Response Inhibition in Adolescents Diagnosed With Attention Deficit Hyperactivity Disorder During Childhood: An Event-Related fMRI Study
Abstract
OBJECTIVE: Frontostriatal neural abnormalities have been implicated in the response inhibition impairments that are characteristic of attention deficit hyperactivity disorder (ADHD). However, reports of such abnormalities in adolescents are inconsistent. The present study used behavioral and functional neuroimaging techniques to examine inhibitory control processes in adolescents who had been diagnosed with ADHD during childhood. METHOD: The authors used functional magnetic resonance imaging (fMRI) during performance of a Go/No-Go task to scan 10 male adolescents who were diagnosed with DSM-III-R ADHD when they were 7 to 11 years old and nine age-, sex-, and IQ-matched comparison subjects with no history of ADHD. Response inhibition was tested by contrasting neural activation during No-Go trials with that during Go trials. RESULTS: The inhibition of a prepotent tendency to respond produced markedly greater activation of the left anterior cingulate gyrus, bilateral frontopolar regions, bilateral ventrolateral prefrontal cortex, and left medial frontal gyrus in the adolescents with childhood ADHD than in the adolescents with no history of ADHD. Activity in the first two regions was inversely related to task performance across the study group. CONCLUSIONS: Compared with adolescents who had no history of ADHD, adolescents who were diagnosed with ADHD during childhood exhibited enhanced responses during inhibition in ventrolateral prefrontal cortical areas that subserve response inhibition, as well as in anterior cingulate and frontopolar regions implicated in other executive functions.
Attention deficit hyperactivity disorder (ADHD) is characterized by developmentally inappropriate symptoms of inattention, impulsiveness, and hyperactivity that arise in childhood and often persist into adolescence. Many investigators view these symptoms as varied manifestations of core impairments in inhibitory control (1, 2), a self-regulatory process that has been conceptualized as the ability to withhold a prepotent response, interrupt an ongoing response, and protect cognitive activity from interference (1). Deficits in inhibitory processes have been found in individuals with ADHD across the lifespan (3), and the similarity of these deficits to those of patients with frontal lobe lesions first implicated prefrontal cortical abnormalities in the pathophysiology of ADHD (4). More recently, morphometric neuroimaging studies have consistently reported subtle volumetric reductions of the prefrontal cortex and caudate nucleus in children with ADHD (5, 6), and functional studies have found reduced metabolic activity in these same regions in children (7) and adults (8) with ADHD but not in adolescents (9, 10) with ADHD.
The introduction of functional magnetic resonance imaging (fMRI) provides an ideal opportunity to examine the neural correlates of inhibitory control deficits in ADHD. However, initial results have been mixed. Steady-state perfusion was reduced in the striatum of children with ADHD (11), and striatal activation was attenuated in young children (12), latency-aged boys (13), and adolescents (14) with ADHD performing the Go/No-Go and Stop tasks. Prefrontal cortical activity during these same inhibitory tasks was enhanced in young children (12) and latency-aged boys (13) with ADHD but reduced in adolescents with ADHD (14). Further, activation of the anterior cingulate cortex was reduced in adolescents (14) and adults (15) with ADHD performing the Stop and Stroop tasks.
Thus, convergent findings from neuroimaging studies provide evidence of frontostriatal abnormalities in ADHD but cannot pinpoint the precise nature of the pathophysiology. The developmental course of the frontostriatal abnormalities and their relation to ADHD symptoms during adolescence are also unclear. Several frontostriatal regions are smaller in children with ADHD, but only the cerebellum continues to show persistent decreased size through adolescence (6). Moreover, functional neuroimaging studies of adolescents with ADHD have found either no significant results (9, 10) or results (14) that contradicted those reported in children (12, 13). These discrepancies may reflect the fact that adolescent neuroimaging studies have based their diagnoses on retrospective reports of childhood behavior, which are of questionable validity, or focused on a somewhat selective group of the ADHD population, namely, adolescents who continue to exhibit the full disorder. Most children with ADHD experience a diminution of symptoms as they grow older (16), and a substantial proportion no longer meet full diagnostic criteria for the disorder in adolescence (17). As such, investigation of adolescents diagnosed with ADHD during childhood, irrespective of current diagnosis, may yield answers regarding the developmental course of neural abnormalities in ADHD.
