar
18. Morin CM, Stone J, McDonald K, Jones S: Psychological management of insomnia: a clinical replication series with 100 patients. Behav Ther 1994; 25:291–309Crossref, Google Scholar
19. Di Nardo PA, Moras K, Barlow DH, Rapee RM, Brown TA: Reliability of the DSM-III-R anxiety disorder categories: using the Anxiety Disorders Interview Schedule—Revised (ADIS-R). Arch Gen Psychiatry 1993; 50:251–256Crossref, Medline, Google Scholar
20. Smith LJ, Nowakowski S, Soeffing JP, Orff HJ, Perlis ML: The measurement of sleep, in Treating Sleep Disorders: Principles and Practice of Behavioral Sleep Medicine. Edited by Perlis ML, Lichstein KL. New York, John Wiley & Sons, 2003, pp 29–76Google Scholar
21. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ: The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28:193–213Crossref, Medline, Google Scholar
22. Carpenter JS, Andrykowski MA: Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 1998; 45:5–13Crossref, Medline, Google Scholar
23. McNally RJ: Remembering Trauma. Cambridge, Mass, Harvard University Press, 2003Google Scholar
24. Schacter DL, Scarry R: Memory, Brain, and Belief. Cambridge, Mass, Harvard University Press, 2000Google Scholar
25. Morin CM, Espie CA: Insomnia: A Clinical Guide to Assessment and Treatment. New York, Kluwer Academic/Plenum, 2003Google Scholar
26. Bonnet MH: The perception of sleep onset in insomniacs and normal sleepers, in Sleep and Cognition. Edited by Bootzin RR, Kihlstrom JF, Schacter DL. Washington, DC, American Psychological Association, 1990, pp 148–158Google Scholar
27. Ogilvie RD, Harsh JR: Sleep Onset: Normal and Abnormal Processes. Washington, DC, American Psychological Association, 1994Google Scholar
28. Reid K, Dawson D: Correlation between wrist activity monitor and electrophysiological measures of sleep in a simulated shiftwork environment for younger and older subjects. Sleep 1999; 22:378–385Crossref, Medline, Google Scholar
29. Sadeh A, Sharkey KM, Carskadon MA: Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17:201–207Crossref, Medline, Google Scholar
30. Ancoli-Israel S. Clopton P, Klauber MR, Fell R, Mason W: Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep 1997; 20:24–27Crossref, Medline, Google Scholar
31. Korszun A, Young EA, Engleberg NC, Brucksch CB, Greden JF, Crofford LA: Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. J Psychosom Res 2002; 52:439–443Crossref, Medline, Google Scholar
32. Harvey AG: Sleep hygiene and sleep-onset insomnia. J Nerv Ment Dis 2000; 188:53–55Crossref, Medline, Google Scholar
33. Bootzin RR, Perlis ML: Nonpharmacologic treatments of insomnia. J Clin Psychiatry 1992; 53(June suppl):37–41Google Scholar
34. Bootzin RR, Rider SP: Behavioral techniques and biofeedback for insomnia, in Understanding Sleep: The Evaluation and Treatment of Sleep Disorders. Edited by Pressman MR, Orr WC. Washington, DC, American Psychological Association, 1997, pp 315–338Google Scholar
35. Lacks P, Rotert M: Knowledge and practice of sleep hygiene techniques in insomniacs and good sleepers. Behav Res Ther 1986; 24:365–368Crossref, Medline, Google Scholar
36. Morin CM: Insomnia: Psychological Assessment and Management. New York, Guilford, 1993Google Scholar
37. Espie CA, Brooks DN, Lindsay WR: An evaluation of tailored psychological treatment of insomnia. J Behav Ther Exp Psychiatry 1989; 20:143–153Crossref, Medline, Google Scholar
38. Espie CA, Inglis SJ, Harvey L, Tessier S: Insomniacs’ attributions: psychometric properties of the Dysfunctional Beliefs and Attitudes About Sleep Scale and the Sleep Disturbance Questionnaire. J Psychosom Res 2000; 48:141–148Crossref, Medline, Google Scholar
39. Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S: Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging 1993; 8:463–467Crossref, Medline, Google Scholar
40. Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R: Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry 1976; 133:1382–1388Link, Google Scholar
41. Mercier JD, Bootzin RR, Lack LC: Insomniacs’ perception of wake instead of sleep. Sleep 2002; 25:559–566Crossref, Google Scholar
42. Tang NKY, Harvey AG: Effects of cognitive arousal and physiological arousal on sleep perception. Sleep 2004; 27:69–78Crossref, Medline, Google Scholar
43. Harvey AG: A cognitive model of insomnia. Behav Res Ther 2002; 40:869–893Crossref, Medline, Google Scholar
44. Tang NKY, Harvey AG: Correcting distorted perception of sleep in insomnia: a novel behavioural experiment? Behav Res Ther 2004; 42:27–39Crossref, Medline, Google Scholar
45. Martell CR Addis ME, Jacobson NS: Depression in Context: Strategies for Guided Action. New York, WW Norton, 2001Google Scholar
46. Bootzin RR, Epstein DR: Stimulus control, in Treatment of Late-Life Insomnia. Edited by Lichstein KL, Morin CM. Thousand Oaks, Calif, Sage, 2000, pp 167–184Google Scholar
47. Bootzin RR: Stimulus control treatment for insomnia, in Proceedings of the 1972 Meeting of the American Psychological Association. Washington, DC, APA, 1972, pp 395–396Google Scholar
48. Edinger JD, Fins AI, Glenn M, Sullivan RJ, Bastian LA, Marsh GR, Dailey D, Hope TV, Young M, Shaw E, Vasilas D: Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol 2000; 68:586–593Crossref, Medline, Google Scholar
49. Harvey AG: Unwanted thought in insomnia, in The Nature and Treatment of Unwanted Intrusive Thoughts in Clinical Disorders. Edited by Clark DA. New York, Guilford, 2004, pp 86–118Google Scholar
50. Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE: Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA 2001; 285:1856–1864Crossref, Medline, Google Scholar
51. Ree MJ, Harvey AG: Behavioural experimental in chronic insomnia, in The Oxford Handbook of Behavioural Experiments. Edited by Bennett-Levy J, Butler G, Fennell MJV, Hackmann A, Mueller M, Westbrook D. Oxford, UK, Oxford University Press, 2004, pp 287–308Google Scholar