The present investigation used fMRI in conjunction with a Go/No-Go task to examine response inhibition in adolescents who were diagnosed with DSM-III-R ADHD during childhood and now reflect the diverse developmental outcomes characteristic of the disorder (16, 17). Previous studies have implicated the caudate nucleus and ventral prefrontal cortex in response inhibition in children (18) and adults (18, 19); activation of anterior cingulate regions that may reflect other aspects of the task has also been reported (19). On the basis of these data and the results of previous studies that used Go/No-Go tasks in children with ADHD (12, 13), we predicted that adolescents with childhood ADHD would exhibit greater activation of ventral prefrontal regions and less activation in the striatum and anterior cingulate than adolescents with no history of ADHD.
Method
Subjects
Participants were 10 male adolescents who were diagnosed with DSM-III-R ADHD when they were 7–11 years old and nine male adolescents with no history of ADHD. The patients were recruited from a larger pool of participants in a study of ADHD conducted between 1990 and 1994 (20, 21). Nine of the patients were right-handed, and one was left-handed. The childhood diagnosis was based on parental responses to the Diagnostic Interview Schedule for Children—Parent Version (22). Patients with a diagnosis of schizophrenia, pervasive developmental disorder, major affective disorder, or Tourette’s syndrome were excluded from the initial study, as were those with a full-scale IQ below 70. Although patients were initially diagnosed according to DSM-III-R criteria, all would have met DSM-IV criteria for ADHD, combined type, during childhood. Two patients had a comorbid diagnosis of conduct disorder in childhood, and one of these children also met diagnostic criteria for separation anxiety disorder.
The patients were reevaluated on average 8.8 years (SD=1.1) following their childhood assessment. Their mean age at reevaluation was 17.9 years (SD=1.6, range=15.0–19.8). Patients and their parents were interviewed separately with the National Institute of Mental Health Diagnostic Interview Schedule for Children Version IV (23), and the two reports were combined by using an either-parent-or-adolescent algorithm (i.e., adding symptoms reported by either source) to diagnose ADHD and other psychiatric disorders (24).
All 10 patients met diagnostic criteria for ADHD during childhood, but their adolescent psychiatric status reflects the diverse outcomes characteristic of the disorder (16, 17): five patients met criteria for DSM-IV ADHD in partial remission as defined by fewer than six symptoms in both the inattentive and hyperactive-impulsive domains; one met full criteria for the combined type of ADHD; three met criteria for the predominately inattentive type of ADHD; and one met criteria for the predominately hyperactive-impulsive type. However, the four patients who met criteria for inattentive or hyperactive-impulsive ADHD should not truly be considered to have the predominately inattentive or hyperactive-impulsive type of ADHD. Rather, they were children with ADHD, combined type, who experienced a diminution of symptoms with age (16), had high numbers of both inattentive and hyperactive-impulsive symptoms, and are more likely to resemble adolescents with ADHD in partial remission than those who had the inattentive or hyperactive-impulsive type during childhood.
One adolescent also met criteria for conduct disorder, but there were no reports of any other axis I disorders. The mean attention problems score on the Child Behavior Checklist (25) completed by parents was 63.6 (SD=9.8). The mean full-scale IQ, as assessed with the WISC-III or WAIS-III, depending on age, was 88.4 (SD=15.7). Seven patients had a previous history of treatment with stimulant medications, but no patient received medication for ADHD in the 6 months preceding this study.
The nine adolescent comparison subjects were recruited by means of advertisements placed in the communities where the patients resided. These adolescents were all right-handed and had a mean age of 17.5 (SD=1.2, range=16.1–19.9). Comparison subjects and their parents were interviewed separately with the disruptive behavior disorders module of the Diagnostic Interview Schedule for Children Version IV (23), and the two reports were combined using an either-or algorithm to screen for a past or present history of ADHD. Comparison subjects with a history of two or more symptoms of ADHD during any 6-month period were excluded from the study. The comparison subjects were not systematically interviewed for the presence of other psychiatric symptoms or disorders. Thus, they most likely did not constitute a “supernormal” group (i.e., free of all pathology or psychiatric symptoms) that is unrepresentative of the rate of “normality” found in the urban population from which the patients were recruited. Nevertheless, those with a previous psychiatric diagnosis or a history of treatment were excluded. Mean estimated IQ of the comparison subjects, as assessed with the Vocabulary and Block Design subtests of the WISC-III/WAIS-III, was 91.9 (SD=16.0). None of the comparison subjects had been exposed to psychotropic medication. Patients and comparison subjects did not differ in age (t=0.63, df=17, p=0.54) or estimated IQ (t=0.61, df=17, p=0.55).
The study was approved by the institutional review boards of Queens College of the City University of New York and the Mount Sinai School of Medicine. After complete description of the study procedures, written informed consent was obtained from the adolescents and, when appropriate, their parents. The adolescents were compensated for their participation in the study.
Experimental Procedures
The Go/No-Go task was conceptualized as a measure of the ability to inhibit responses to rare nontargets (No-Go trials) in the context of frequent targets (Go trials). The task consisted of three 200-second blocks. Each block contained 120 stimuli, with 99 (83%) Go trials and 21 (17%) No-Go trials, resulting in a total of 63 No-Go trials in the task. The stimulus for the No-Go trials was “X”; the stimuli for the Go trials was “A” through “F.” Trial order was pseudorandomized so that the occurrence of No-Go trials was jittered from 3 seconds to 12 seconds (i.e., preceded by two to eight Go trials). Each block began with a 20-second central fixation-cross, after which the stimuli were presented at fixation for 500 msec followed by a 1000-msec interstimulus interval demarcated by a central fixation-cross. Participants were reminded at the beginning of each block to respond as quickly as possible while trying not to make mistakes.
Stimuli were generated on an IBM-compatible personal computer and projected by means of an SVGA projector system onto a rear-projection screen mounted over the participants’ legs. Participants viewed the stimuli through a mirror on the head coil positioned above their eyes and responded with an optical button held in the right hand. Responses were recorded on a personal computer and provided measures of reaction time and accuracy.
Image Acquisition
Structural and functional MRI scans were acquired on the same 1.5-T General Electric Horizon scanner (GE Medical Systems, Milwaukee) modified with hardware for echo planar imaging. A series of T1-weighted three-dimensional structural images (spoiled-gradient recall echo in a steady state, repetition time [TR]=24 msec, echo time [TE]=5 msec, flip angle=40°, 124 slices, slice thickness=1.2 mm contiguous, field of view=230 mm, matrix=256×256 pixels) were acquired for cross-subject registration. Subsequently, 14 axial spin-echo T2-weighted structural images encompassing the whole brain (TR=600 msec, TE=18 msec, slice thickness=5 mm, spacing=2.5 mm, field of view=230 mm, matrix=256×256 pixels) were obtained to localize the functional activity and to align images to a reference brain. Functional scans of the whole brain were acquired at the same 14-slice locations by using a multislice two-dimensional echo planar imaging sequence depicting the blood-oxygenation-level-dependent (BOLD) signal (TR=2000 msec, TE=40 msec, flip angle=90°, slice thickness=5 mm, spacing=2.5 mm, field of view=230 mm, matrix=64×64 pixels). The participants completed three runs of 200 seconds each, resulting in 100 time points per participant.
Data Analysis
Image preprocessing and analyses were conducted by using statistical parametric mapping (SPM 99, Wellcome Department of Cognitive Neurology, London). The first 10 volumes of each functional time series were discarded. The functional scans were realigned to the remaining first volume to correct for interscan movements by means of a rigid body transformation with three rotation and three translation parameters. The functional scans, the T2-weighted anatomical scan, and the high-resolution three-dimensional spoiled-gradient recall echo image were coregistered. The functional scans were subsequently spatially normalized to a standard template (Montreal Neurological Institute) by using normalization parameters estimated from the three-dimensional spoiled-gradient recall echo image and were then resampled by using a sinc interpolation, resulting in a voxel size of 2×2×2 mm. Finally, the functional images were smoothed with a 7.5×7.5×15-mm full width at half maximum Gaussian kernel.
General linear modeling was conducted for the functional scans from each subject by modeling the observed event-related BOLD signals and regressors to identify the relationship between the experimental parameters and the hemodynamic response. Event-related analyses were conducted by using the default statistical parametric mapping basis function, which consists of a synthetic hemodynamic response function composed of two gamma functions and its derivative (26). Regressors were created by convolving a train of delta functions (representing the sequence of individual trials) with the base function. The linear combination of all the regressors was used to model the hemodynamic response to four conditions: correct and incorrect No-Go and Go trials. The six realignment parameters generated during motion correction were entered as covariates.
The images for each participant were collapsed into a single image for each of the four conditions. The specific effects of response inhibition were tested by applying appropriate linear contrasts to the parameter estimates for the correct No-Go minus correct Go contrast, resulting in a contrast map for each participant. The contrast images of all participants were entered into second-level group analyses conducted with a random effects statistical model that accounted for intrasubject variability and permitted population-based inferences to be drawn. These analyses examined group-by-condition interactions highlighting changes in activation patterns during correct No-Go trials versus correct Go trials in adolescents with and without a history of ADHD.
The resultant voxel-wise statistical maps were then thresholded for significance by using a cluster-size algorithm that protects against an inflation of the false positive rate with multiple comparisons. Results for a priori regions of interest are reported at an uncorrected height (intensity) threshold of p<0.01 and an extent threshold of kappa=120 voxels. A Monte Carlo simulation (http://www.wjh.harvard.edu/~slotnick/scripts/cluster_threshold_ beta.m) of the brain volume in the present study indicated that these thresholds correspond to a whole brain false positive rate of approximately 0.01. Post hoc analyses were conducted to test the effects of response inhibition separately in the adolescents with and without a history of ADHD. Coordinates of activation were converted from the Montreal Neurological Institute coordinates to the Talairach and Tournoux coordinates (27) by using the mni2tal algorithm (M. Brett, Cambridge, Mass.) before designation of anatomical localization and Brodmann’s areas.
Group differences in the behavioral data were analyzed by using Student’s t test, in which the percent of commission errors on No-Go trials and the percent of omission errors and mean reaction time on Go trials served as the dependent variables. Finally, Pearson product-moment correlations between percent change in MRI signal intensity, Child Behavior Checklist attention problems score, and behavioral performance were calculated for all subjects and within the two groups separately for regions that demonstrated significant activation.
Results
Task Performance
Adolescents with childhood ADHD made significantly more commission errors on No-Go trials (mean=28.6%, SD=14.0%) than adolescents with no history of ADHD (mean=14.7%, SD=13.5%) (t=–2.19, df=17, p=0.04). However, group differences in the percent of omission errors on Go trials (patients’ mean=4.3%, SD=6.2%, versus comparison subjects’ mean=1.3%, SD=2.7%) and mean reaction time (patients’ mean=340 msec, SD=24, versus comparison subjects’ mean=386 msec, SD=91) were not significant (t=1.34, df=17, p=0.20, and t=1.54, df=17, p=0.14, respectively). Task performance did not correlate with the attention problems score of the Child Behavior Checklist in the adolescents with childhood ADHD (all p>0.10). There were no significant group differences in mean translational movement along the echo planar time series (patients’ mean=0.42 mm, SD=0.09, versus comparison subjects’ mean=0.41 mm, SD=0.09) (t=0.11, df=17, p=0.92). Mean rotational displacement for both groups was less than 0.01 (t=0.56, df=17, p=0.58).
fMRI Data
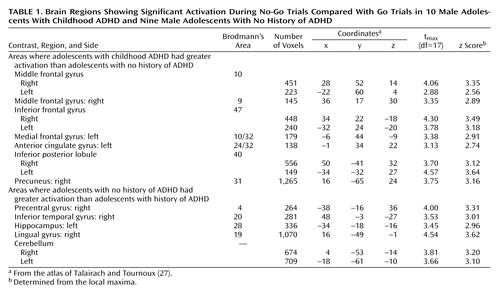
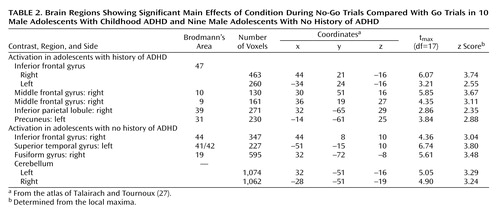
Significant group-by-condition interactions for the comparison of correct No-Go trials and correct Go trials in adolescents with and without a history of ADHD were seen in several regions of interest (Table 1 and Figure 1). Specifically, adolescents with childhood ADHD exhibited markedly greater activation of the left and right ventrolateral inferior frontal gyrus (Brodmann’s area 47), left and right frontopolar regions of the middle frontal gyrus (Brodmann’s area 10), right dorsolateral middle frontal gyrus (Brodmann’s area 9), left anterior cingulate gyrus (Brodmann’s area 24/32), and left medial frontal gyrus (Brodmann’s area 10/32) than adolescents with no history of ADHD. Closer examination of the interaction effects revealed that patients had greater activation of these regions for No-Go than Go trials and that comparison subjects showed greater activation for Go than No-Go trials. Patients also showed greater activity of the right precuneus (Brodmann’s area 31) and bilateral inferior parietal lobule (Brodmann’s area 40). In contrast, stimulus-induced activation of the left precentral gyrus (Brodmann’s area 4), right lingual gyrus (Brodmann’s area 19), right inferior temporal gyrus (Brodmann’s area 20), left hippocampus, and bilateral cerebellum was greater in adolescents with no history of ADHD than in those with childhood ADHD (Table 1).
Post hoc analyses of the effects of condition (correct No-Go trials versus correct Go trials) separately in patients and comparison subjects revealed divergent patterns of activation in the two groups (Table 2). Adolescents with childhood ADHD activated the left and right ventrolateral inferior frontal gyrus (Brodmann’s area 47), right frontopolar cortex (Brodmann’s area 10), right dorsolateral middle frontal gyrus (Brodmann’s area 9), right inferior parietal lobule (Brodmann’s area 39), and left precuneus (Brodmann’s area 7). In contrast, adolescents with no history of ADHD activated a neural network that included the right inferior frontal gyrus (Brodmann’s area 44), left superior temporal gyrus (Brodmann’s area 41/42), right fusiform gyrus (Brodmann’s area 19), and left and right cerebellum.
The magnitude of the signal change at the voxel of maximal activation in bilateral frontopolar regions (mean of left and right) and left anterior cingulate gyrus correlated with the percent of commission errors in all subjects (frontopolar: r=0.57, N=19, p=0.01; cingulate: r=0.64, N=19, p=0.003) (Figure 2). The relationship between anterior cingulate activation and errors was evident but not significant because of the small number of subjects in each group (r=0.59, N=10, p=0.08, for adolescents with childhood ADHD and r=0.56, N=9, p=0.12, for adolescents with no history of ADHD). In contrast, frontopolar activation was related to commission errors only in adolescents with no history of ADHD (r=0.63, N=9, p=0.07). There were no significant correlations between activation in the adolescents with childhood ADHD and the attention problems score of the Child Behavior Checklist (all p>0.10).
Discussion
These findings provide evidence of prefrontal cortical abnormalities that are clearly linked to impaired performance on a response inhibition task in adolescents who were diagnosed with ADHD during childhood. These adolescents performed more poorly on the Go/No-Go task and exhibited differential activation in several neural regions previously implicated in the pathophysiology of ADHD compared with adolescents matched for age, gender, and IQ who had no history of ADHD. Specifically, the inhibition of a prepotent response produced markedly greater activation of the left anterior cingulate gyrus, left medial frontal gyrus, left and right frontopolar, left and right ventrolateral, and right dorsolateral regions of the prefrontal cortex in patients than comparison subjects. In fact, activation of these regions in comparison subjects increased when they were responding and decreased during response inhibition.
Activation of the anterior cingulate gyrus was inversely related to performance on the Go/No-Go task, with greater activation in individuals who had more difficulty inhibiting the prepotent response. A similar relationship between performance and frontopolar activation was found only in the adolescents with no history of ADHD. These findings are somewhat surprising given that these regions are not generally associated with response inhibition per se but, rather, with interrelated executive processes (28–31). The anterior cingulate gyrus seems to monitor for conflict (28, 29), and the frontopolar regions purportedly mediate “cognitive branching” or the maintenance of a primary goal during the performance of a concurrent task (30, 31). Anterior cingulate and frontopolar activation is usually associated with responding (28, 32), and the inverse correlations with task performance in adolescents with no history of ADHD indicate that successful response inhibition involved decreased activation of these regions. Similar correlations between anterior cingulate activation and performance in adolescents with childhood ADHD suggest that they used comparable conflict monitoring processes but with less efficiency.
In contrast, the fact that frontopolar activity was related to performance in adolescents with no history of ADHD but not those who had the disorder suggests differential engagement of higher-order executive processes by the two groups. These data suggest that the inhibitory control deficits in adolescents with childhood ADHD may be related to difficulties with overriding executive processes generally associated with responding. Alternately, the unexpected finding of reduced bilateral cerebellar activation in adolescents with childhood ADHD raises the possibility that enhanced frontopolar and anterior cingulate activity in these individuals might represent adaptive mechanisms to compensate for impairments in the cognitive processes mediated by the cerebellum.
The present results are largely consistent with two previous fMRI studies that used the Go/No-Go task in young children (12) and latency-aged adolescents (13) with ADHD. The increased ventral prefrontal cortical activity in children with ADHD reported in these studies is comparable to the present findings of greater activation of the ventrolateral prefrontal cortex in adolescents with childhood ADHD. The ventral prefrontal cortex is thought to subserve response inhibition in both children and adults (18, 19). Together, these data suggest that the abnormal neural activation seen in the adolescents with childhood ADHD in the present study may reflect a continuation of response inhibition deficits from childhood.
The current findings of enhanced ventrolateral prefrontal and anterior cingulate activation in adolescents with childhood ADHD differ from previous reports of reduced activation of the inferior prefrontal cortex in adolescents with ADHD performing the Stop task (14) and the anterior cingulate gyrus in adults with ADHD during the Stroop task (15). These discrepancies may be related to the different tasks used in the studies or to differences between the subjects in each study.
The patients in our current study were unique in several ways. First, they were all diagnosed with ADHD as children but demonstrated different degrees of symptoms in adolescence. Many of these adolescents would not have qualified for the neuroimaging studies of adolescents (14) and adults (15) with ADHD, which focused on the relatively selective group of adults and adolescents who continue to have the full disorder. Second, and closely related, the patients in our study were not self-referred during adolescence and adulthood, as were the subjects in other neuroimaging studies of ADHD (14, 15). Self-referral of adolescents and adults with ADHD is associated with numerous selection biases as well as concerns regarding the veracity of retrospective recall of childhood symptoms (33) and has consistently generated different findings from those reported in longitudinal studies (34). Finally, none of the participants in the present study had received stimulant treatment in the 6 months before being scanned, but many or all of the participants in previous studies of adolescents and adults with ADHD were being treated with stimulants and were scanned after limited washout periods (14, 15).
These findings must be considered in the context of the methodological limitations of the Go/No-Go task used in this study. First, the comparison of Go trials, which required motor responses, and No-Go trials, which did not involve responses, introduced motor activity as a potential confounding factor in the analyses. However, this is less of an issue in group comparisons like the current study than in single-group designs, since the two groups serve as controls for each other. Second, the preponderance of Go trials over No-Go trials required to create the prepotent tendency to respond in the task also yielded more data points for Go trials than No-Go trials, which may have skewed the analyses toward the effects of Go trials. Thus, we cannot completely rule out that our findings reflect the effects of motor control processes rather than inhibitory control processes.
Notwithstanding these limitations, the current results indicate that adolescents who were diagnosed with ADHD during childhood have difficulty inhibiting a prepotent response and correspondingly generate greater activation of ventrolateral prefrontal cortex, which mediates response inhibition, as well as higher-order anterior cingulate and frontopolar cortical areas, which subserve executive processes. Inverse correlations between anterior cingulate and frontopolar activation and performance indicate that successful response inhibition involves decreased activity in these regions. The data suggest that the enhanced activation in adolescents with childhood ADHD may be related to difficulties with overriding response processes or may reflect adaptive mechanisms to compensate for impairments in other brain regions.
 |
 |
Received Aug. 2, 2003; revision received Dec. 16, 2003; accepted Dec. 23, 2003. From the Departments of Psychiatry and Radiology, Mount Sinai School of Medicine; the Sackler Institute for Developmental Psychobiology, Weill Medical College of Cornell University, New York; the Neuropsychology Subprogram of the Ph.D. Program in Psychology, Graduate Center of the City University of New York; and the Department of Psychology, Queens College of the City University of New York. Address reprint requests to Dr. Schulz, Department of Psychiatry, Box 1230, Mount Sinai School of Medicine, One Gustave Levy Place, New York, NY 10029; [email protected] (e-mail). Supported by grant 97183497 from the William T. Grant Foundation (Dr. Halperin) and NIMH collaborative grant R21 MH-066360-01 (Dr. Newcorn).
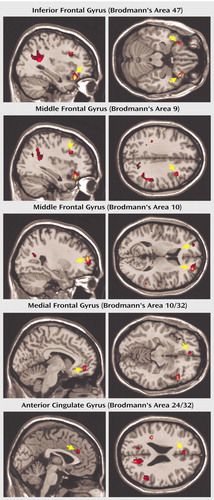
Figure 1. Brain Regions of Significantly Greater Activation During No-Go Trials Compared With Go Trials in 10 Male Adolescents With Childhood ADHD Than in Nine Adolescent Males With No History of ADHDa
aArrows indicate activation in region of interest.
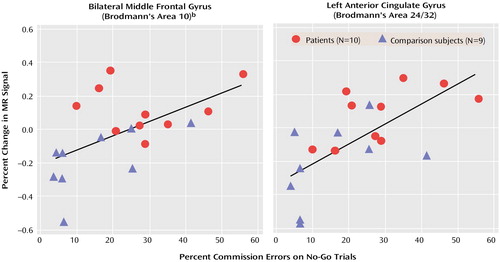
Figure 2. Change in Magnetic Resonance (MR) Signal in Left and Right Middle Frontal Gyrus and Left Anterior Cingulate Gyrus as a Function of Percent Commission Errors in 19 Male Adolescents With or Without Childhood ADHDa
aThe percent of commission errors on No-Go trials correlated significantly with the magnitude of the MR signal change at the voxel of maximal activation during No-Go trials compared with Go trials in left and right frontopolar regions (r=0.57, N=19, p=0.01) and left anterior cingulate gyrus (r=0.64, N=19, p=0.003).
bMean of the activation in right and left hemispheres.
1. Barkley RA: Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121:65–94Crossref, Medline, Google Scholar
2. Schachar R, Tannock R, Logan G: Inhibitory control, impulsiveness, and attention deficit hyperactivity disorder. Clin Psychol Rev 1993; 13:721–739Crossref, Google Scholar
3. Sergeant JA, Geurts H, Oosterlaan J: How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res 2002; 130:3–28Crossref, Medline, Google Scholar
4. Mattes JA: Role of frontal lobe dysfunction in childhood hyperkinesis. Compr Psychiatry 1980; 21:358–369Crossref, Medline, Google Scholar
5. Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J: Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology 1997; 48:589–601Crossref, Medline, Google Scholar
6. Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL: Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 2002; 288:1740–1748Crossref, Medline, Google Scholar
7. Kim BN, Lee JS, Shin MS, Cho SC, Lee DS: Regional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci 2003; 252:219–225Google Scholar
8. Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM: Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990; 323:1361–1366Crossref, Medline, Google Scholar
9. Zametkin AJ, Liebenauer LL, Fitzgerald GA, King AC, Minkunas DV, Herscovitch P, Yamada EM, Cohen RM: Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1993; 50:333–340Crossref, Medline, Google Scholar
10. Ernst M, Cohen RM, Liebenauer LL, Jons PH, Zametkin AJ: Cerebral glucose metabolism in adolescent girls with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:1399–1406Crossref, Medline, Google Scholar
11. Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF: Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nat Med 2000; 6:470–473Crossref, Medline, Google Scholar
12. Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti I-M, Yang Y, Ulug AM, Casey BJ: Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 2003; 53:871–878Crossref, Medline, Google Scholar
13. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998; 95:14494–14499Crossref, Medline, Google Scholar
14. Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET: Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry 1999; 156:891–896Link, Google Scholar
15. Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J: Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry 1999; 45:1542–1552Crossref, Medline, Google Scholar
16. Biederman J, Mick E, Faraone SV: Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry 2000; 157:816–818Link, Google Scholar
17. Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA: Hyperactive boys almost grown up. Arch Gen Psychiatry 1991; 48:77–83Crossref, Medline, Google Scholar
18. Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman R, Casey BJ: A neural basis for development of inhibitory control. Dev Sci 2002; 5:9–16Crossref, Google Scholar
19. Garavan H, Ross TJ, Stein EA: Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA 1999; 96:8301–8306Crossref, Medline, Google Scholar
20. Halperin JM, Sharma V, Siever LJ, Schwartz ST, Matier K, Wornell G, Newcorn JH: Serotonergic function in aggressive and nonaggressive boys with attention deficit hyperactivity disorder. Am J Psychiatry 1994; 151:243–248Link, Google Scholar
21. Halperin JM, Newcorn JH, Schwartz ST, Sharma V, Siever LJ, Koda VH, Gabriel S: Age-related changes in the association between serotonergic function and aggression in boys with ADHD. Biol Psychiatry 1997; 41:682–689Crossref, Medline, Google Scholar
22. Shaffer D, Fisher P, Piacentini J, Schwab-Stone M, Wicks J: Diagnostic Interview Schedule for Children—Parent Version (DISC-2.1P). New York, New York State Psychiatric Institute, 1989Google Scholar
23. Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME: NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 2000; 39:28–38Crossref, Medline, Google Scholar
24. Schwab-Stone ME, Shaffer D, Dulcan MK, Jensen PS, Fisher P, Bird HR, Goodman SH, Lahey BB, Lichtman JH, Canino G, Rubio-Stipec M, Rae DS: Criterion validity of the NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC 2.3). J Am Acad Child Adolesc Psychiatry 1996; 35:878–888Crossref, Medline, Google Scholar
25. Achenbach TM: Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, University of Vermont, Department of Psychiatry, 1991Google Scholar
26. Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R: Event-related fMRI: characterizing differential responses. Neuroimage 1998; 7:30–40Crossref, Medline, Google Scholar
27. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
28. Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI: Cognitive and brain consequences of conflict. Neuroimage 2003; 18:42–57Crossref, Medline, Google Scholar
29. Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung H-C, May J, Gore JC: An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cogn Brain Res 2002; 13:427–440Crossref, Medline, Google Scholar
30. Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J: The role of the anterior prefrontal cortex in human cognition. Nature 1999; 399:148–151Crossref, Medline, Google Scholar
31. Braver TS, Bongiolatti SR: The role of frontopolar cortex in subgoal processing during working memory. Neuroimage 2002; 15:523–536Crossref, Medline, Google Scholar
32. Picard N, Strick PL: Imaging the premotor areas. Curr Opin Neurobiol 2001; 11:663–672Crossref, Medline, Google Scholar
33. Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P: Accuracy of adult recall of childhood attention deficit hyperactivity disorder. Am J Psychiatry 2002; 159:1882–1888Link, Google Scholar
34. Marks DJ, Newcorn JH, Halperin JM: Comorbidity in adults with attention-deficit/hyperactivity disorder. Ann NY Acad Sci 2001; 931:216–238Crossref, Medline, Google Scholar



